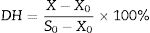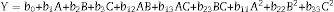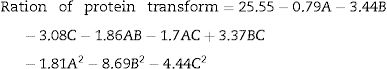Cordyceps militaris 202 is a potential fungus for biotransformation zein, due to its various proteases, high tolerance and viability in nature. In this article, single factor experiment and response surface methodology were applied to optimize the liquid fermentation conditions and improve the ability of biotransformation zein. The optimized fermentation conditions were as follows: inoculum concentration of 19%, volume of liquor of 130mL/500mL and pH of 4.7. Under this condition, the degree of hydrolysis (DH) was 27.31%. The zein hydrolysates from fungi fermentation maintained a high thermal stability. Compared to the original zein, the zein hydrolysates were found to have high solubility, which most likely results in improved foaming and emulsifying properties. Overall, this research demonstrates that hydrolysis of zein by C. militaris 202 is a potential method for improving the functional properties of zein, and the zein hydrolysates can be used as functional ingredients with an increased antioxidant effect in both food and non-food applications.
Zein is the major proportion (65%) of corn gluten meal (CGM), and CGM is major co-product of corn wet-milling process. China is the second largest country for production and consumption of corn. Zein is excellent materials with unique nature, and its poor solubility in water, which contributes to zein difficult in food industry application.1 Protein modification may be carried out to improve its nutritional and/or functional properties. Modification of protein usually contain physical,2 chemical3 and biological treatments,4,5 which change the physicochemical and functional properties by changing its conformation and structure. This has been reviewed by several researchers.6–8 The biological treatment is one of the most used methods among the researches for modification, which consists of enzyme treatment and fermentation. The hydrolysates may be expected to have increased solubility, foaming and emulsifying properties compared with native protein, and there are many studies have proved these.9–11 Compared with the single enzyme hydrolysis, the hydrolysis by liquid fermentation has advantage on several aspects, such as low cost, and food safety, thus making it more suitable for protein modification. Zhang12 thought that the product of polypeptide by liquid fermentation can save 40–50% cost, compared with enzyme hydrolysis.
Cordyceps militaris is considered as one of the most expensive mushrooms in China, because of its attractive beneficial effects and rarity in nature.13 The submerged culture for producing mycelium can be used as ingredients in functional foods, which have conduct to meet the demand for commercial applications.14–16 Xiao17 found the C. militaris can degrade chickpea protein and improve water holding capacity, fat absorption capacity and emulsifying properties of flours by solid-state fermentation. The utilization of liquid fermentation of zein with C. militaris 202 can transform zein into soluble protein, thus improving the utilization and bioavailability in the aqueous phase. However, the existent researches about C. militaris mostly focus on its metabolite,18–20 no available reports about use of enzymatic proteolysis by C. militaris 202 for modifying the functionality of zein could be found.
Thus, our subject is to optimize the fermentation condition of transforming zein to soluble protein by liquid fermentation with C. militaris 202. The degree of hydrolysis (DH) was used as the evaluation to obtain optimal fermentation conditions by response surface methodology (RSM). Moreover, we investigated the effects of fermentation time on the physicochemical and functional properties (solubility, emulsification, foaming properties and antioxidative activity) of zein hydrolysates. The information is essential in modifying zein by liquid fermentation, which can provide a potential method to improve function properties and biological activity of zein, and improve its utilization value and field.
Materials and methodsMaterialsZein was obtained from Shanghai Ryon Biological Technology, China (purity>92%). C. militaris 202 was preserved in National Engineering Laboratory for Wheat and Corn Deep Processing. All other chemicals were of analytical grade
MediaThree media were used in this experiment, as follows: PDA, fermentation seeding, fermentation media. The composition of three media was as follows:
PDA medium (g/L): potatoes 200.0, glucose 20.0 and agar 18.0.
Fermentation seeding (g/L): potatoes 200.0, glucose 20.0, MgSO4 1.5, KH2PO4 3.0, VB1 0.2.
Fermentation media (g/100mL): glucose 7.0, zein 6.0, MgSO4 0.15, KH2PO4 0.3, VB1 0.2.
MethodsPreparation of liquid fermentationC. militaris 202 was incubated in the agar medium at 24°C for about 7 d, until the white mycelia covered on the surface of the agar culture media. Three truffles were inoculated in 100mL conical flasks containing 30mL liquid medium. Mediums had been sterilized at 121°C for 20min before inoculation (YXQ-LS-SII, Boxun Corp, Shanghai, China). The conical flasks were kept at 24±1°C for 3 d, and then were used as the C. militaris 202 seeding culture for zein fermentation. C. militaris 202 seeding was broken using a sterilized Blender, then inoculated 10mL seeding [equivalent to 10% of the fermentation medium (100mL)] to 500mL conical flasks, the conical flasks were shaking with 160 r/min at 24°C for 8 d.
