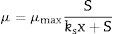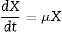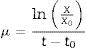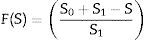Kinetic behaviors of five Lactobacillus strains were investigated with Contois and Exponential models. Awareness of kinetic behavior of microorganisms is essential for their industrial process design and scale up. The consistency of experimental data was evaluated using Excel software. L. bulgaricus was introduced as the most efficient strain with the highest biomass and lactic acid yield of 0.119 and 0.602gg−1 consumed lactose, respectively. The biomass and carbohydrate yield of L. fermentum and L. lactis were slightly less and close to L. bulgaricus. Biomass and lactic acid production yield of 0.117 and 0.358 for L. fermentum and 0.114 and 0.437gg−1 for L.actobacillus lactis were obtained. L. casei and L. delbrueckii had the less biomass yield, nearly 11.8 and 22.7% less than L. bulgaricus, respectively. L. bulgaricus (R2=0.9500 and 0.9156) and L. casei (R2=0.9552 and 0.8401) showed acceptable consistency with both models. The investigation revealed that the above mentioned models are not suitable to describe the kinetic behavior of L. fermentum (R2=0.9367 and 0.6991), L. delbrueckii (R2=0.9493 and 0.7724) and L. lactis (R2=0.8730 and 0.6451). Contois rate equation is a suitable model to describe the kinetic of Lactobacilli. Specific cell growth rate for L. bulgaricus, L. casei, L. fermentum, L. delbrueckii and L. lactis with Contois model in order 3.2, 3.9, 67.6, 10.4 and 9.8-fold of Exponential model.
Kinetic models are useful tools in design and control of biotechnological processes to obtain improved knowledge about microbial growth behavior using mathematical models along with specifically accurate and repeatable detailed experiments. Cell growth time-courses are involved individual growth phases: lag phase, exponential growth phase, stationary phase and the phase with exponential decay. Nonlinear mathematical models used to identify growth parameters.1
Lactobacillus is a genus of gram-positive facultative anaerobic bacteria also known as one of the main groups of probiotic organisms involved in functional foods.2–5 A common feature of these microorganisms is their antagonistic activity against some pathogens such as salmonella spp., Escherichia coli, Listeria monocytogenes, Clostridium perfringens and Helicobacter hepaticus.6–11 The capability of L. plantarum and L. reuteri in phytic acid hydrolysis is valuable in bread processing technology.12 Inoculating hetero-fermentative lactic acid bacteria to alfalfa silages cause to increase the production of lactic and acetic acids, hence decrease pH, reduce the number of yeasts and molds, and inhibit Enterobacterium and Klebsiella pneumoniae.13 Lactobacilli are widely applied in industrial enzyme production processes, for example glucose-forming amylase 14 and lactase.15 Different strains of Lactobacilli are the main fermentative lactic acid and γ-aminobutyric acid producers used in commercial processes as well as starters in dairy products.16–18
Kinetic behavior of Lactobacilli was studied by some previous researchers. Ghaly et al.,19 reported that high lactose concentrations had an inhibitory effect on L. helveticus growth rate. They also emphasized that adding yeast extract to culture medium and using micro aeration could caused to increase the specific growth rate and lactose consumption of Lactobacilli.19 Vasudha and Hari20 investigated Gompertz and Logistic kinetic models for L. plantarum NCDC 414. The viable cell counts increased from 4×105 to 7×1010CFUmL−1 at 24h.20 Cock and Stouvenel21 studied lactic acid production by L. lactis subs lactis and showed that up to 35gL−1 lactic acid was obtained in fermentation with using 60gL−1 of initial glucose.21 Amrane22 evaluated the growth kinetic of L. helveticus on whey permeate. He characterized and described five separate phases during L. helveticus growth.22L. rhamnosus cell dry weight was obtained; which was equal to 23gL−1 after 18h incubation at appointed bioreactor conditions.23 Gupta et al.,24 found that L. plantarum cell growth rate was improved with an increase in agitation speed.24 It was also found that malic acid as carbon source enhanced the specific growth rate of L. plantarum from 0.2 to 0.34h−1.25 The study of cell growth and substrate utilization kinetic of L. casei and L. rhamnosus showed as strong exponentially dependent on product inhibition at low lactic acid concentrations.26 In our best of knowledge, there is not any documented report on Lactobacilli kinetics with Contois and Exponential kinetic models.
