The present study conducted a genetic characterization and determined growth rate and biomass production in solid and liquid media, using strains obtained from wild edible sporomes of Lyophyllum that grow in high mountains. Vegetative isolation was used to obtain a total of four strains, which were divided into two clades within the section Difformia: Lyophyllum sp. and Lyophyllum aff. shimeji. Growth rate and biomass production were influenced by both the culture media and the strains. In a potato dextrose agar medium, the strains presented a higher growth rate, while in a malt extract-peptone and yeast agar medium, the growth rate was lower, but with a higher biomass production that was equal to that in the malt extract-peptone and yeast liquid medium.
Wild edible mushrooms play an important role in natural ecosystems and have great economic potential. Management of their natural populations should therefore be based on sustainable development, through local knowledge and the study of species domestication techniques, that allows long-term conservation of the wild germplasm.1–3 Technological development surrounding strains of wild edible mushrooms should preferentially be focused on species that are adapted to local climatic conditions and to the existing substrates of each region in order to be successfully cultivated.4
Worldwide, 92 species of wild edible and medicinal mushrooms have been reported that can be cultivated.5 The genus Lyophyllum is of great economic, culinary and medicinal importance, as well as being used for auto-consumption, and some of its edible species are characterized by their excellent flavor and texture.6–11 These species are phylogenetically classified within the section Difformia, although their taxonomic position can be confusing as a result of their morphological plasticity.12,13 In general, they are saprobic species; however, some can be facultative ectomycorrhizal.10
Commercially cultivated species of Lyophyllum include L. shimeji (Kawam.) Hongo, L. descastes (Fr.) Singer and L. fumosum (Pers.) P.D. Orton.5,9,11,14–17 Despite the fact that L. shimeji forms ectomycorrhizae, it has been cultivated successfully in synthetic medium thanks to the capacity of the mycelium for rapid growth in generalist media, such as potato dextrose agar.13 There is controversy in the case of L. decastes and L. fumosum, since both have been reported as saprobic or ectomycorrhizal.10,13,14,18,19 This could be related to the geographic origin of the strains and to the habitat in which they develop. This inconsistency could also be a consequence of incorrect species identification.10,12 In Mexico, both species are considered edible and are of cultural and dietary importance. They are commonly known as “clavitos”, “enterrados” or “macoyitas” and are rare and difficult to find. They grow in forests of Pinus, Pinus-Alnus, Pinus-Abies and Abies religiosa.6,7,20–23
Commercial cultivation of Lyophyllum spp. is a long and complex process. Most studies are focused on the acquisition of biomass in submerged cultures, which is considered a viable alternative for obtaining high quality mushrooms, rich in immunostimulant polysaccharides.8 The necessity of generating knowledge regarding the genetic resources of native fungi of central Mexico is therefore proposed. The objectives of the present study were to characterize native high mountain strains of the genus Lyophyllum, as well as to determine their growth rate and biomass production in both solid and liquid media. It is intended to use this information to lay a base groundwork that help to know optimum conditions for the growth and conservation of germplasm (strains), for further work on fruiting bioassays.
Materials and methodsBiological materialStrains were obtained by vegetative isolation from sporophores of the genus Lyophyllum. Mushrooms were collected through directed sampling in forests of Pinus spp. and Abies religiosa within the Nevado de Toluca Flora and Fauna Protection Area (APFFNT, by its Spanish acronym) in State of Mexico, Mexico. Collections were conducted with the help of key informants, who provided information during the fieldwork about the use of these mushrooms as food. The specimens were herborized,24 characterized with basic mycology techniques, identified11,25,26 and deposited in the Fungi Collection of the Nacional Herbarium of Mexico at the Institute of Biology, UNAM (MEXU), with herbarium numbers 28148, 28147 and 28149. The strains were deposited in the Strain Collection of the Institute of Ecology (INECOL) in Xalapa, Veracruz, Mexico, with the keys IE 981, IE 982, IE 983. Along with the strains obtained, analysis was conducted on strain IE 975, which had already been deposited in the strain collection after being obtained in a previous study.27 The DNA of the strains was extracted and sequenced and their growth rate and biomass production in solid media and biomass production in liquid media evaluated.