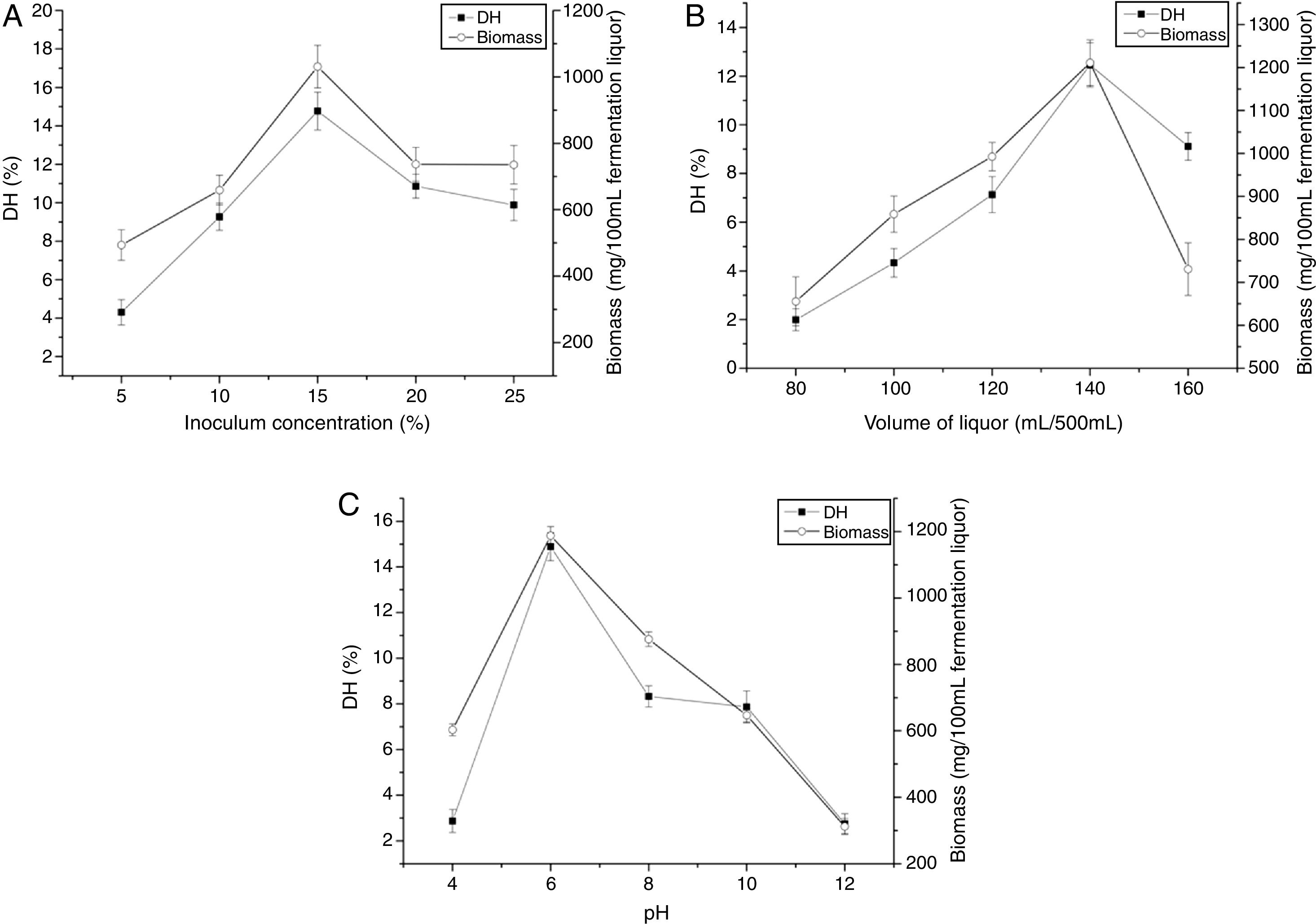
Optimization of zein hydrolysates fermentation conditionsThe fermentation conditions were optimized to improve degree of hydrolysis (DH), including inoculum concentration (v/v, 5%, 10%, 15%, 20%, 25%), volume of liquor (equivalent to total volume of the fermentation medium) (mL/mL, 80/500, 100/500, 120/500, 140/500, and 160/500), pH (2.0, 4.0, 6.0, 8.0, 10.0). The biomass and degree of hydrolysis were determined. The experiments were conducted in three replicates.
BiomassThe mycelia in the fermented broth were washed for 4 times by distilled water, and then were obtained by filtering using absorbent gauze. The mycelia was dried to a constant weight in the hot air oven at 105°C (YLA-6000, Shanghai, China), then measuring dry weight for biomass. The experiments were conducted in three replicates.
Degree of hydrolysis (DH)The DH was measured according to the method of Yang.21 The supernatant of fermented broth was obtained by centrifugation, then, mixing 15mL 10% trichloroacetic acid solution (TCA) with 15mL supernatant, and then the mixture was put at 25°C for 30min. The sediment was removed by centrifugation at 4000×g. The soluble nitrogen in supernatant and total nitrogen content of substrate were determined by microKjeldahl. The experiments were conducted in three replicates. The DH was calculated as follows:
DH: degree of hydrolysis
X: soluble nitrogen content in 10% TCA of fermented liquor, g
X0: soluble nitrogen content in 10% TCA of unfermented liquor, g
S0: total nitrogen content of substrate, g
Preparation of zein hydrolysates by liquid fermentationAfter fermentation under the optimized fermentation conditions (optimized by RSM), the reaction was terminated by boiling for 5min. In order to extract the zein hydrolysates, the fermented broth was centrifuged at 4000×g for 20min and the zein hydrolysates were obtained by fraction precipitation with ammonium sulfate at 50% saturation. The precipitations were dialyzed against water at 4°C for 24h in order to remove salt and then were freeze-dried. The purified zein hydrolysates were used for characterization of functional properties.
Functional properties of zein and zein hydrolysatesThe solubility of zein and its hydrolysates were determined using the methods previously reported.22 The foam capacity and stability were determined by Ruth and Groninger.23 Emulsifying activity and stability were determined by Zayas.24 The experiments were conducted in three replicates.
Thermal propertiesThe thermal properties of zein and its fermentative hydrolysates were measured using a differential scanning calorimetry (DSC) (Q20 DSC, TA, USA). A procedure presented by Zhao,25 with some modifications, was utilized. The samples (2mg) were heated from 0°C to 160°C at a heating rate of 10°C/min. An empty pan was used as a reference. The peak temperature (Tp) and total calorimetric enthalpy (△H) were calculated. The experiments were conducted in three replicates.
Determination of antioxidant activitiesThe scavenging effect of zein and its fermentative hydrolysates on DPPH free radical was measured according to the method of Xia et al.26 The determination of hydroxyl radical scavenging activity, was presented by Halliwell,27 with some modifications. A procedure presented by Xia et al.26 with some modification was applied to determine Fe2+-chelating activity. A procedure presented by Lu et al.28 with some modification was applied to measure the reducing power. The experiments were conducted in three replicates.
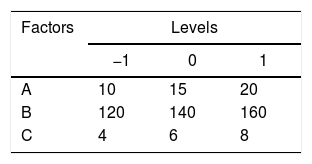
Statistical analysisRSM was used to perform the experimental designs, statistical analysis and regression model by Design Expert Version 8.0.6. Box–Behnken designs (BBD) at three levels was applied to evaluate their combined effect, as shown in Table 1. The range and center point values of three independent variables were selected based on the results of preliminary experiments (Fig. 1). Table 3 shows the experimental design consisting of 17 factorial points. The polynomial quadratic equation (1) for the optimal point was fitted to analyze the effect of each independent variable to the response:
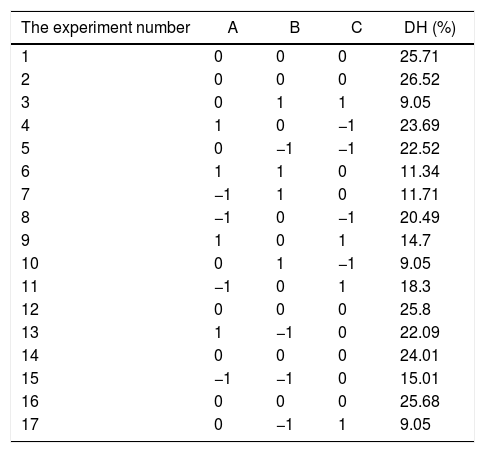
where Y is the DH calculated by response. A, B and C are the independent variables. The coefficients of the polynomial were represented by the following: b0 (constant term); b1, b2 and b3 (linear effects); b11, b22 and b33 (quadratic effects); and b12, b13 and b23 (interaction effects). The statistical differences in parameters were evaluated by Duncan's multiple range tests in Table 2.The experimental design and RSM results.