In this article, kinetic behavior, cell growth and substrate consumption trends of five different strains of Lactobacilli were investigated. Based on Contois and Exponential kinetic models in a batch submerged cheese whey as the main nutrients of the medium was used. In each case, influential kinetic parameters were determined.
Materials and methodsMicroorganisms and inoculum preparationLactobacillus species: L. casei subsp. casei PTCC1608, L. delbrueckii subsp. delbrueckii PTCC1333, L. delbrueckii subsp. bulgaricus PTCC1737, L. fermentum PTCC1744 and L. delbrueckii subsp. lactis PTCC1743 were prepared from Iranian Research Organization for Science and Technology.
The stock culture of each strain was separately prepared on MRS medium. Inoculated cultures were incubated at 37°C for 48h and then stored in a refrigerator at 4°C. Batch submerged fermentation process was performed in separate laboratory shake flasks contained of deproteinized sterile whey as the main substrate. The culture media was enriched by adding some growth factors including (gL−1): lactose, 50; yeast extract, 10; sodium acetate, 5; KH2PO4, 2; MgSO4, 0.2; MnSO4, 0.05; FeSO4, 0.03; peptone, 10.
Culture preparation100mL of deproteinized and enriched sterile whey was added to a 250mL shake flask. Before autoclaving, pH was adjusted to 6.5 by a 2M NaOH solution of or 2N HCl. To prevent undesired reactions, deproteinized whey and the enrichment media were separately autoclaved at 121°C for 15min and then were mixed together in sterile conditions to obtained 100mL final culture medium in each shake flask.
Batch submerged fermentationInoculation was performed by adding 2.5mL of Lactobacillus stock culture to each shake flask. Then, the flasks were incubated at 37°C mixed with agitation speed of 50rpm for 50h. At this period, samples were removed at proper 5h intervals to assay lactic acid, lactose and cell dry weight concentrations.
AssessmentsLactic acid and lactose concentrations were analyzed by high performance liquid chromatography (HPLC, Perkin Elmer 200, Shimadzu, Japan) with Aminex HEX-87H column. The mobile phase consisted of 5mM sulfuric acid solution at 40°C and flow rate of 0.6mLmin−1 was used.27
Cell dry weight was assayed using a spectrophotometer (Shimadzu, 1601, Japan) at a wavelength of 480nm. Standard dilute solutions of bacterial cell were prepared from stationary phase of cell growth. To determine cell dry weight calibration curve, 15mL of each standard sample was passed through a cellulose acetate filter with 0.45micron pore size. Filters then washed with distilled water and dried at 100°C for 24h. Cell dry weight was calculated based on differences between the initial and the final filter weights. For each Lactobacillus species, a separate standard curve of cell dry weight versus absorption value was recorded; which was applied to determine cell dry weight in actual experimental samples.
Kinetic modelsContois (Eq. (1)) is an un-structured kinetic model based on substrate and biomass concentration and Exponential (Eq. (2)) kinetic models is another un-structured kinetic model based on only substrate concentration.
where μ and μmax is the specific growth rate and the maximum specific growth rate of bacterial strain in term of h−1, respectively. Ks, S, S0, S1, X and Xm is the semi-saturated coefficient, the limiting substrate (lactose) concentration, initial lactose concentration, required lactose for initial cell biomass forming, cell dry weight and the maximum cell dry weight in term gL−1, respectively.ResultsCell growthFive different Lactobacilli species were studied for cell growth evaluation for incubation period of 50h. Deproteinized whey as carbon sources was used in a submerged batch culture. Almost for all studied strains, a 6h lag phase period and 25–45h (depend on the strain) exponential growth phase was observed. Table 1 presents biomass production and lactose consumption trends for five different strains of Lactobacilli.
Biomass and lactose concentrations and specific growth rate for five studied Lactobacilli in a submerged batch culture medium of lactose fortified whey at 37°C with 50rpm agitation speed for 52h.