Genetic characterizationAn XNAP kit (Sigma–Aldrich) was used to extract the DNA from the strains. A dissection needle was used to take 1–2mm2 of aerial mycelium from each strain in the PDA medium. The mycelium was incubated for 10min at 65°C and 10min at 95°C in 20μL of extraction solution in a thermocycler (Bio-Rad, model T100). Following incubation, 20μL of dilution solution was added and the mixture incubated at ambient temperature for 30min.28 The ribosomal internal transcribed spacer (ITS) region was amplified with the primers and ITS1F and ITS4,29 following the protocol of Izzo et al.30 The quality of the PCR products was reviewed by electrophoresis in 1% agarose gels. Good quality products were selected and cleaned using a mixture of 1μL of ultrapure water and 1μL of ExoSAP-IT (USB-Affimetrix) for each 3.5μL of PCR product. The sequencing reaction was conducted with a BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems), following the manufacturer's instructions. The DNA was sequenced in both directions, using the same primers used in the PCR, in a sequencer ABI 3100 (Applied Biosystems) in the Laboratory of Genomic Sequencing of Biodiversity and Health of the Institute of Biology, UNAM. The sequences were edited and assembled in Geneious Pro R7 (Biomatters). The sequences of each strain were deposited in GenBank with the accession numbers KY195930, KY195931, KY195932 and KY195933.
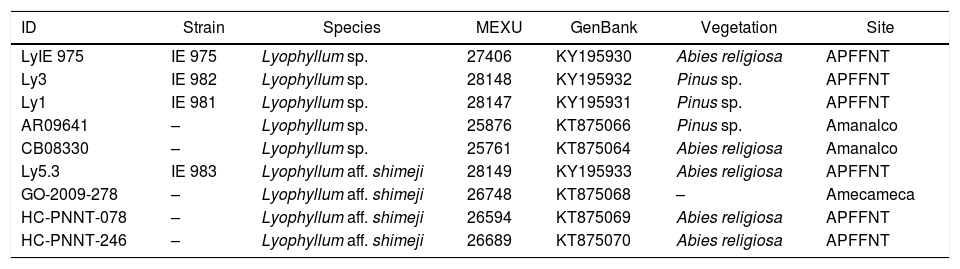
The phylogenetic analyses used the DNA sequences of clade V (section Difformia) for phylogenetic analysis of the family Lyophyllaceae.31 Sequences of specimens of Lyophyllum collected previously in State of Mexico were also used (Table 1). The outgroup was Lyophyllum leucophaeatum (AF357032). The ITS sequences were aligned in Muscle32 and analyzed using methods of maximum likelihood and by Bayesian inference in Geneious Pro R7 (Bio-matters). The final alignment had 573bp and an overall nucleotide pairwise identity of 94%. The best substitution models were chosen with MrModelTest version 2.33 The analyses of maximum likelihood used the PhyML algorithms,34 searching for the tree topology with BEST and GTR as a substitution model. Bootstrap (MLb), with 1000 replications, was used to test the support of the branches. In the Bayesian analysis, the algorithms of MrBayes were applied,35 using the radial of the Gamma variation and the GTR model of substitution with four Monte Carlo chains over 1,000,000 generations. Each 400 generations was sampled with a temperature value of 0.2. In order to test the support of the branches, the branch length values were stored and posterior probabilities (BPp) calculated. ITS sequences were used in the Bayesian and the maximum likelihood analysis.