| The experiment number | A | B | C | DH (%) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 25.71 |
| 2 | 0 | 0 | 0 | 26.52 |
| 3 | 0 | 1 | 1 | 9.05 |
| 4 | 1 | 0 | −1 | 23.69 |
| 5 | 0 | −1 | −1 | 22.52 |
| 6 | 1 | 1 | 0 | 11.34 |
| 7 | −1 | 1 | 0 | 11.71 |
| 8 | −1 | 0 | −1 | 20.49 |
| 9 | 1 | 0 | 1 | 14.7 |
| 10 | 0 | 1 | −1 | 9.05 |
| 11 | −1 | 0 | 1 | 18.3 |
| 12 | 0 | 0 | 0 | 25.8 |
| 13 | 1 | −1 | 0 | 22.09 |
| 14 | 0 | 0 | 0 | 24.01 |
| 15 | −1 | −1 | 0 | 15.01 |
| 16 | 0 | 0 | 0 | 25.68 |
| 17 | 0 | −1 | 1 | 9.05 |
The effects of inoculum concentration on DH and biomass were presented in Fig. 1A. The DH and biomass increased with increasing inoculum concentration and maximum point was recorded at 15%. Then, the DH and biomass decreased at higher inoculum concentration. The volume of liquor had significant influence on DH and biomass, as shown in Fig. 1B. The DH and biomass were achieved at 140mL/500mL. With higher or lower values, the DH and biomass were comparatively reduced. The effects of pH on the DH and biomass (Fig. 1C) show that the highest DH and biomass were achieved at the initial pH 6.0. With higher or lower values, the DH and biomass were comparatively reduced.
Optimization of fermentation parameters for DH by the RSM analysisA total of 17 experimental points for optimizing the three individual parameters (the inoculum concentration (A), the volume of liquor (B) and pH (C)) were shown in Table 2. Results were fitted with a second-order polynomial equation. The second-order polynomial equation was used to analyze the ration of protein transform, and describe the relationship between the variable as follows;
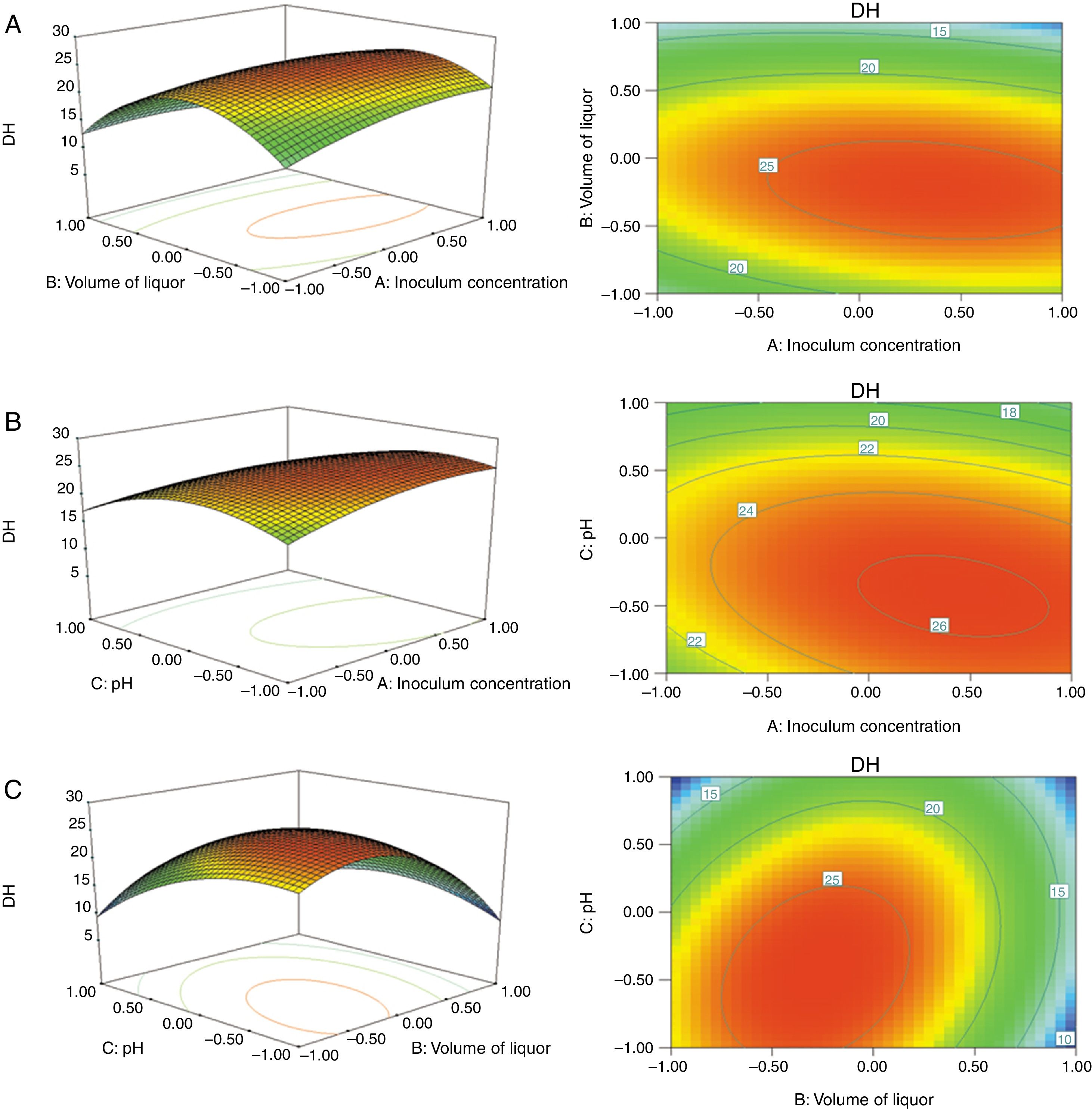
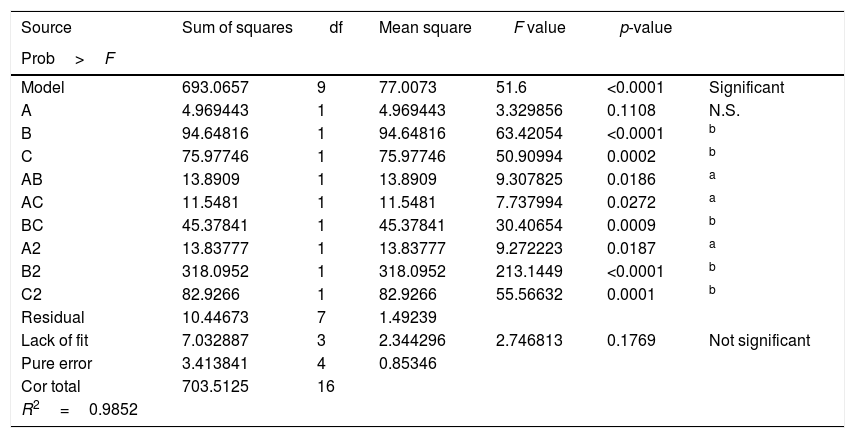
The results of ANOVA for RSM are shown in Table 3. The determination coefficient for the model was 0.9852, which indicated that the model fit well with the data. The lack of fit was 0.1769, which was not significant relative to the pure error. These results mean that the DH of C. militaris 202 could be analyzed and predicted by the model. The 3D response surface plots and the 2D contour plots of the responses of DH are shown in Fig. 2. At a fixed pH, when inoculum concentration was at a higher level, DH increased and then decreased sharply with increasing volume of liquor, which could be ascribed to the dissolved oxygen restriction in the culture medium.29 When volume of liquor was at a higher level, DH changed insignificantly. However, when volume of liquor was at a lower level, DH increased and then tended to constant with increasing inoculum concentration. It was indicated that volume of liquor had significantly effect on protein transform. At a fixed volume of liquor, the DH increased and then decreased with increasing pH and inoculum concentration. It was noticed that protein transform of inoculum at lower level increased quickly than that at higher level with increasing pH. At a fixed inoculum concentration, DH of volume of liquor at higher level increased markedly than that at lower level with increasing of pH.