| Time (h) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 52 | ||
| L. bulgaricus | X (gL−1) | 0 | 0.2 | 0.9 | 2.5 | 4.2 | 5.1 | 5.1 | 5 | 5.1 | 5.2 |
| S (gL−1) | 50 | 47.9 | 40.1 | 26.6 | 13.5 | 7.3 | 7 | 6.7 | 6.1 | 5.8 | |
| μ (h−1) | – | 0 | 0.250 | 0.210 | 0.169 | 0.135 | 0.108 | 0.089 | 0.077 | 0.071 | |
| L. casei | X (gL−1) | 0 | 0.2 | 0.8 | 2.6 | 3.9 | 4.2 | 4.3 | 4.3 | 4.2 | 3.8 |
| S (gL−1) | 50 | 48.2 | 42.1 | 31.9 | 17.8 | 10.5 | 9.2 | 9.1 | 8.7 | 8 | |
| μ (h−1) | – | 0 | 0.231 | 0.214 | 0.165 | 0.127 | 0.102 | 0.085 | 0.072 | 0.064 | |
| L. lactis | X (gL−1) | 0 | 0.2 | 0.6 | 1.9 | 3.1 | 3.2 | 3.1 | 3.4 | 3.9 | 3.1 |
| S (gL−1) | 50 | 49.2 | 38.8 | 27.3 | 21.4 | 19.6 | 18.2 | 16.4 | 15.8 | 13.2 | |
| μ (h−1) | – | 0 | 0.183 | 0.188 | 0.152 | 0.115 | 0.091 | 0.079 | 0.071 | 0.059 | |
| L. delbrueckii | X (gL−1) | 0 | 0.2 | 0.6 | 1.7 | 2.4 | 2.6 | 2.9 | 3.2 | 3.1 | 3 |
| S (gL−1) | 50 | 48.1 | 36.4 | 25.3 | 22.1 | 18.2 | 16.4 | 15.1 | 13.6 | 12.4 | |
| μ (h−1) | – | 0 | 0.183 | 0.178 | 0.138 | 0.107 | 0.089 | 0.077 | 0.065 | 0.058 | |
| L. fermentum | X (gL−1) | 0 | 0.1 | 0.7 | 2.1 | 2.5 | 2.7 | 2.7 | 2.9 | 3.3 | 3.5 |
| S (gL−1) | 50 | 49.3 | 44.2 | 35.6 | 29.1 | 26.8 | 25.3 | 23.2 | 22.6 | 20.1 | |
| μ (h−1) | – | 0 | 0.324 | 0.254 | 0.179 | 0.137 | 0.110 | 0.093 | 0.083 | 0.077 | |
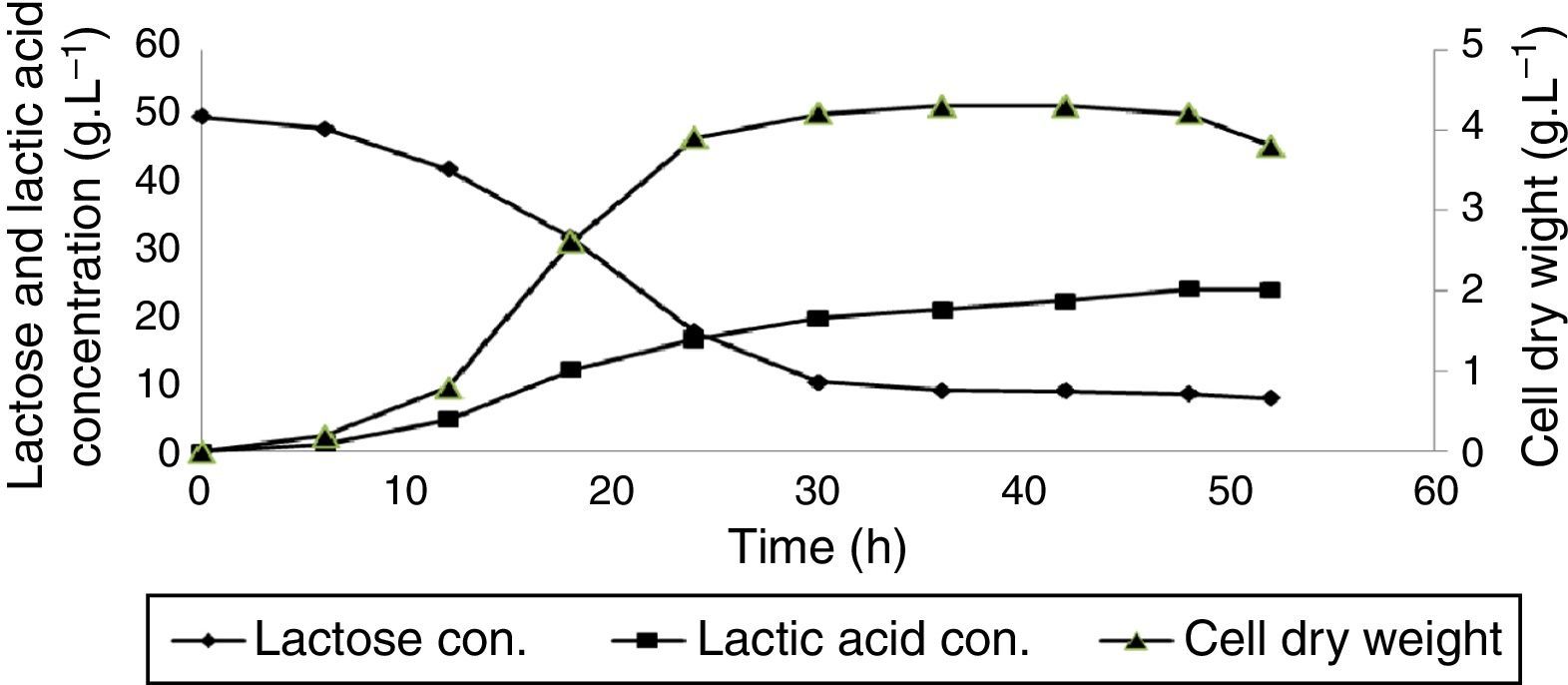
Fig. 1 presents cell dry weight and lactic acid production as well as lactose consumption profile for L. delbrueckii subsp. bulgaricus PTCC1737. After incubation period of (the real end of the exponential growth phase) maximum cell dry weight (5.1gL−1) was obtained. The