Strains and specimens of Lyophyllum studied.
| ID | Strain | Species | MEXU | GenBank | Vegetation | Site |
|---|---|---|---|---|---|---|
| LyIE 975 | IE 975 | Lyophyllum sp. | 27406 | KY195930 | Abies religiosa | APFFNT |
| Ly3 | IE 982 | Lyophyllum sp. | 28148 | KY195932 | Pinus sp. | APFFNT |
| Ly1 | IE 981 | Lyophyllum sp. | 28147 | KY195931 | Pinus sp. | APFFNT |
| AR09641 | – | Lyophyllum sp. | 25876 | KT875066 | Pinus sp. | Amanalco |
| CB08330 | – | Lyophyllum sp. | 25761 | KT875064 | Abies religiosa | Amanalco |
| Ly5.3 | IE 983 | Lyophyllum aff. shimeji | 28149 | KY195933 | Abies religiosa | APFFNT |
| GO-2009-278 | – | Lyophyllum aff. shimeji | 26748 | KT875068 | – | Amecameca |
| HC-PNNT-078 | – | Lyophyllum aff. shimeji | 26594 | KT875069 | Abies religiosa | APFFNT |
| HC-PNNT-246 | – | Lyophyllum aff. shimeji | 26689 | KT875070 | Abies religiosa | APFFNT |
Vegetative isolation was conducted in the potato dextrose agar (PDA) medium. For evaluation of the strains (growth rate and biomass production) in solid media, malt extract-peptone and yeast agar (ME-PYA) and PDA were used. The PDA medium was prepared with 39% w/v potato dextrose agar BD BioxonMR; the medium ME-PYA was prepared with 33.6% w/v malt extract agar BD Difco™, 2% w/v yeast extract BD BioxonMR and 1% w/v gelatin peptone BD BioxonR. For the liquid media, malt extract with peptone and yeast (ME-PY) and potato dextrose (PD) were used. The medium ME-PY was prepared with 33.6% w/v malt extract BD Bacto™, 2% w/v yeast extract BD BioxonMR and 1% w/v gelatin peptone BD BioxonR. The medium PD was prepared with 39% w/v potato dextrose broth BD Difco™. All culture media were sterilized in an autoclave at 121°C, 15 Ibs psi for 15min and 0.05g of Chloramphenicol (Sigma®) were added.
Evaluation of strains in solid mediaStrain characterizationMorphological characterization of the strains was carried out according to Cruz-Ulloa,36 using the color key “HTML color codes” (html-color-codes.info/). For microscopic characterization, a sample of mycelium was taken from the periphery of the strain and temporary preparations were made with Congo red dye at 10%. In these, the presence or absence of clamps was determined and the diameter of the hyphae measured at 100× (20 hyphae per treatment) using a Motic® optical microscope and the software for the Motic Digital Microscope DMB3-223 (Motic China Group Co., Ltd., 2001–2004).
Growth rate and biomass productionA cork borer was used to take a sample of agar with mycelium 5mm in diameter. Five replicates were taken of this agar sample per culture medium and strain and each was placed in the center of a Petri dish (9cm in diameter). The Petri dishes were incubated in darkness at 18°C taken from that reported by a previous study27 and every third day the colony diameter was measured with a vernier (5″ metal vernier caliper, Pretul®, China). The growth rate (GR) was obtained from the equation GR=(Fd−Id)/(Ft−It) (where “Fd” is the final diameter of growth, “Id” the initial diameter of growth and “Ft−It”=the days of mycelial growth).37 At the end of the incubation period of the strains, the agar was eliminated by immersion in boiling water and the mycelium was then rinsed with hot water. Once washed, it was oven dried at 80°C for 24h and its dry biomass recorded.27
Evaluation of strains in liquid mediumProduction of biomassIn 250mL Erlenmeyer flasks, 100mL of the ME-PY and PD media were placed with five replicates per strain and culture medium. The media were inoculated with mycelium of each strain of 0.5cm in diameter and incubated in darkness for 20 days at 18°C and at 120rpm in a PolyScience® agitating water bath. At the end of the incubation period, the mycelium was filtered and washed with distilled water then oven dried at 80°C for 24h and then weighed. The pH of the medium was measured at the beginning and end of the incubation period.