ANOVA for response surface quadratic model.
| Source | Sum of squares | df | Mean square | F value | p-value | |
|---|---|---|---|---|---|---|
| Prob>F | ||||||
| Model | 693.0657 | 9 | 77.0073 | 51.6 | <0.0001 | Significant |
| A | 4.969443 | 1 | 4.969443 | 3.329856 | 0.1108 | N.S. |
| B | 94.64816 | 1 | 94.64816 | 63.42054 | <0.0001 | b |
| C | 75.97746 | 1 | 75.97746 | 50.90994 | 0.0002 | b |
| AB | 13.8909 | 1 | 13.8909 | 9.307825 | 0.0186 | a |
| AC | 11.5481 | 1 | 11.5481 | 7.737994 | 0.0272 | a |
| BC | 45.37841 | 1 | 45.37841 | 30.40654 | 0.0009 | b |
| A2 | 13.83777 | 1 | 13.83777 | 9.272223 | 0.0187 | a |
| B2 | 318.0952 | 1 | 318.0952 | 213.1449 | <0.0001 | b |
| C2 | 82.9266 | 1 | 82.9266 | 55.56632 | 0.0001 | b |
| Residual | 10.44673 | 7 | 1.49239 | |||
| Lack of fit | 7.032887 | 3 | 2.344296 | 2.746813 | 0.1769 | Not significant |
| Pure error | 3.413841 | 4 | 0.85346 | |||
| Cor total | 703.5125 | 16 | ||||
| R2=0.9852 |
N.S., no significant (p>0.05); R2, correlation coefficient.
The optimized conditions were as follows: inoculum concentration of 18.6%, volume of liquor of 132.04mL/500mL and pH of 4.73. Under the fermentation conditions, the DH in theory was 27.49%. However, considering the operability in actual production, the optimal conditions were adjusted as follows: inoculum concentration of 19%, volume of liquor of 130mL/500mL and pH of 4.7. In order to verify the accuracy of this model, verification experiments were carried out. The average DH of strains reached 27.31%. The relative error was 0.65% in comparison with the predicted value. These data proved that the model was accurate and reliable for predicting the experimental results. Thus, this model has a practical guiding significance. These results suggested that the hydrolysis of zein by liquid fermentation with C. militaris 202 was effective.
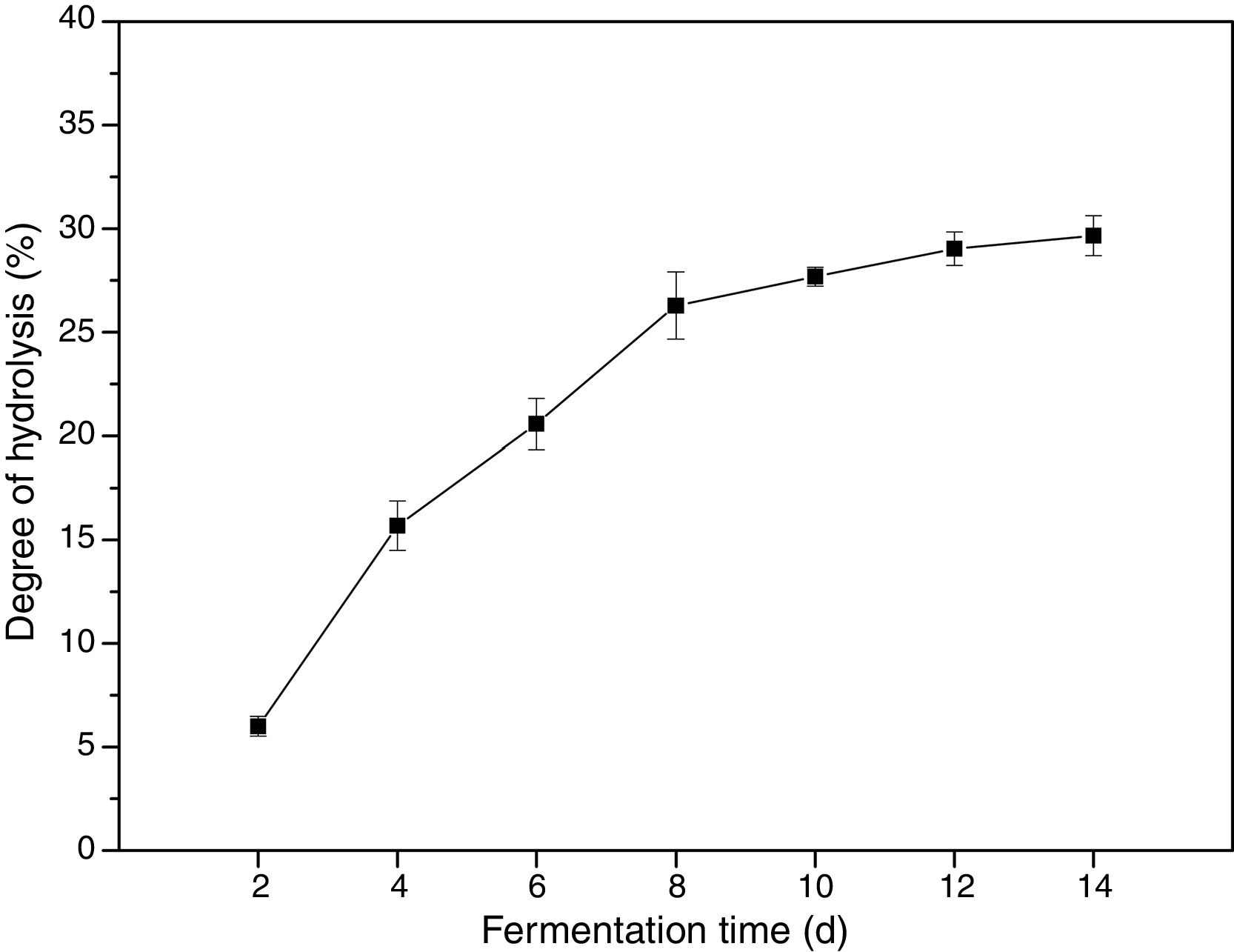
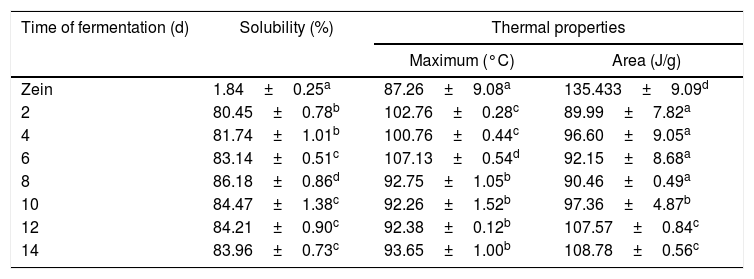