rate of biomass productivity was calculated as 0.17gL−1h−1 for this strain. Maximum produced lactic acid by L. delbrueckii subsp. bulgaricus PTCC1737 was defined to be 26.6gL−1. For this strain, maximum lactic acid yield and productivity was determined of 0.602gg−1 consumed lactose (after 52h incubation) and 0.804gL−1h−1 (after 24h incubation), respectively. Results showed that cell dry weight was increased from 0.9 to 5.1gL−1 (more than 466% increase in cell density) at a period of 18h in exponential growth phase.
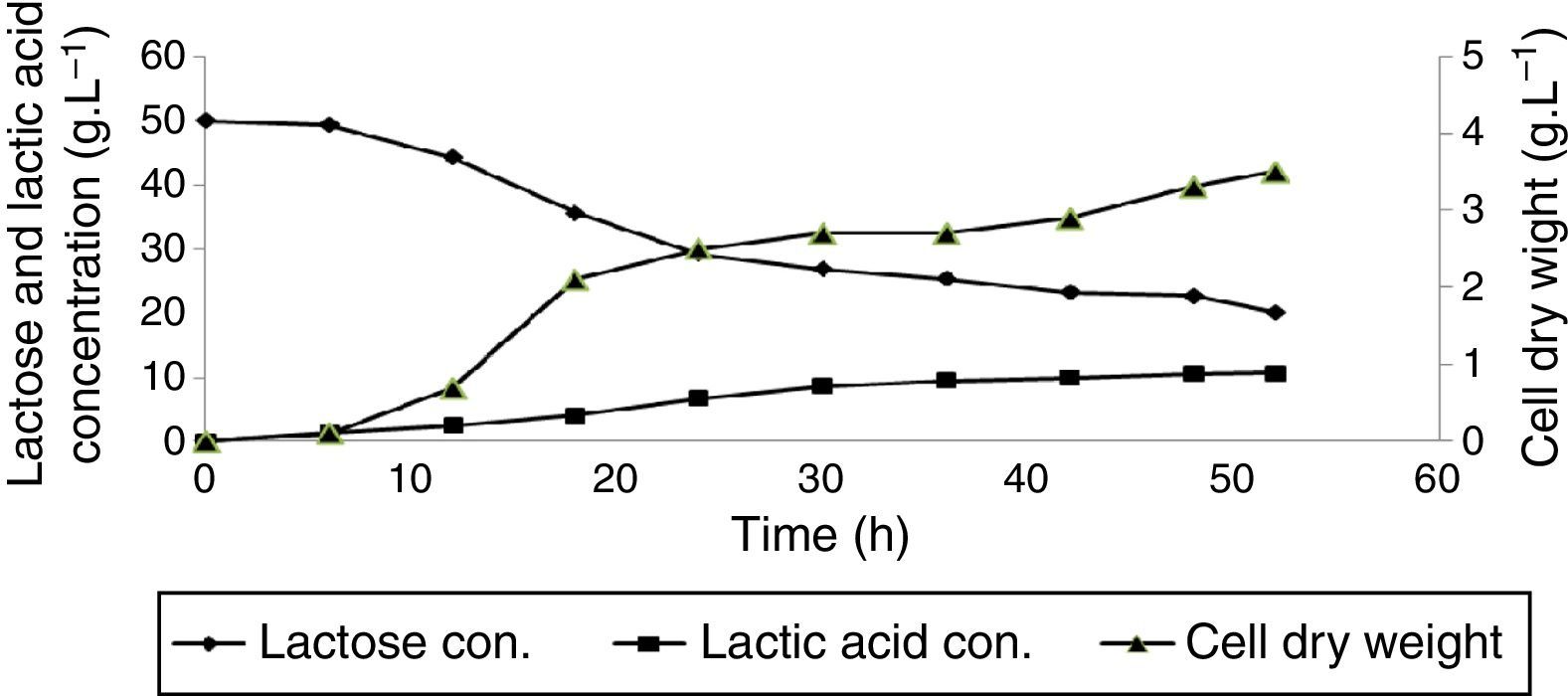
Similar behavior, of course with lower efficiency was observed for L. casei subsp. casei PTCC1608 (Fig. 2). For this strain, produced cell biomass has reached to 3.4gL−1 after 36h incubation. Biomass productivity of L. casei subsp. casei PTCC1608 was calculated 0.119gL−1h−1. Maximum lactic acid concentration of 24.2gL−1was obtained. For this strain, maximum lactic acid yield and productivity was determined as 0.586gg−1 consumed lactose (48h after incubation) and 0.696gL−1h−1 (after 24h incubation), respectively. However, cell dry weight was increased from 0.8 to 4.3gL−1 (more than 437% increase in cell density) at a period of 24h in exponential growth phase.
L. delbrueckii subsp. lactis PTCC1743 reached to its end of exponential growth phase after 24h incubation. At this time, cell dry weight was 3.1gL−1 and then, remained constant for a period of 18h. But at the end of the stationary phase (after 48h incubation) suddenly increased to 3.9gL−1 and then decreased again to 3.1gL−1 after 52h incubation (Fig. 3). Biomass productivity of L. delbrueckii subsp. lactis