Pan-drug resistant Gram-negative bacteria, being resistant to most available antibiotics, represent a huge threat to the medical community. Colistin is considered the last therapeutic option for patients in hospital settings. Thus, we were concerned in this study to demonstrate the membrane permeabilizing activity of colistin focusing on investigating its efficiency toward those pan-drug resistant isolates which represent a critical situation. We determined the killing dynamics of colistin against pan-drug resistant isolates. The permeability alteration was confirmed by different techniques as: leakage, electron microscopy and construction of an artificial membrane model; liposomes. Moreover, selectivity of colistin against microbial cells was also elucidated. Colistin was proved to be rapid bactericidal against pan-drug resistant isolates. It interacts with the outer bacterial membrane leading to deformation of its outline, pore formation, leakage of internal contents, cell lysis and finally death. Furthermore, variations in membrane composition of eukaryotic and microbial cells provide a key for colistin selectivity toward bacterial cells. Colistin selectively alters membrane permeability of pan-drug resistant isolates which leads to cell lysis. Colistin was proved to be an efficient last line treatment for pan-drug resistant infections which are hard to treat.
Recently, it has been witnessed worldwide that Gram-negative bacteria resistant to many classes of antibiotics represents a fearful situation toward the emergence of a future medical disaster.1 There are 2 terms commonly describing those superbugs; which are multi-drug resistant (MDR) and pan-drug resistant (PDR). An isolate is considered MDR if it exhibited resistance toward 5 out of the 7 anti-pseudomonal classes of antimicrobial agents, i.e. anti-pseudomonal penicillins, cephalosporins, carbapenems, monobactams, quinolones, aminoglycosides, and colistin, while it is a PDR if it showed resistance toward all 7 anti-pseudomonal antimicrobial agents, including colistin.2 There was another view considering that PDR isolates were those resistant to all antibiotics but only susceptible to polymyxins.3,4 Although there is no apparent definition for the term PDR throughout literature, it generally denotes resistance against a variety of antibiotics excluding polymyxins.5 Such view is adopted in the present work. In the past few decades there have been a tremendous increase in resistance to currently available antibiotics and a significant decline in development of new ones.6 This leads to the revival of older agents as polymyxins, for the treatment of such PDR infections.2
Polymyxins are a group of polypeptide cationic antibiotics that were isolated from Bacillus polymyxa in the 1940s.7 Since then, polymyxin E (colistin) and polymyxin B were extensively used in clinical practice for Gram-negative organisms.8,9 However, they were gradually withdrawn from the market and abandoned during the last two decades due to claimed reports of toxicity. Therefore, during that time, there have been limited studies on the clinical use, pharmacokinetics and pharmacodynamics of colistin.10 Emergence of the PDR pathogens, necessitated the re-evaluation of polymyxin therapies.11
Colistin has been recently considered as last option treatment for patients with nosocomial PDR infections, which have become an important public health issue, owing to its favorable properties of rapid bacterial killing, a narrow spectrum of activity, and slow development of resistance.12,13 Colistin interacts electrostatically with the outer membrane of Gram-negative bacteria and competitively displaces divalent cations which stabilize the lipopolysaccharide layer thus disrupting the membrane integrity. It is then subsequently taken up via the self-promoted uptake pathway.13 It is believed that colistin forms cracks in the outer membrane which promotes its uptake inside the cell and permits the passage of different molecules.14 Thus, polymyxins produce a disruptive detergent effect, leading to increased permeability in the outer membrane, leakage of the absorbing cytoplasmic contents, cell lysis and finally death.15 The chemical composition of bacterial membranes being rich in phosphatidylethanolamine and negatively charged lipids allows such electrostatic attraction with cationic peptides in contrast to eukaryotic cells in which cholesterol is the predominant component providing a clue for the selectivity of action toward microbial versus host cells.16 Such a situation prompted the present microbiological study to investigate the membrane permeability alteration of colistin and it bactericidal effect on PDR Gram-negative clinical isolates including Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli.