SolubilityIn the present study, a food grade fungus was selected for producing zein hydrolysates, which was able to secrete efficient proteases for the hydrolysis of the insoluble protein. Under the optimized conditions, the DH reached 27.31%, indicating C. militaris 202 was effective for zein proteolysis. DH is generally used as a proteolysis monitoring parameter, indicating the extent of hydrolysis degradation of protein. The DH of zein at fermentation time of 2, 4, 6, 8, 10, 12 and 14 day is shown in Fig. 3. The DH of zein increased as the fermentation time increased, increasing rapidly within the initial 8 d. The highest DH was 29.66%, at fermentation time of 14 day. Since the DH of zein corresponded to its fermentation time under fixed fermentation condition, the fermentation time was taken as the factor for evaluating the physicochemical properties of zein hydrolysates.
Solubility is one of important prerequisite for the functional properties of proteins and directly influences their applications in the food and non-food industry.25 The solubilities of zein and fermentative hydrolysate are shown in Table 4. The zein showed poor solubility in pH 7.0 distilled water, and the solubility was 1.84%. The poor solubility was due to the rigid structure of zein which contained higher intermolecular, intramolecular disulphide bonds and hydrophobic interactions. However, the liquid fermentation of zein can significantly improve solubility (p<0.05). The maximum solubility was at fermentation for 8 d (86.18%). Compared with the original zein, the improved solubility of hydrolysate could be attributed to the unfolding of the peptide chains, the decrease of size caused by protease hydrolysis, and the exposure of more charged residues and polar groups into the surrounding aqueous phase. These structural changes resulted in an improvement of protein–water interaction, thus increasing the solubility.30
Solubility and thermal properties of zein and its hydrolysates.
| Time of fermentation (d) | Solubility (%) | Thermal properties | |
|---|---|---|---|
| Maximum (°C) | Area (J/g) | ||
| Zein | 1.84±0.25a | 87.26±9.08a | 135.433±9.09d |
| 2 | 80.45±0.78b | 102.76±0.28c | 89.99±7.82a |
| 4 | 81.74±1.01b | 100.76±0.44c | 96.60±9.05a |
| 6 | 83.14±0.51c | 107.13±0.54d | 92.15±8.68a |
| 8 | 86.18±0.86d | 92.75±1.05b | 90.46±0.49a |
| 10 | 84.47±1.38c | 92.26±1.52b | 97.36±4.87b |
| 12 | 84.21±0.90c | 92.38±0.12b | 107.57±0.84c |
| 14 | 83.96±0.73c | 93.65±1.00b | 108.78±0.56c |
Mean±SD (n=3) and different letters in one column are significantly different (p<0.05).