PTCC1743 was evaluated equal to 0.081gL−1h−1 after 48h. In exponential growth phase, cell dry weight was increased from 0.6 to 3.1gL−1 (more than 416% increase in cell density) at a period of 12h. Maximum lactic acid concentration was obtained as 14.7gL−1 at after 42h incubation. Maximum lactic acid yield and productivity was obtained 0.437gg−1 consumed lactose (after 42h incubation) and 0.47gL−1h−1 (after 30h incubation), respectively.
L. delbrueckii subsp. delbrueckii PTCC1333 had an extended exponential growth phase until 42h after incubation. At this time, cell dry weight reached to 3.2gL−1 (Fig. 4). Biomass productivity of L. delbrueckii subsp. delbrueckii PTCC1333 was obtained as 0.076gL−1h−1 after 52h incubation. Lactic acid production was observed both at exponential and the stationary growth phases. Maximum lactic acid concentration (13.2gL−1) was obtained after 52h incubation (end of the stationary phase). Maximum lactic acid yield and productivity was obtained 0.351gg−1 consumed lactose (after 52h incubation) and 0.317gL−1h−1 (after 36h incubation), respectively.
L. fermentum PTCC1744 had the longest exponential growth phase; after 52h incubation period, maximum cell dry weight of 3.5gL−1 was obtained (Fig. 5). In addition, its biomass productivity was 0.067gL−1h−1. Lactic acid production was observed at both exponential and the stationary growth phases. Maximum lactic acid concentration (10.7gL−1) was obtained at the end of the stationary phase. Maximum lactic acid yield and productivity was defined as 0.358gg−1 consumed lactose (after 52h incubation) and 0.29gL−1h−1 (after 30h incubation), respectively.
