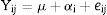The purpose of this study was to investigate the production of flavor compounds from olive mill waste by microbial fermentation of Rhizopus oryzae and Candida tropicalis. Olive mill waste fermentations were performed in shake and bioreactor cultures. Production of flavor compounds from olive mill waste was followed by Gas Chromatography–Mass spectrometry, Gas chromatography- olfactometry and Spectrum Sensory Analysis®. As a result, 1.73-log and 3.23-log cfu/mL increases were observed in the microbial populations of R. oryzae and C. tropicalis during shake cultures, respectively. C. tropicalis can produce a higher concentration of d-limonene from olive mill waste than R. oryzae in shake cultures. The concentration of d-limonene was determined as 185.56 and 249.54μg/kg in the fermented olive mill waste by R. oryzae and C. tropicalis in shake cultures respectively. In contrast, R. oryzae can produce a higher concentration of d-limonene (87.73μg/kg) d-limonene than C. tropicalis (11.95μg/kg) in bioreactor cultures. Based on sensory analysis, unripe olive, wet towel, sweet aromatic, fermented aromas were determined at high intensity in olive mill waste fermented with R. oryzae meanwhile olive mill waste fermented with C. tropicalis had only a high intensity of unripe olive and oily aroma.
The food and agricultural industries produce annually million tons of waste, resulting from production and consumption of food, peels, pulps, aqueous residues and others, many of which raise serious disposal issues and, consequently, considerable costs to various industries.1 Therefore, using agro-wastes is the most popular aspect in biotechnological processes for production of high value-added products in terms of the reducing production cost.2,3 Recently, agro-wastes have been focused in biotechnological flavor production by using microbial fermentation or biotransformation owing to their high amount of reusable components for microorganisms.1,4–6 Several studies were conducted on the production of natural flavor compounds from agro-wastes by microbial fermentation. Cassava bagasse, sugar beet, beet molasses, coffee husk, soy bean waste, apple pomace, cacao bagasse were extensively used in these studies for the production of natural flavors by yeast or molds.7–11 For instance, de Oliveira et al.12 reported that 2-phenylethanol was produced from cassava wastewater by Saccharomyces cerevsiae, Geotrichum fragrans and Kluveromyces marxianus in the following yields order: 0.74g/L, 0.19g/L and 0.08g/L. Zheng et al.13 produced vanillin from waste residue of rice bran oil by both strains of Aspergillus niger CGMCC0774 and Pycnoporus cinnabarinus CGMCC1115. In the more recently, Wilkowska et al.14 showed that the production of esters and alcohols including ethyl acetate, isoamyl acetate, isoamyl alcohol and 2-phenylethanol from apple pomace with chokeberry and cranberry pomaces can be achieved by immobilized K. marxianus LOCK0024 in shake culture. Fadel et al.15 reported that a high concentration of 6-pentyl-α-pyrone (3.62mg/g DM) associated with coconut aroma can be produced from a sugarcane bagasse by using T. viride EMCC-107. Olive mill waste (OMW) and olive mill waste water (OMWW) are the most important wastes for olive oil industry in Mediterranean regions. One to 2.5 million tons was produced annually during olive oil season in Andalusia region (Spain)16 while 200–250 thousand tons of OMW are produced in Turkey.17 OMW is a solid phase by-product resulting from extraction of olive oil by pressure or centrifugation. OMW approximately has 25–55% water; 25–50% of fiber with a great degree of lignifications, 5–8% of residual oil, 2–6% of ash and 6–10% of nitrogen associated with the insoluble fiber fraction.18–20 Microbial fermentation has been taken over by many researchers due to possibility of valorization of agro-wastes, low-cost production steps and the possibility of using several types of microorganisms.21,22 From this perspective, utilization of the filamentous fungi such as Rhizopus species and the thermo and ethanol tolerance of Candida species have been widely examined in bioenergy and bioproduct industries.23–25 When we take into the consideration the nutritional content of OMW for microbial growth, OMW might be used as raw material in the production of flavor compounds by both microorganisms via microbial fermentation. Therefore, this study focuses to investigate the production of natural flavor compounds from OMW by microbial fermentation of Rhizopus oryzae and Candida tropicalis.