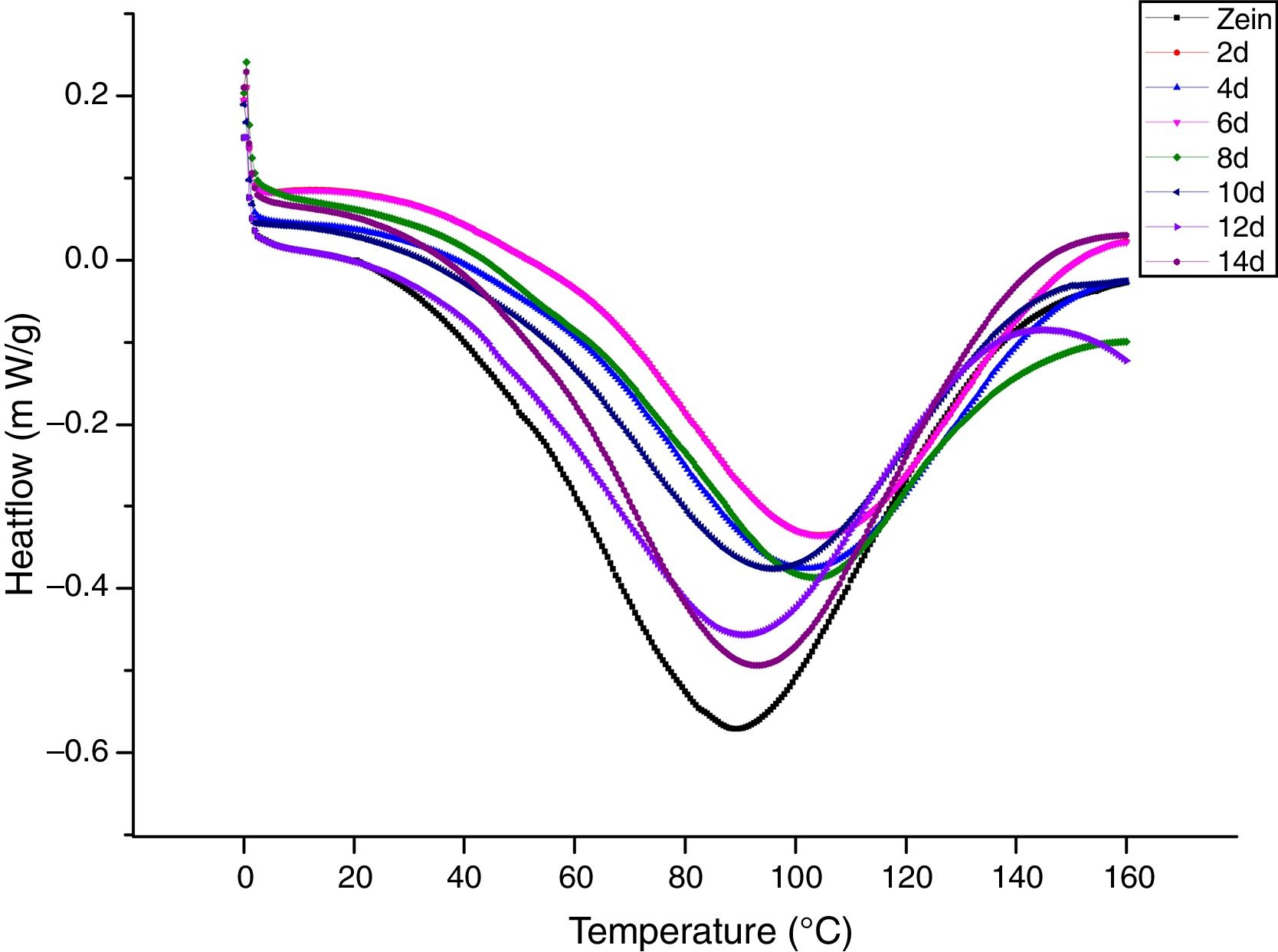
Thermal property is closely related to the structure and functional properties of proteins.31 Variations in the thermal behavior of zein and its fermentative hydrolysates obtained by different fermentation time were measured, and are shown in Fig. 4. The peak temperatures (Tp) of zein was 87.25°C, which is similar with the result by Li,32 who also found the Tp of zein was 90°C. The Tp of zein fermentative hydrolysate were found to be significantly different from the control (p<0.05). With increasing fermentation time (8 d–14 d), there were no significant differences among the hydrolysates (p>0.05). The high Tp value usually implies high thermal stability. Thus, the high Tp value of zein hydrolysates indicated good thermal stability. As shown in Table 2, the thermal enthalpy (△H) of the zein (135.43°C) was significantly higher than that of zein hydrolysates (p<0.05), which indicated that fermentation could markedly decrease the ordered structures and aggregation tendency of the protein were decreased during the fermentation period. Many factors can influence the thermal properties of proteins, such as, hydrophobic interactions, –SH/–SS–, hydrogen bonds and hydration.33 The synergism among these factors resulted in the complex changes observed in Tp and △H.
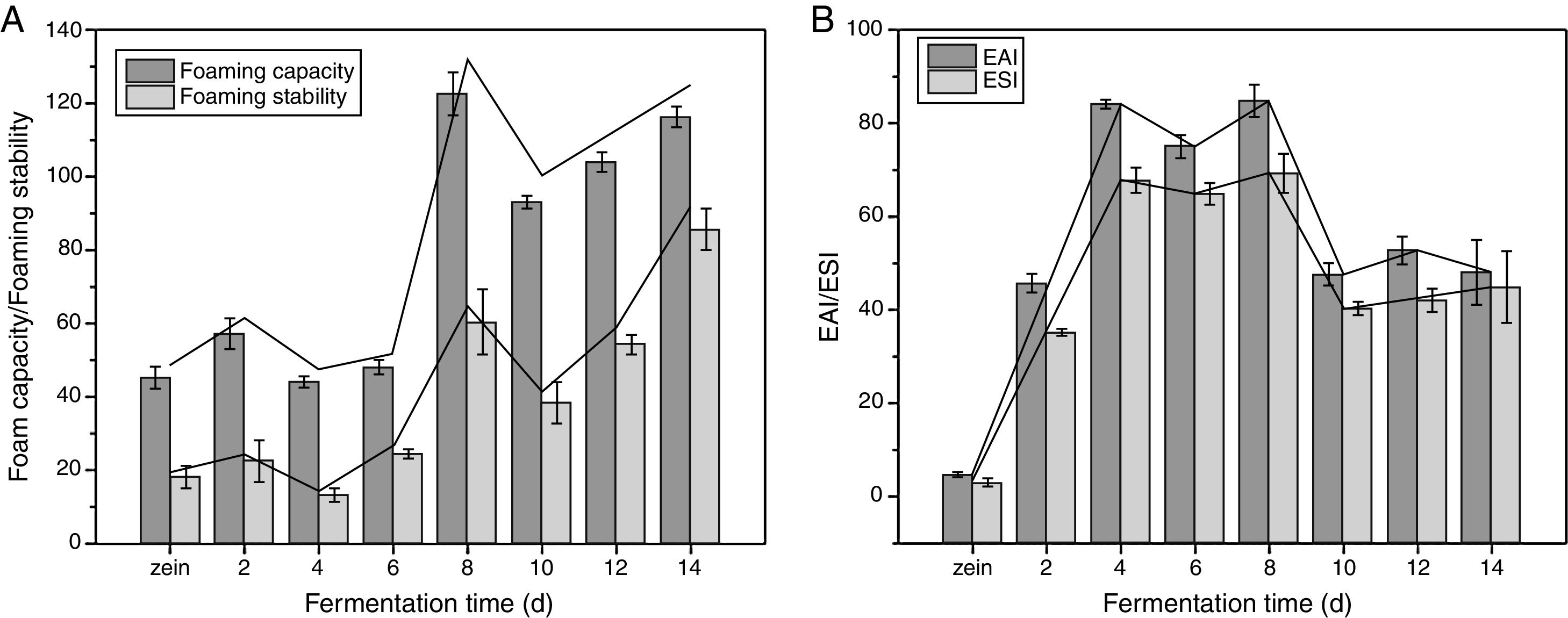
Foaming properties and foaming stabilityThe foam capacity (FC) and foam stability (FS) of zein and its hydrolysates at pH 7.0 are shown in Fig. 5A. The FC and FS of zein hydrolysates were higher (p<0.05) than those of zein, and the FC and FS of zein hydrolysates were also affected by fermentation time. Their maximum FC and FS were obtained at 8 d and 14 d, respectively (122.56% and 85.54%). Thus, controlling of the fermentation time should be conduct as it leads to undesirable property changes.