Mycelial characterizationCharacterization of the morphology of the mycelium was conducted prior to drying using a Motic® stereoscopic microscope. The number of pellets formed was quantified and their diameter measured with a vernier. For microscopic characterization, samples of the pellets were taken at the end of the period of agitation and temporary preparations made with Congo red dye at 10% for observation under a Motic® optical microscope and corroboration of the presence of clamps.
Statistical analysisThe statistical analyses were conducted with the program Statgraphics® Centurion XVI (Statpoint Technologies, Inc., 2009). The effect of the interaction between the strains and the culture media on the growth rate and biomass production was evaluated using a multivariate analysis of variance (MANOVA). Significant differences among culture media were determined with a Tukey multiple range test (p≤0.05) and correlation analysis was performed between biomass production and growth rate in the solid medium. The data presented a normal distribution.
ResultsBiological materialThe sporophores that were collected had different morphotypes. The sporophores of strain IE 983 (MEXU 28149) had a size of the pileus of 1.9–7cm, convex, smooth surface and incurved margin or plane, dark brown (#2A1B0A) a brown-greyish (#2A1B0A-#1C1C1C). Lamellae slightly decurrent or free, white (#FFFFFF) to cream (#FBF8EF). Stipite of 2.7–6×0.9–2cm, cylindrical, fibrous-cartilaginous and white-cream (#FAFAFA-#F8ECE0). Subglobose spores of 6.2 (4.5–7)×3.8 (3.4–4.2)μm and basidia of 32 (30.4–34.6)×6.5 (5.4–7.3)μm.
The sporophores morphology of the strain IE 975 (MEXU 27406), IE 981 (MEXU 28147) and IE 982 (MEXU 28148) is varied, the size of the pileus ranges from 2 to 9cm across, of form flat-convex, slightly umbonate, with smooth and incurved edge, crema-greyish (#FBF8EF-#D8D8D8), light brown (#61380B) to brown (#3B240B). Lamellae from free adhered to slightly decurrent, white (#FFFFFF), cream (#FBF8EF) and grayish (#D8D8D8). Stipe cylindrical of 3–9cm in length, smooth or slightly striate, cream-grayish (#FBF8EF-#D8D8D8), or brown (#613F0B). In terms of size of the spores and the basidia, the specimens of the strains IE 981 and IE 975 are different to IE 982. The specimens of strains IE 981 and IE 975 have globose to subglobose spores of 3.5–6.5×3.8–6μm with basidia of 23–36×5–8μm, the specimen of strain IE 982 has spores of 4.9–7×5.3–7μm and basidia of 36–42×3.4–4.5μm.