Materials and methodsMicroorganisms and antibioticsFour clinical bacterial isolates, identified by classical microscopic and biochemical procedures,17,18 were used in this study. These are: A. baumannii (A182), P. aeruginosa (P103), E. coli (E9) and K. pneumoniae (K103). The identified isolates were maintained by freezing in 15% glycerol broth 19 (Oxoid Ltd.; Basingostok; Hampshire, England). Colistin sulphate was obtained as powder from Pharco pharmaceutical Co., Egypt. It was dissolved in water to prepare stock solutions. The susceptibility of the tested isolates, to the different classes of antibiotics was determined by the standard disc agar diffusion technique according to Bauer et al.20 with some modifications.21 MIC of colistin was determined by the standard broth dilution technique.22
Bactericidal activity of colistin using the viable count techniqueFor each tested isolate, two concentrations of colistin (1/2MIC and MIC) were prepared in sterile nutrient broth. Each concentration was inoculated with overnight culture to give a final inoculum of 106cfu/mL. A control without antibiotic was prepared for each of the tested isolates. The systems were mixed well and incubated at 37°C with shaking. Samples were aseptically withdrawn from each test tube at 0, 1, 3, 6 and 24h and serially diluted with sterile saline. Then, 10μL portions were dropped onto the surface of overdried nutrient agar plates. The plates were left to dry and incubated inverted at 37°C for 24h, the resulting colonies were counted and the original viable count was determined.23
Effect of colistin on the cytoplasmic membrane by leakage techniqueBacterial suspensions of the selected isolates were prepared by streaking an overnight broth culture onto nutrient agar slants (Oxoid Ltd.; Basingostok; Hampshire, England). The slants were incubated at 37°C for 16–18h. The resulting growth of 3 slants was resuspended in 5mL sterile 0.9% saline to produce heavy inoculum (O.D600 adjusted to 2) and transferred into sterile test tubes. The obtained bacterial suspensions were centrifuged at 12,000×g for 5min. The formed pellets were washed twice with sterile saline and then were resuspended in 5mL sterile saline. Aliquots of the prepared bacterial suspension of each isolate were treated with 50mg/L of colistin. A control was included in each test containing untreated bacterial suspension. Both the treated and untreated bacterial suspensions were incubated at 37°C for 24h. After incubation, the bacterial suspensions were centrifuged at 12,000×g for 5min. The absorbance of the clear supernatant was estimated at 260 and 280nm against saline solution using the spectrophotometer (Thermospectronic, Helios alpha, NC 9423UV A 1002E, England).24
Effect of colistin on the leakage of red blood cellsOne milliliter of fresh human blood was centrifuged at 2000×g for 5min and the cells were washed 4× with sterile 0.9% saline discarding the supernatant every time. The sedimented red blood cells (RBCs) were resuspended in 1mL buffer (5mM sodium phosphate and 150mM sodium chloride [pH 7.4]). The RBCs suspension in buffer was distributed in sterile eppendorf tubes in 25μL aliquots and 1mL of colistin solution dissolved in the same buffer was added to each eppendorf in concentrations ranging from 0.78mg/L to 100mg/L. The resulting suspensions were incubated at room temperature for 2h. The systems were then centrifuged at 2000×g for 10min. The release of hemoglobin was monitored by measuring the absorbance of the supernatant at 540nm by spekol (Carl Zeiss, Jena, Germany). Absorbance of control untreated cells in saline was measured and used as a blank. Total haemolysis was also measured by lysing colistin-untreated RBCs with distilled water.25
Effect of colistin on the ultrastructure of bacterial cells using the transmission electron microscopeTwo clinical isolates were used in this study; a sensitive and an induced resistant population for each isolate. The resistance was induced by serial passaging in increasing colistin concentrations. 0.5mL of overnight broth culture was inoculated in flasks containing 50mL sterile nutrient broth. Flasks were incubated in the orbital shaking incubator (100rpm) at 37°C till reaching acceptable turbidity for about 4–5h. The selected clinical isolates were treated with 100mg/L of colistin for 1h. Proper controls lacking colistin were included for each isolate population. The obtained bacterial suspensions were centrifuged at 6000×g for 5min. The supernatants were discarded. The obtained colistin-treated and control bacterial pellets were then resuspended in 3mL Trump's fixative solution and further processed as previously described.26,27 The samples were examined and photographed at an accelerating voltage of 80kV using Joel CX 100 transmission electron microscope at the Electron Microscope Unit, Faculty of Science, Alexandria University.
Effect of colistin on the cytoplasmic membrane using artificial cytoplasmic membrane modelNegatively-charged unilamellar cholesterol free liposomes were prepared by reverse-phase evaporation method.28 The liposomes were then treated by colistin (100mg/L) for 24h and were morphologically examined by the phase contrast microscope (Olympus, CX 41 RF) using oil-immersion objective lens (100×) for any damage in their shape following colistin treatment.