Emulsifying activity and emulsifying stabilityThe emulsifying activity index (EAI) and emulsifying stability index (ESI) of the zein and its hydrolysate at pH 7.0 are shown in Fig. 5B. The EAI of hydrolysates was improved and was significant higher (p<0.05) than that of zein. The EAI was increased from 4.5% to 84.82%, when the fermentation time was increased from 0 d to 8 d. The ESI of the fermentative hydrolysate showed the similar trend. The increase of EAI and solubility reflected the enhanced solubility of protein after fermentation, enabling protein diffusion to the oil/water surface easily. However, further increasing the fermentation time from 10 d to 14 d, the EAI of fermentative hydrolysates were maintained at a constant value (more than 60%). This phenomenon was similar with the observation for corn glutelin hydrolysates by protease, in which extensive hydrolysis did not increase the protein emulsifying activity.34 This was due to the extensive hydrolysis prevents the formation of a continuous protein film at the oil/water interface to stabilize the emulsions.26
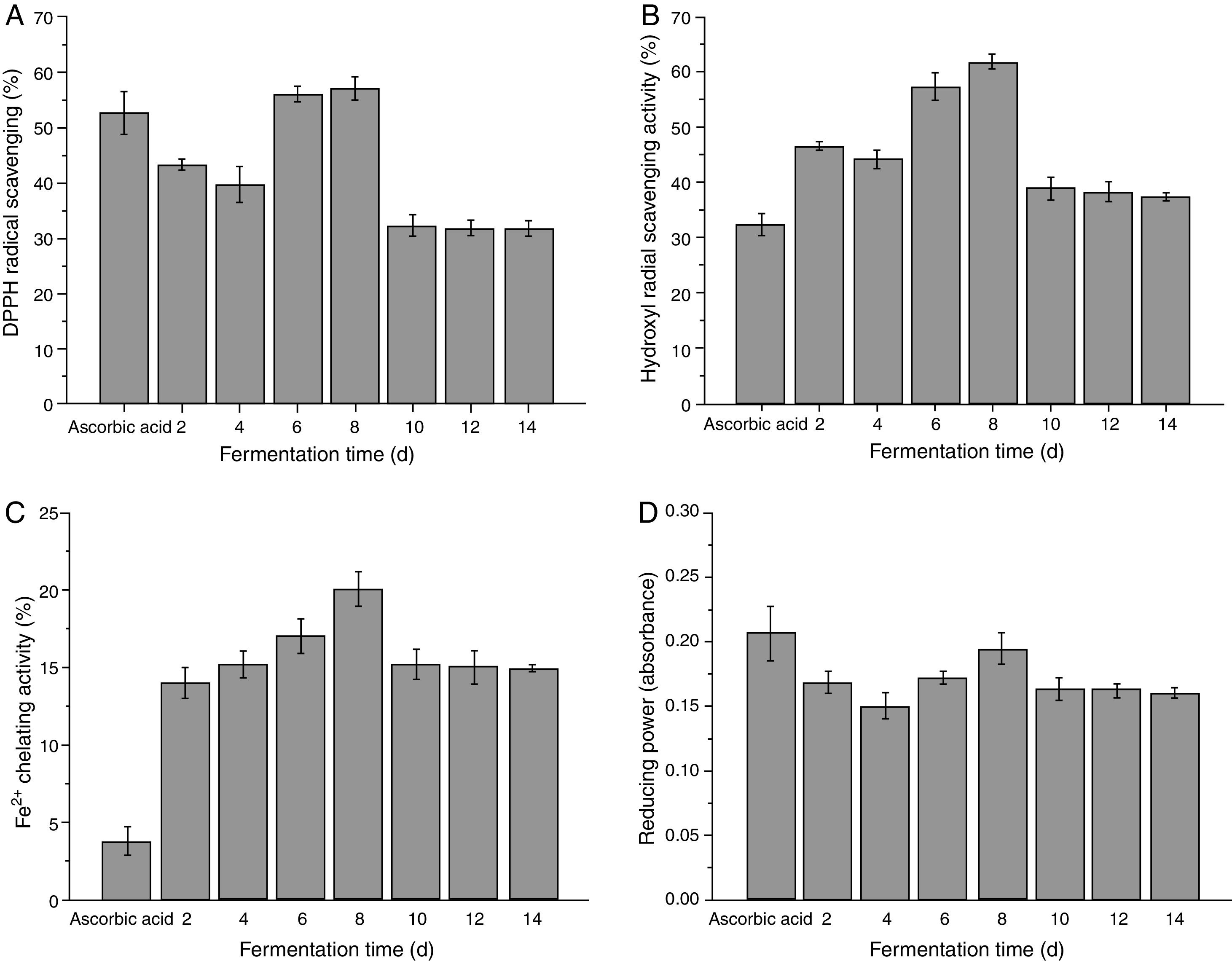
Antioxidant properties of zein hydrolysatesThe antioxidant activity of zein was not tested because it barely dissolved in pH 7.0 distilled water. The DPPH radical scavenging capacities of zein hydrolysates are presented in Fig. 6 A. All of the samples tested exhibited good scavenging ability against DPPH (31.60–56.98%). In particular, the activity of hydrolysates (56.98%) at fermentation time of 8 d was higher than that of others (p<0.05). The DPPH radical scavenging of zein hydrolysates by alkaline protease and pepsin hydrolysates were 12–28% and 22–59%, respectively.35,36 Thus, the activity of fermentative hydrolysates was comparable to the hydrolysates reported in these literatures. The result indicated that fermentative hydrolysates contained substances that were electron donors and could react with free radicals to convert them to stable products and terminate the radical chain reaction.
Hydroxyl radical can activate lipid peroxidation and react with almost any adjacent biomolecules such as DNA, proteins and amino acids.26 Since this damage can cause several diseases, its removal may be one of the most effective method to prevent putrefaction of food and occurrence of some diseases.37 The hydroxyl radical scavenging activities of fermentative hydrolysates are shown in Fig. 6B. The fermentative hydrolysates exhibited strong inhibitory effect against hydroxyl radicals (37.28–61.58%). The results suggested that fermentative hydrolysates have the potential to protect food against hydroxyl radical-induced damages.