Genetic characterizationThe strains and sporophores were divided into two lineages of Lyophyllum within clade V (section Difformia). The first was formed by the strain IE 983, by sporophores bought in the market of Amecameca, State of Mexico and by sporophores collected in the APFFNT (in the zone from which all the strains were obtained). This clade was recognized both by the Bayesian and maximum likelihood analyses with a high level of support (BPp=0.99, MLb=91.6). In both cases, this is the brother clade of L. shimeji. The second clade was formed by the strains IE 981, IE 982 and IE 975, together with specimens collected in Amanalco, State of Mexico. This clade had good support in the Bayesian analysis (BPp=0.87) (Fig. 1), but no support in the analysis of maximum likelihood (MLb=31.5). In both analyses, the position of this clade is uncertain within the clade of L. decastes s.l. (L. decastes, L. fumosum, L. conglobatum). Table 1 shows the data of the strains generated in the present research, as well as their ID was used in the Bayesian inference analysis (Fig. 1)
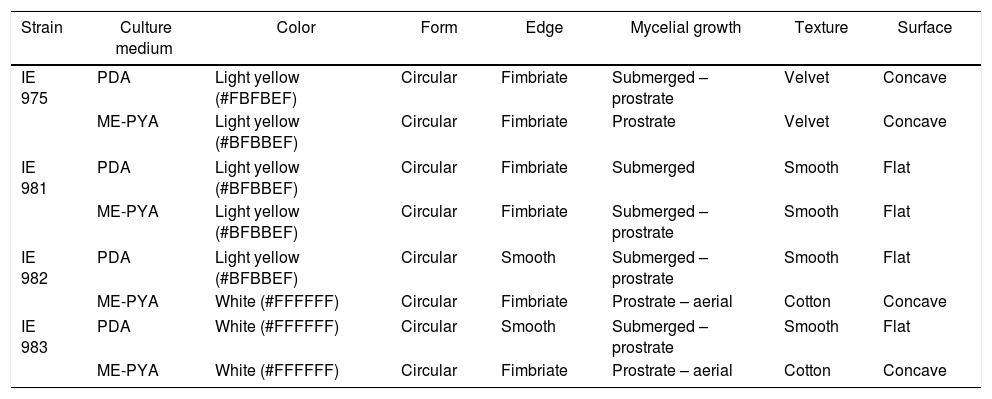
Characterization of the strainsFour native strains of Lyophyllum were evaluated, of which three were obtained in the present study (IE 981, IE 982 and IE 983) and one was previously deposited in INECOL (IE 975). These strains presented morphological differences on culturing in different media (Fig. 2).
The strain IE 975 presented prostrate mycelium in ME-PYA and submerged-prostrate mycelium in PDA, while in IE 981, the mycelium was submerged in PDA and submerged-prostrate in ME-PYA. The strain IE 982 presented differences between both media, from color to surface type. This was also true of IE 983, where only the circular form was similar in both media. Exudates were absent in all the cases (Table 2, Fig. 2).
Macromorphological characterization of four Lyophyllum strains.
| Strain | Culture medium | Color | Form | Edge | Mycelial growth | Texture | Surface |
|---|---|---|---|---|---|---|---|
| IE 975 | PDA | Light yellow (#FBFBEF) | Circular | Fimbriate | Submerged – prostrate | Velvet | Concave |
| ME-PYA | Light yellow (#BFBBEF) | Circular | Fimbriate | Prostrate | Velvet | Concave | |
| IE 981 | PDA | Light yellow (#BFBBEF) | Circular | Fimbriate | Submerged | Smooth | Flat |
| ME-PYA | Light yellow (#BFBBEF) | Circular | Fimbriate | Submerged – prostrate | Smooth | Flat | |
| IE 982 | PDA | Light yellow (#BFBBEF) | Circular | Smooth | Submerged – prostrate | Smooth | Flat |
| ME-PYA | White (#FFFFFF) | Circular | Fimbriate | Prostrate – aerial | Cotton | Concave | |
| IE 983 | PDA | White (#FFFFFF) | Circular | Smooth | Submerged – prostrate | Smooth | Flat |
| ME-PYA | White (#FFFFFF) | Circular | Fimbriate | Prostrate – aerial | Cotton | Concave | |
PDA, potato dextrose agar; ME-PYA, malt extract-peptone and yeast agar.
Microscopically, the four strains developed branched, septate, smooth hyphae with rotundate hyphal termination and abundant and frequent presence of clamps. The average diameter of the hyphae for the strain IE 975 was 1.9μm in PDA, and 1.6μm in ME-PYA, the strain IE 983 presented an average diameter of 2.9μm in PDA and 3.1μm in ME-PYA, the strain IE 981 was 2.4μm in PDA and 2.2μm in ME-PYA and the strain IE 982 was 2.3μm in PDA and 2.7μm in ME-PYA.