Effect of colistin on the formation of spheroplastsOne hundred μL of exponential cell culture, adjusted to have O.D600 of 0.05 were inoculated into 3mL Müller-Hinton broth containing 0.3M of sucrose and were mixed well. Then, aliquots of 100μL were distributed in sterile eppendorf tubes. Ten μL of colistin were added to 3 eppendorfs in concentration of 1/2 MIC. Ten μL of ceftazidime were added to 3 other eppendorfs in concentration of 1/2 MIC. A control eppendorf was included containing 0.9% saline instead of antibiotic solution. The eppendorfs were incubated in the orbital shaking incubator at 37°C, 100×g for 90min. Samples were then taken and examined by the phase contrast microscope using oil-immersion objective lens (100×).
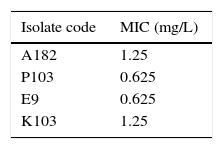
Results and discussionGlobally, there is a growing threat from the emergence of MDR and PDR organisms especially Gram-negative bacteria, such as P. aeruginosa, A. baumannii, Klebsiella and Enterobacter species in hospital settings.29 PDR pathogens represent a fearful clinical situation with tremendous implications. So, this study aimed at investigating the deleterious effects of colistin on those PDR pathogens. The four bacterial isolates used in this study were confirmed to be pan-drug resistant by the antibiotic susceptibility testing being resistant to all antibiotic classes except colistin (data not shown). The MIC of colistin against the tested isolates ranged from 0.625 to 1.25mg/L as determined by broth dilution method (Table 1). All the tested isolates were sensitive to colistin according to the CLSI resistance breakpoint which is 4mg/L (Table 1).
The dynamics of killing of colistin was determined by the viable count technique against the tested isolates using ½ MIC and MIC of colistin. Colistin was shown to be a rapid bactericidal agent in a concentration dependent manner in all the tested PDR isolates. Rapid and significant declines (>2log) in bacterial survival were observed after 3h in all the tested isolates at 1/2 MIC level reaching almost 6logs at the MIC level. The rapid bactericidal activity of colistin is related to its permeabilizing action on the cell membrane following self-promoted uptake.30 However, re-growth was observed as early as 6h and substantial re-growth occurred at 24h in all the tested PDR clinical isolates at 1/2MIC while only 2 isolates showed re-growth at the MIC level (Fig. 1). This might be caused by the heteroresistant subpopulations which grow probably at a slower rate than the sensitive subpopulation and hence, temporary inhibition could be mistaken. This phenomenon is of potential risk as it can lead to therapy failure if colistin monotherapy is used.31
Bactericidal activity of colistin against PDR isolates. (A) A. baumannii (A182), (B) P. aeruginosa (P103), (C) K. pneumonie (K103) and (D) E. coli (E9). Filled circles represent the control untreated cells, filled squares represent 1/2 MIC of colistin, filled triangles represent MIC of colistin.
A leakage study was conducted to confirm the membrane permeability alteration effect of colistin. The effect of 50mg/L of colistin on the cytoplasmic membrane against 4 representative PDR clinical isolates was determined spectrophotometrically. The difference between the values of absorbance at 260 and 280nm of the supernatant of both the treated cells and the untreated cells corresponding to the net leakage due to treatment is illustrated in Fig. 2.
Colistin resulted in a net loss of 260 and 280nm-absorbing materials. The extent of leakage differed from one organism to another. The maximum leakage was observed for the P103 isolate.
This leakage effect is due to the destabilization of membrane integrity arising after the electrostatic interaction of colistin with the outer membrane displacing the divalent cations from the LPS layer.32 This results in an increase in the permeability of cell membrane, leakage of cell contents and finally cell death.
Furthermore, to demonstrate the selectivity of colistin toward microbial membranes, the effect of colistin on the haemolysis of human RBCs was studied. The release of hemoglobin was monitored by measuring the absorbance of the supernatants of the different reaction mixtures at 540nm by the spekol. Then, the per cent of haemolysis of RBCs was calculated. It is shown in Fig. 3 that the per cent of RBCs haemolysis increased by increasing colistin concentration. It produced about 1.3% haemolysis at a concentration of 12.5mg/L which is nearly more than ten times the average MIC of the tested PDR clinical isolates. At low concentrations, it produced insignificant haemolysis less than 1%. However, there was a sharp increase in the per cent of haemolysis at concentrations above 50mg/L which are unachievable in vivo. This confirms the selectivity of colistin toward the cytoplasmic membrane of the bacterial cells and denotes its in vivo safety at the normal administered doses. The composition of bacterial cells being rich in anionic phosphatidylglycerol and cardiolipin likely provides an important determinant for antimicrobial cationic peptides to target microbial membranes.33
Another evidence for the damaging effect of colistin on the cell envelope is obtained by electron microscope examination. The effect of colistin on the ultrastructure of 2 representative isolates (A182 and P103) was studied. The cells (sensitive and induced resistant) were treated by 100mg/L of colistin for 30min, centrifuged, fixed and prepared by encapsulating and cutting ultra thin sections from treated and untreated bacterial cell sediments followed by examination of the prepared section under the transmission electron microscope. A smooth continuous cell wall and cytoplasmic membrane structures were observed in the untreated sensitive bacterial strains. However, a slightly wavy cytoplasmic outline was observed for the induced resistant one (Fig. 4). Colistin-treated cells showed numerous projections and pores on the cell wall which was almost dissolved whereas the cytoplasmic membrane was partially damaged. The contents of some treated cells appeared depleted as part of the cytoplasmic material was released through cracks. Such phenomenon was more apparent with the sensitive isolates than with the corresponding isolates with induced colistin resistance (Fig. 5).