The Fe2+-chelating ability of zein fermentative hydrolysates is shown in Fig. 6C. The Fe2+-chelating capacity of the hydrolysates increased from 15.98% to 20.02% with increasing of fermentation time, and then reduced slightly. Liu et al.34 reported a similar Fe2+-chelating ability for protease hydrolysates (about 10–30%) derived from corn glutelin. Kong previously reported that the type of proteases used and the DH affect the antioxidant activity.38 Thus, the difference of Fe2+-chelating capacity in our results may be ascribed to the type of proteases or/and DH compared with literature. Compared with the native zein, the improvement of the metal ion binding power of the fermentative hydrolysates might be ascribed to an increased concentration of carboxylic groups in the side chain of the acidic and basic amino acids.39
The reducing power assay is often used to evaluate the potential antioxidant activity of natural products. Fig. 6D shows the reducing power of fermentative hydrolysates. The fermentative hydrolysates exhibited good reducing power (0.15–0.19%), which were more effective reducing capacity than those of barley glutelin hydrolysates (0.121 at 2mg/mL).26 These results revealed that zein hydrolysates by C. militaris 202 have better reducing power.
DiscussionTo improve the utilization and bioavailability of zein in the aqueous phase, we utilized liquid fermentation of zein with C. militaris 202 to transform zein into soluble protein. In this study, the effects of inoculum concentration, volume of liquor and pH on the DH and biomass were investigated. The DH increased with increasing inoculum concentration and maximum point was recorded at 15%, while it decreased at higher inoculum concentration. The hydrolysate inoculated with higher inoculum concentration had lower yield, which was similar with the report by Ferchichi.40 This phenomenon may be explained by that the transformation of zein into products was accompanied by some metabolic activities, and this action results in negative impact on the fermentation process. Besides, this may result from cell were unable to attain complete growth due to nutritional inhibition before secreting protease. Thus, it was observed that the DH was low at higher inoculum concentration. C. militaris 202 exhibited better ability to hydrolyze zein in an acid condition. As shown in Fig. 1C, the DH increased with increase in the initial pH from 4.0 to 6.0 and maximum point was recorded at pH 6.0. Then, it decreased at higher pH values. Previous studies evaluating the effect of pH on the hydrolysis of zein reported the high DH mostly at alkaline condition.36 Nevertheless, acidic fermentation medium induced elevation in the DH in our study. Such similar acidic pH values (5.0–8.0) were demonstrated to maximize biomass from entomopathogens, such as Paecilomyces farinosus, Beauveria bassiana, and Metarhizium anisopliae.41 Since the maximum biomass of C. militaris 202 is achieved at acidic condition,42 therefore, acidic condition can promote the growth and enzyme production in the medium, which results in enhancement of zein transform. High cell density, is also required for efficient protease biosynthesis.43,44 The determination coefficient for the model was 0.9852, which indicated that the model fit well with the data. The lack of fit was 0.1769, which was not significant relative to the pure error. The RSM can be accurately used for prediction and optimization of zein hydrolysis by fungi under the experimental conditions employed in this study.
There are several reports on modifying the functionality of isolated glutelin from barley and corn.31,34 However, no available reports about use of enzymatic proteolysis by C. militaris 202 for modifying the functionality of zein could be found. Thus, we evaluated the feasibility of improving the functional properties of corn zein in an aqueous solution using of enzymatic proteolysis by C. militaris 202. Under the optimized conditions, the DH reached 27.31%, indicating C. militaris 202 was effective for zein proteolysis. Since the DH of zein corresponds to its fermentation time under fixed fermentation condition, the fermentation time was taken as the factor for evaluating the physicochemical properties of zein hydrolysates, thus providing useful information on potential commercial applications. The fermentative hydrolysates of zein showed significantly improved solubility (p<0.05) in pH 7.0 aqueous phase. Even limited time hydrolysis by fermentation was able to significantly improve the zein solubility. Solubility is important prerequisite for the functional properties of protein; the increased solubility can affect their applications in the food industry. The FC of zein was 45%, which was lower (p<0.05) than that of rice protein (118.8%).25 This might be due to the poor solubility of zein in pH 7.0 water. The FC and FS of zein hydrolysate protein were higher (p<0.05) than those of zein, accompany with the increasing solubility. Zheng45 also reported that the FC and FS of protein increased after enzymatic hydrolysis. The hydrolysis by fermentation significantly improved protein solubility (p<0.05), which facilitates the formation of an interfacial membrane and foam production. Nevertheless, the FC and FS of zein hydrolysates fermented for 2–6 day changed not significantly. According to the results, zein hydrolysates exhibited solubility of 80.45% but poor foam capacity and stability at pH 7.0. This showed that solubility is not the only requirement to improve foam properties or other functional properties. This phenomenon may be ascribed to the low DH. As shown in Fig. 5A, the FS and FC of protein samples were significantly changed with the increase of fermentation time. Enzymatic hydrolysis has shown to be a suitable method for modifying the foaming properties of protein as a function of the molecular weight profile and composition of the different fractions which, in turn, depends on the DH.46
The results (Fig. 6) indicated that zein hydrolysates prepared by fermentation possessed excellent antioxidant activity, and there was significant difference detected among different fermentation time. Liu et al.47 discovered that the DH of porcine plasma hydrolysates increased with increasing hydrolysis time, observed from 0.5 to 5h of hydrolysis. The study of the hydrolysis of peanut peptides thought there was correlation between DH and antioxidant.48 In general, researchers thought the antioxidant activities of hydrolysates increased with increasing DH. Our research also showed the similar results, which the antioxidant activities of hydrolysates (2–8 day) increased with increasing fermentation time. Nevertheless, the antioxidant activities of hydrolysates (10–14 day) decreased, which indicated there may other factors affect the antioxidant activities of hydrolysates, such as molecular weight distribution and amino acid composition.48 In our study, the result revealed that fermentative hydrolysates can be used for minimizing or preventing lipid oxidation in food products, and prolonging the shelf life of foods.
ConclusionSingle factor experiment and RSM were applied to optimize the liquid-state fermentation conditions and improve the ability of zein transform ability. The optimized fermentation conditions were as follows: inoculum concentration of 19%, volume of liquor of 130mL/500mL and pH of 4.7. Under this condition, the DH was 27.31%. The hydrolysates maintained a high thermal stability. The zein hydrolysates were found to have a higher solubility which most likely resulted in improved foaming and emulsifying properties compared to the original zein. The results indicated that zein fermentative hydrolysates prepared by fermentation possessed excellent antioxidant activity. Overall, this research indicated that zein hydrolysates can be potentially used as functional ingredients with an increased antioxidant effect in both food and non-food application.
Conflicts of interestThe authors report no conflicts of interest in this work.
This work was supported by the National Key R&D Program of China (Grant Number: 2016YFD0400702), and Jilin Province “Double Ten Project” Major Scientific and Technological Projects – Key Technology Research and Product Development of Health Staple Food of Coarse Grains (Grant Number: 20150201010NY).