Evaluation of the strains in solid mediumThe mycelium presented significant differences in growth rate (F1,39=128.23, p≤0.0001) in the two culture media used, with that of the PDA medium reaching up to 0.4cm día−1; with a maximum diameter of 9cm reached in approximately 21 days, and that of the ME-PYA medium presenting a minimum rate of 0.31cm/day. Differences in growth rate not only depended on the culture medium but also on the strain (F3,39=59.69, p≤0.0001). Table 3 shows that, according to the Tukey multiple range test, four groups were formed; the higher growth in the medium PDA was present in three (IE 975, IE 982 and IE 983) of the four strains, followed by IE 982 and 983 in ME-PYA with 0.37 and 0.34cm/day low respectively. The strain IE 981 in both culture media and IE 975 in ME-PYA presented, on average, the lowest growth rate. The formation of the groups is generated from the interaction (F3,39=22.00, p≤0.0001) between the factors: culture media and strains, which affects the growth rate of the mycelium. For this reason, each strain requires a different medium to favor its development.
Growth rate and biomass production in solid and liquid media.
| Strains | Solid medium | Liquid medium | |||
|---|---|---|---|---|---|
| Culture medium | Growth rate (cm/day) | Biomass (g/Petri dish) | Culture medium | Biomass (g/l) | |
| IE 975 | PDA | 0.40 aa | 0.09 c | PD | 5.79 cd |
| ME-PYA | 0.32 cd | 0.22 b | ME-PY | 8.52 abcd | |
| IE 981 | PDA | 0.32 cd | 0.38 a | PD | 11.12 ab |
| ME-PYA | 0.31 d | 0.36 a | ME-PY | 11.98 a | |
| IE 982 | PDA | 0.38 ab | 0.20 b | PD | 5.54 d |
| ME-PYA | 0.37 b | 0.30 a | ME-PY | 9.62 abcd | |
| IE 983 | PDA | 0.40 a | 0.16 bc | PD | 7.48 bcd |
| ME-PYA | 0.34 c | 0.18 b | ME-PY | 9.92 abc | |
PDA, potato dextrose agar; ME-PYA, malt extract-peptone and yeast agar; PD, potato dextrose; ME-PY, malt extract with peptone and yeast.
In terms of the biomass production in the solid medium, significant differences were also presented among the strains (F3,39=54.11, p≤0.0001) and the culture media (F1,39=19.73, p≤0.0001) and there was an interaction between both factors (F3,39=7.28, p≤0.05) that influenced biomass production. The highest biomass production was presented in the strain IE 981 in both culture media (Table 3), and in IE 982 in the medium ME-PYA, with an average maximum of up to 0.383g/Petri dish, followed by IE 975 and IE 983 in the medium ME-PYA, IE 982 and IE 983 in PDA and a lower production with IE 975 in PDA. There was a negative and statistically significant correlation (p≤0.0001, r=−0.6334) between growth rate and biomass production. That is, the strains that developed the highest biomass were generally those that presented the lowest average growth rate.
Evaluation of strains in liquid mediumFor biomass production in liquid medium, significant differences were presented among strains (F3,39=9.55, p≤0.0001), with IE 982 the strain that generated the highest quantity of biomass in both culture media, followed by IE 975, IE 982 and IE 983 in ME-PY and these latter strains, with a lower production, in PD. These results show that there are significant differences between the culture media (F1,39=15.61, p≤0.05), with the ME-PY the most efficient in terms of biomass production (11.98g/L), which is up to double that of the PD (5.54g/L) (Table 3). In contrast to the biomass production in the solid medium, there was no relationship between the culture medium and the strains in the liquid medium (F3,39=1.07, p≥0.05) that would affect biomass production.
In general, in terms of both biomass production and growth rate, the strains behaved differently among themselves and among culture media. In PD, the strains had a higher growth rate, while in ME-PY the rate was slow but with a greater biomass production, as was the case in ME-PY. In the liquid media, the four strains formed pellets; however, in ME-PY, where there was greater biomass production (Table 3) after 20 days in agitation, the pellets were saturated and formed a conglomerate of gelatinous consistency.