Material and methodsMicroorganisms and inoculum preparationStrains of Rhizopus oryzae NRLL 395 and Candida tropicalis ATCC 665 were obtained from Department of Bioengineering, Ege University (Izmir, Turkey). Both microorganisms were grown on slant Potato Dextrose Agar (PDA) in Petri plate at 30°C for 7 days. Then, R. oryzae spores and C. tropicalis cells were collected by washing with 0.1% (w/v) Tween 80 from agar surface, separately. The spore suspension of R. oryzae was filtered through two layers cheesecloth and centrifuged at 3000rpm for 5min. The cell suspension of C. tropicalis was centrifuged at 3000rpm for 5min. Both microbial suspensions were counted with Thoma counting chamber by using light microscope.7,26 The suspensions contain 107–8spores or cells/mL for R. oryzae and C. tropicalis.
Experiments of shake cultures and bioreactor culturesInitially, 200g of OMW were weighed and grinded by using knife mill Restch GM 200 (Haan, Germany) for 15min. Then, 2L of OMW solution (10%, w/v) was prepared for shake cultures. The OMW solution was homogenized at 24,000rpm by Ultraturax (IKA-WERKE GmbH, Germany). The solution was divided into six groups (300mL) and initial pH was adjusted to 5.0, for R. oryzae and pH 7.0, for C. tropicalis by using 1N HCl and 1N NaOH. Initial pHs were selected based on previous studies and these pHs are optimum for microbial growth.27,28 30mL of OMW solution was poured into 100mL Erlenmeyer and tops were closed with cotton wool and aluminum foil. The OMW solutions were sterilized in an autoclave (Hirayama, Saitama, Japan) at 121°C for 15min and then inoculated with C. tropicalis and R. oryzae at a level of 107–8 cell or spores/mL OMW suspension. The flasks then were incubated at 120rpm for 288h at 30°C in a rotary incubator (Sartorius-Certomat IS, Goettingen-Germany). The control groups without microorganism were prepared by following the same procedure. Duplicate samples were prepared for each treatment.
Bioreactor cultures were conducted in a 5L stirred tank bioreactor (STR) (Biostat A-plus®, Sartorius, Melsungen, Germany) with 4L working volume. Fermentation conditions used for microbial growth and flavor production were based on the results obtained in shake cultures. The STR was equipped with two six-blade impellers, pH probe (Hamilton, Easyferm K8/325) and PT 100 temperature sensor. The aeration rate, agitation speed and temperature for both microbial cultures were set as 0.325vvm, 120rpm and 30°C respectively.
Specific growth rate and microbial countSpore count of R. oryzae and cell count of C. tropicalis during fermentation were determined by pour plate technique with Potato Dextrose agar (PDA).26 The sample was taken from shake flask and STR intermittently in aseptic conditions.
Analysis of flavor compoundsFlavor compounds from fermented OMW were determined by gas chromatography–olfactometry (GCO), gas chromatography–mass spectrometry (GC–MS) and sensory analysis.
Extraction of flavor compoundsFlavor compounds in fermented and unfermented OMW were extracted by solid-phase microextraction (SPME).29 Three grams of OMW were weighed in a 40mL amber colored screw top vial with hole cap PTFE/silicon septa (Supelco, Bellafonte, USA) to which 1g of NaCl was added to the vial. The vial was kept at 40°C in a water bath (GFL, Grossburgwedel, Germany) for 20min to equilibrate the volatiles in headspace. Then, a SPME (2cm to 50/30μm DVB/Carboxen/PDMS, Supelco, Bellafonte) needle was inserted into the vial. The SPME fiber was exposed at a depth of 2cm in the headspace of the vial for 20min at 40°C in water bath. Then, the sample was injected into GC–MS, immediately.30
Gas chromatography olfactometry (GCO) analysisThe GCO analysis was performed for 5-days fermented OMW to determine the changes in flavor profile. The GCO was conducted by HP 6890 GC (Agilent Technologies, Wilmington, DE, USA) equipped with a flame ionization detector (FID), a sniffing port and splitless injector system. A nonpolar column (HP-5 30m length, 0.32mm i.d., 0.25μm df; J&W Scientific) was used for sniffing. Column effluent was split 1:1 between FID and olfactory port using deactivated fused silica capillaries (90cm length, 0.25mm i.d.). Helium was used as the carrier gas. Inlet pressure was 7.07psi, and flow 1.2mL/min. The GC oven temperature was programmed from 40 to 230°C at a rate of 10°C/min, with initial hold of 5min and final hold time of 20min. The FID and sniffing port were maintained at the temperatures of 250°C and 200°C, respectively. GCO procedure was duplicated.30 Post-peak intensity method was used for the determination of aroma intensity by using 10-point scale anchored to the left with ‘not’ and to the right with ‘very’.31 Sniffer had 100h of experience with GCO technique, scale using and odor description. Aroma-active compounds were identified by comparing retention indices (RI) and odor quality of unknowns with those of references analyzed at the same experimental conditions by sniffer during GCO procedure. Retention indices were calculated using n-alkane series.32