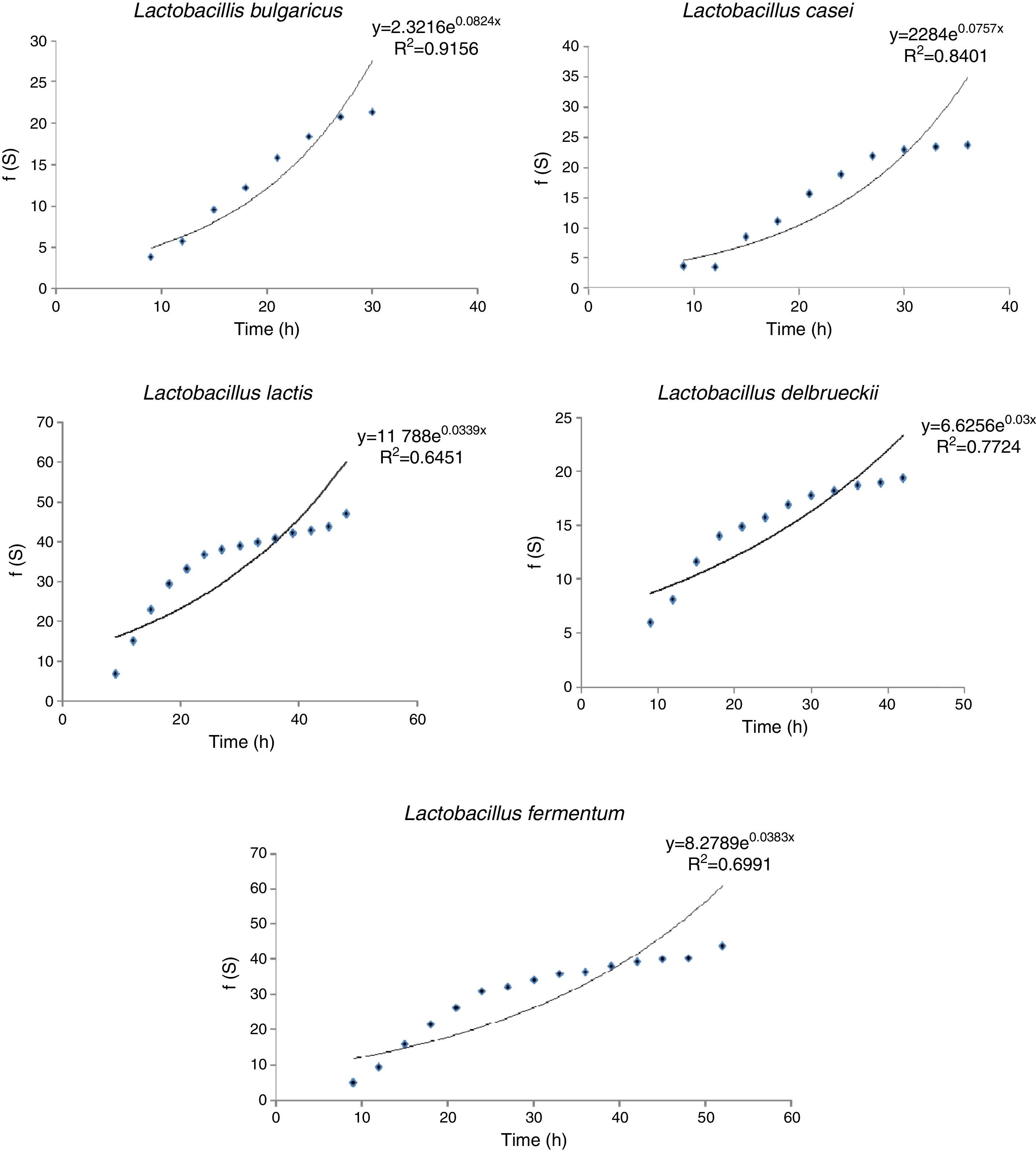
Contois kineticIn order to evaluate the consistency of five studied strains with Contois kinetic model, experimental data of lactose consumption and cell dry weight production in exponential growth phase were used (Table 1). Fig. 6 represents the linear fitted experimental data using Contois kinetic model for five investigated Lactobacilli strains.
Malthus law explains the exponential growth phase of Lactobacillus species in a batch culture (Eq. (3)). Integration of Eq. (3) using suitable initial condition (X=X0 at t=t0) resulted in Eq. (4) that demonstrates specific cell growth rate (μ). The values for initial biomass concentration and lag phase time delay (X0 and t0) were considered 0.2gL−1 and 6h, respectively.