In PD, the pellets were spongy with short filaments on the periphery, a characteristic known as the “hairy length”.38 The number and diameter of pellets differed in each strain; in IE 975, a minimum of 2 and a maximum of 9 were formed, with diameters from 1 to 3.2cm; in IE 982, there were from 6 to 24, with diameters of 0.6cm to 4.2cm; in IE 983, from 8 to 12 with diameters of 1.6 to 4cm, and in IE 981, from five to 11, with diameters of 0.9 to 4.5cm.
The initial pH of the culture media was 5 for ME-PY and 5.7 for PD. At the end of the 20 days of incubation and agitation, this was different in the four strains and in the two culture media. The strain IE 975 presented a final pH of 4.3 in ME-PY and 4.8 in PD, the strain IE 983 was 4.8 in both media, IE 981 was 4.2 in ME-PY and 5.5 in PD and the strain IE 982 was 5.9 in ME-PY and 6.1 in PD. As was the case in the solid medium, the four strains in the liquid medium presented abundant clamps.
DiscussionThe two clades into which the strains and sporophores of Lyophyllum collected in the State of Mexico were differentiated, are found in the section Difformia, which is located in the clade Vb that resolves 5 phylospecies, 9 morphospecies and 5 morphogenetic species.4 Clade 1, formed by the strain IE 983 (Ly5.3) and specimens obtained in markets and by collection in the APFFNT (GO-2009-278, HC-PNNT-078, HC-PNNT-246), is brother to the clade/Vb-6 shimeji, which comprises one strain31,39 and a wild specimen associated with coniferous forest,12,31 with a high level of support both in the Bayesian analysis and in the maximum likelihood. The morphological characteristics of the specimen from which the strain was obtained, as well as its spore size, match the characteristics reported for L. shimeji.11 The position as a brother clade to that of L. shimeji (Fig. 1) could be attributed to different conditions such as probable recent evolution or to the geographic origin or habitat in which they develop, since L. shimeji has been reported in association with Pinus in Asia,10,12 while the specimens and strains in the present study are associated with Abies religiosa. This agrees with that reported for the clade of L. fumosum and L. decastes, who separated these into two subclades because they develop in different vegetation types (deciduous and coniferous forests, respectively).12
The position of clade 2 formed by the three other strains: IE 975, IE 982 and IE 981 and two specimens collected (AR09641 and CB08330) is uncertain (MLb=31.5) within clade Vb, and the morphological characterization of the sporophores does not provide the data that would allow a more accurate classification. The specimens of those that were obtained are genetically similar among themselves; however, their morphology and microscopy is varied, but the size of the spores is similar to that of L. decastes.26 However, the basidia do not match those of any of the other species within the section Difformia. Likewise, there are differences in terms of habitat (Table 1), since they are found both in the forest of Abies religiosa and Pinus sp. Confusion in the identification of the species is generated by the intraspecific plasticity of the species classified in the complex L. decastes within the section Difformia.11,12,26
The specific clades within the clade/Vb are not clearly differentiated, due to the paraphyletic characteristic of the clade Vb-7 supported by a cryptic species (AF357060), while the clade Vb-8 is monophyletic and phylogenetically more distant from Vb-7.31 Other studies have found the same problem in delimitation of species within this clade.12 The identification must be accompanied by a phylogenetic analysis and examination of ecological aspects, as well as a characterization at strain level.10,40,41 For this reason, delimitation of these species requires a comprehensive taxonomic study, in which the possibility of describing them as new species is given consideration.