Ultrastructure of colistin treated P. aeruginosa cells. (A1,2,3): Sensitive P. aeruginosa P103 cells, (B1,2,3): Resistant (induced) P. aeruginosa P103 cells, A1 and B1 represent the control untreated cells for each category. Magnification: 30,000×: A3, B1, B2, 40,000×: A1, B3 and 50,000×: A2.
Moreover, the effect of colistin on an artificial cytoplasmic membrane model was determined by examination under the phase contrast microscope. The effect of colistin (100mg/L) on the negatively-charged unilamellar cholesterol free liposomes compared to the control untreated ones is illustrated in Fig. 6. Colistin resulted in an overall deformation in the structure of the artificial cytoplasmic membrane model. Various effects were observed on the phospholipid membrane ranging from roughness and distortion of the outline, pore formation and complete rupture of the membrane and liberation of the internal contents.
It was a thought of interest whether colistin affects only the outer membrane or it also affects the cell wall. So, the effect of colistin on the cell wall was compared to that of ceftazidime which is a β-lactam antibiotic known to act on the cell wall by using the isolate P103. Ceftazidime causes spheroplast formation prior to cell lysis in Gram-negative bacteria due to inhibition of transpeptidases (Penicillin-Binding Proteins) which catalyze the cross linking of peptidoglycan chains. Thus, it disrupts the synthesis of peptidoglycan resulting in the release of autolysins which enzymatically degrade the cell walls forming spheroplast, which is osmotically-sensitive cell lacking rigidity of the cell wall.34 Sucrose 0.3M acts as a stabilizer for the formed spheroplasts. By using sub-inhibitory concentrations of ceftazidime in the present study, spheroplasts were formed and viewed by the phase contrast microscope (Fig. 7). When comparing that to colistin, similar structures were obtained at 1/2 MIC level. Formation of such osmotically unstable structures indicates that colistin not only acts on the cytoplasmic membrane but also on the cell wall. Therefore, it could be assumed that treatment of sensitive Gram-negative cells with colistin in the presence of hypertonic solution might have resulted in the release of autolysins which degraded most of the cell wall leaving spheroplasts supported by the surrounding high osmotic pressure.35 Hence, it can be elucidated that colistin acts on multiple layers of Gram-negative cells, initially on the outer membrane as demonstrated by the electron microscopy study and then on the inner membrane as shown by the spheroplasts data.
In conclusion, it can be demonstrated that colistin is still a promising drug for the treatment of infections due to PDR clinical isolates. It induces alterations in the permeability of the bacterial cytoplasmic membrane, leakage of the intracellular contents and ultimately cell death. However, colistin should not be misused to avoid the global problem of development of resistance. Further in vivo investigations are still required concerning the pharmacokinetics, pharmacodynamics and toxicodynamics of colistin.
Conflict of interestNone declared.
The work was conducted in Pharmaceutical Microbiology Department, Faculty of Pharmacy, Alexandria University, Egypt.
















![Morphology of liposomes examined under oil-immersion objective lens (total magnification 1000×). (A) Untreated control, (B) Colistin-treated [additional optical zoom: A1 and B1 (2×), A2 and B2 (3×)]. Morphology of liposomes examined under oil-immersion objective lens (total magnification 1000×). (A) Untreated control, (B) Colistin-treated [additional optical zoom: A1 and B1 (2×), A2 and B2 (3×)].](https://static.elsevier.es/multimedia/15178382/0000004700000002/v2_201703300117/S151783821600054X/v2_201703300117/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