Specific cell growth rate values were calculated according to the cell dry weight as biomass concentration (X) and average lactose concentration as limiting substrate concentration (Save) for the exponential growth phase using Eq. (4). Kinetic constant coefficients (μmax, Ks) were calculated using the curve fitting method.
Exponential modelThe consistency of the practical data for the cell growth and lactose consumption of five studied Lactobacillus species with Exponential kinetic model is presented in Fig. 7. F(S) was defined as Eq. (5).
Specific cell growth rate was calculated for each of studied Lactobacilli in exponential and the stationary growth phases are presented in Table 1.
DiscussionThe analysis of obtained results (presented in Table 1) showed that L. delbrueckii subsp. bulgaricus PTCC1737, L. fermentum PTCC1744 and L. delbrueckii subsp. lactis PTCC1743 had the highest biomass yield of 0.119, 0.117 and 0.114gg−1 of consumed lactose, respectively. L. casei subsp. casei PTCC1608 and L. delbrueckii subsp. delbrueckii PTCC1333 showed lower biomass production yield, relatively 11.8 and 22.7% less than L. delbrueckii subsp. bulgaricus PTCC1737, respectively. Fig. 1 represents that lactic acid production rate in exponential growth phase was higher than the stationary phase. Biomass productivity of L. casei subsp. casei PTCC1608 was calculated 30% less than L. delbrueckii subsp. bulgaricus PTCC1737. Maximum lactic acid concentration was 9% less than L. delbrueckii subsp. bulgaricus PTCC1737. For this strain, maximum lactic acid yield and productivity was in order 2.65 and 8.7% less than L. delbrueckii subsp. bulgaricus PTCC1737. Biomass productivity of L. delbrueckii subsp. lactis PTCC1743 was 52.35% less than L. delbrueckii subsp. bulgaricus PTCC1737. Maximum lactic acid concentration was approximate 45% less than L. delbrueckii subsp. bulgaricus PTCC1737. Maximum lactic acid yield and productivity was in order of 27.4 and 41.5% less than L. delbrueckii subsp. bulgaricus PTCC1737. Cock and Stouvenel21 found 35gL−1 lactic acid productions by L. lactis subs lactis. In the present work, maximum lactic acid concentration was obtained as 26.6gL−1 by L. delbrueckii subsp. bulgaricus PTCC1737. Biomass productivity of L. delbrueckii subsp. delbrueckii PTCC1333 was 55.3% less than L. delbrueckii subsp. bulgaricus PTCC1737 and approximately near to L. delbrueckii subsp. lactis PTCC1743. Lactic acid production was observed at exponential and the stationary growth phases. Maximum lactic acid concentration was obtained after 52h incubation (end of the stationary phase), half of L. delbrueckii subsp. bulgaricus PTCC1737. Maximum lactic acid yield and productivity was in order 41.7 and 60.6% less than L. delbrueckii subsp. bulgaricus PTCC1737. L. fermentum PTCC1744 had the longest exponential growth phase. Its biomass productivity was 60.6% less than L. delbrueckii subsp. bulgaricus PTCC1737. This strain was the same as previous mentioned strain, lactic acid production was observed at both exponential and the stationary growth phases. Maximum lactic acid concentration was about 60% less than L. delbrueckii subsp. bulgaricus PTCC1737. Maximum lactic acid yield and productivity was in order of 40.5 and 63.9% less than L. delbrueckii subsp. bulgaricus PTCC1737. Polak-Berecka et al.,23 reported 23gL−1 of dry cell weight after 18h for L. rhamnosus, much more than our studied strains. This indicate that strain type and culture composition have considerable impact on biomass production.23 Reported data by Berry et al.,28 on L. rhamnosus ATCC10863 growth characteristics and its lactic acid production in batch culture of a defined medium showed a yield of 0.84glactic acidg−1 of consumed substrate.28 Bustos et al.,29 studied lactic acid production by L. pentosus ATCC8041 from vine-trimming wastes. They reported a production yield of 0.77 (glactic acidg−1 consumed substrate) that is relatively less than our obtained data.29 It was found that the quality of substrate and type of organism species are key parameters in product yield. The main reason might be due to existence of high mineral concentration in the whey. Kim et al.,30 reported 13.7gL−1 lactic acid concentrations in a 48h fermentation using L. lactis ssp. lactis that is much lower than our obtained data using glucose as the main substrate.30
Results showed that L. delbrueckii subsp. bulgaricus PTCC1737 and L. casei subsp. casei PTCC1608 had good acceptable consistency using Contois kinetic model. R-square, μmax and Ks for the first strain were in order of 0.95, 0.265h−1 and 1.34gL−1 and for the second one were 0.9552, 0.299h−1 and 3.85gL−1, respectively (Table 2). L. delbrueckii subsp. delbrueckii PTCC1333 also showed an acceptable fitting with Contois kinetic model. For this strain R-square, μmax and Ks were 0.9493, 0.311h−1 and 13.11gL−1, respectively. L. fermentum PTCC1744 was fitted to Contois model with a good R-square (0.9367) and the greatest amounts of specific cell growth rate (2.591h−1). But its Contois semi-saturated coefficient (Ks) was the greatest obtained value of 188.33gL−1. Therefore, it is perceived that Contois kinetic model may not be desired to describe the cell growth and substrate consumption behavior of L. fermentum PTCC1744. Results indicated that the consistency of L. delbrueckii subsp. lactis PTCC 1743 with Contois kinetic model is the less than all other investigated strains. For this strain, R-square was determined to be as low as 0.873.