Regarding the characteristics of the strains of the two species, these are differentiated mainly by the color of the mycelium (Table 2) while sharing some other characteristics, such as the absence of anamorphic structures, the form of mycelium growth and the presence of abundant clamps and a fimbriate edge. This agrees with that reported for strains of different Lyophyllum species within the sections Lyophyllum, Difformia and Tephrophana.41 A detailed study of the strains could generate more accurate information for identification of the species. However, the description of the strains in this study does not provide data regarding distinctive characteristics that would permit differentiation among the species.40,41 The characteristics of the strains (physiology, diameter, growth rate) also provide information about the influence of temperature or pH or even the chemical composition of the culture media on the mycelial growth and production; these are parameters used in bioassays and in physiological studies that could provide data regarding suitable conditions for cultivation.42,43 In this case, the incubation temperature of the strains was 18°C, taken from that reported by a previous study.27 In this respect, the temperature used is lower than that reported for L. decastes which grows at 20°C,44L. fumosum at 25°C17 and Lyophyllum spp. from 20 to 25°C.41 Optimum incubation temperature depends on the climatic conditions of the regions of origin of the strains4; for this reason, it is logical that our strains would have lower optimal temperatures since they develop in high mountain environments and are adapted to lower temperatures.
Regarding the growth rate, the strain IE 983, which corresponds to L. aff. shimeji and IE 975 of Lyophyllum sp. presented a higher growth rate than that reported for L. shimeji and L. decastes, with growths of 47–60mm and 22–85mm, respectively, after four weeks of incubation.41 Growth rate and biomass production are related to nutrient sources and a suitable C:N ratio.45
In the culture media where the mycelium invaded more rapidly, biomass production was much lower and the strains that developed more biomass were those that had, on average, a lower growth rate. This suggests that the best nutritional conditions are provided by the medium based on malt extract, allowing increased hyphal branching and thus a higher quantity of biomass. In contrast, in the potato dextrose medium, the rapid growth rate shows that, under nutrient limiting conditions, the hyphae tend to be less branched and grow at a higher rate in order to maximize exploration of the medium in search of nutrients.45–47
In liquid medium, biomass production, production of polysaccharides and the form of growth (pellets) are influenced by the strain and the culture medium as well as by the method of culture initiation, the size of spawn of the strain and the intensity of agitation.48–51 The culture medium (source of carbon and nitrogen) also intervenes in the density and diameter of the pellets.49,52 The strain that had the greatest biomass developed a higher number of pellets, a result that was very similar to that of Sparassis latifolia Y.C. Dai & Zheng Wang.53 The medium based on malt extract supplemented by peptone and yeast was the most suitable for all four strains of Lyophyllum. This medium is considered one of the best sources of nitrogen for high mycelial growth. The peptone and/or yeast extract are also recognized efficient sources of nitrogen for the production of biomass in Ganoderma lucidum (Curtis) P. Karst., Lentinula edodes (Berk.) Pegler, Pleurotus ostreatus (Jacq.) P. Kumm. and L. decastes with maltose and glucose serving as sources of carbon.8,54 In the case of L. decastes, there was a greater yield and production of polysaccharides in glucose, as a source of carbon, and yeast,8 in contrast to the four strains of Lyophyllum, that present greater biomass production in a medium that is rich in nitrogen.
The biomass generated in a liquid medium can be used as a liquid spawn, as a food and dietetic supplement, and for pharmaceutical applications and enzyme production54,55 due to the production of polysaccharides.8 The pH of the media was not adjusted, but at the end of the period of agitation it had changed in each of the strains in both culture media. This could be due to the high biomass production and, while this was not measured, the production of metabolites; factors that modify the pH at the end of the incubation period.52,53
Mushrooms cultivation using native strains, particularly of Lyophyllum, requires studies that comprise the characterization and identification (morphological and genetic) of the sporophores and strains utilized; knowledge of ecological aspects, life cycles, production and composition of metabolites and identification of substrate types and environmental conditions suitable for fructification.1,3,4
Lyophyllum is a genus with potential for commercial culture and has functional and medicinal properties.9 The strains IE 982 of Lyophyllum sp. and IE 983 of L. aff. shimeji, that presented the best conditions in the in vitro culture, represent an excellent option for studies focused on the acquisition of secondary metabolites and their experimental culture at substrate level. Working with native strains constitutes a good alternative for the regional management of germplasm adapted to the climatic and substrate conditions where they develop naturally, eliminating dependence on strains from other countries.
Conflicts of interestThe authors declare no conflicts of interest.