A comparison of Contois and Exponential kinetic constants for five different species of Lactobacilli.
| Strain | R2 | μmax (h−1) | Ks (gL−1) | ||
|---|---|---|---|---|---|
| Contois | Exponential | Contois | Exponential | Contois | |
| L. delbrueckii subsp. bulgaricus PTCC1737 | 0.9500 | 0.9156 | 0.265 | 0.0824 | 1.34 |
| L. casei subsp. casei PTCC1608 | 0.9552 | 0.8401 | 0.299 | 0.0757 | 3.58 |
| L. delbrueckii subsp. lactis PTCC 1743 | 0.8730 | 0.6451 | 0.333 | 0.0339 | 12.72 |
| L. delbrueckii subsp. delbrueckii PTCC1333 | 0.9493 | 0.7724 | 0.311 | 0.03 | 13.11 |
| L. fermentum PTCC1744 | 0.9367 | 0.6991 | 2.591 | 0.0383 | 188.33 |
Vasudha and Hari 20 studied the Gompertz and Logistic kinetic models for L. plantarum NCDC 414. They found that the viable cell counts increased from 4×105 to 7×1010CFUmL−1, lactic acid concentration increased by about 4.5 folds and 44% w/v of substrate consumption was occurred at an incubation period of 24h.20 In this work, significant lactic acid production observed for the exponential and stationary phases. In addition, the cell dry weight increased by about 10–15 folds (depend on the strain) in 24h. Alvarez et al.,26 studied the kinetics of cell growth, lactic acid production and substrate utilization of L. casei var. rhamnosus. They reported a strong exponentially dependent product inhibition at low lactic acid concentrations. Their results indicated that lactic acid production rate was partially associated with biomass growth as this work demonstrated.26
Based on the calculated kinetic parameters (Table 2), L. delbrueckii subsp. bulgaricus PTCC1737 and L. casei subsp. casei PTCC1608 had good acceptable consistency with Contois kinetic model too. R-square and μmax for the first strain were in order 0.9156 and 0.0824h−1 and for the second strain were 0.8401 and 0.0757h−1, respectively (Table 2).
It was found that other studied strains did not have an acceptable consistency with Exponential kinetic model. Thus, Exponential kinetic model is not a well desired model to describe the cell growth and substrate consumption behavior of these three strains. The obtained specific growth rate for L. delbrueckii subsp. bulgaricus PTCC1737 with Exponential kinetic was about 70% less than the obtained value with Contois kinetic model. Also, for L. casei subsp. casei PTCC1608, 75% decline was documented in this work.
ConclusionThis is the first report on the cell growth and substrate utilization kinetic of five different strains of Lactobacilli with respect to Contois and Exponential kinetic models. L. delbrueckii subsp. bulgaricus PTCC1737 was introduced as the desired strain in fields of biomass and lactic acid production yield. L. delbrueckii subsp. bulgaricus PTCC1737 and L. casei subsp. casei PTCC1608 showed acceptable consistency with both Contois and Exponential kinetic models. We found, Contois is better than Exponential model to describe cell growth and lactose consumption behavior of L. strains.
Conflicts of interestThe authors declare no conflicts of interest.
The authors wish to thank the Offices of Vice Chancellors in Research at Islamic Azad University, Qaemshahr Branch for their support of valued experimental and analytical work conducted during the course of present research.