Identification and quantification of flavor compoundsFlavor compounds were determined by gas chromatography–mass spectrometry (GC–MS). Nonpolar HP5 MS column (30m×0.25mm i.d.×0.25μm film thickness, J&W Scientific, Folsom, CA) was used for separation of flavor compounds. The GC–MS system consisted of an HP 6890 GC and 7895C mass selective detector (Agilent Technologies, Wilmington, DE, USA). The oven temperature was programmed from 40°C to 230°C at a rate of 10°C/min with initial and final hold times of 5 and 20min, respectively. Helium was used as the carrier gas with a constant flow of 1.2mL/min. The Mass Spectrometry Detector (MSD) conditions were as follows: capillary direct interface temperature, 280°C; ionization energy, 70eV; mass range, 35–350amu; scan rate, 4.45scan/s.30 Identification of the flavor compounds was based on the comparison of the mass spectra of unknown compounds with those in the National Institute of Standards and Technology33 and Wiley Registry of Mass Spectral Data, 7th Edition34 mass spectral databases. Quantification of flavor compounds was expressed as relative abundances of flavor compounds by Eq. (1).35 2-Methyl pentanoic acid and 2-methyl-3-heptanone were used as an internal standard (IS) for acidic and neutral-basic compounds, respectively.
Sensory analysisA roundtable discussion was conducted to determine descriptive sensory properties and changes in aroma profiles of fermented OMW versus control samples (unfermented OMW) for shake cultures.36 Panelists were staff and graduate students in the Department of Food Engineering at Çanakkale Onsekiz Mart University. Four female and three male participated for sensory panel. Their ages ranged from 24 to 45 years. The panel received about 300h of training during generation and definition of descriptive terms. Panelists quantified the attributes using 15-point product-specific scale anchored to the left with ‘not’ and on the right with ‘very’.36
Statistical analysisAnalysis of variance (ANOVA) was conducted to determine the differences in the amount of flavor compounds during fermentation time in shake cultures and bioreactor cultures. ANOVA model37 is shown in Eq. (2).
where Yij is the jth observation value in the ith fermentation time, μ is the general population mean, αi is the effect of the ith fermentation, and eij represents the random error term. Tukey's Honestly significant difference (HSD) test was used for separating means; SPSS for Windows (version 15.0) was used for all statistical analyses.Results and discussionMicrobial growth of Rhizopus oryzae and Candida tropicalis in OMWThe microbial growth of R. oryzae and C. tropicalis in OMW during shakecultures and bioreactor cultures were shown in Table 1. Maximum increase in microbial population of R. oryzae and C. tropicalis were determined as 1.73logcfu/mL (1.29 fold) and 3.23logcfu/mL (1.52 fold), respectively. The growths of R. oryzae and C. tropicalis came into stationary phase around 72h in shake cultures. In stationary phase, microbial populations of R. oryzae and C. tropicalis were around 6.5logcfu/mL and 9.0logcfu/mL, respectively. In bioreactor cultures, it was observed that both microorganisms were attained the exponential growth phase within 24h. The microbial population of R. oryzae increased around 3logcfu/mL (3 fold) through 288h of fermentation whereas C. tropicalis populations’ increased 1.4logcfu/mL (1.22 fold) at the same fermentation time (Table 1).
Growth of R. oryzae and C. torpicalis in OMW during shake cultures.
| Fermentation time (h) | Microbial count±S.E. (logcfu/mL OMW solution) | |
|---|---|---|
| Shake cultures | ||
| R. oryzae | C. tropicalis | |
| 0 | 0.92±0.08 | 6.15±0.14 |
| 24 | 6.76±0.65 | 7.97±1.13 |
| 48 | 7.65±0.01 | 7.61±0.01 |
| 72 | 6.68±0.01 | 9.21±2.25 |
| 120 | 6.83±0.04 | 9.38±0.01 |
| 168 | 6.69±0.01 | 9.05±0.14 |
| 288 | 6.84±0.03 | 9.28±0.01 |
| Maximum growth (log cfu/mL) | 1.73 | 3.23 |
In the literature, there are several studies on the growth behavior of R. oryzae and C. tropicalis in solid state and submerged fermentation,38–40 and most of researchers have revealed different results for growth behavior and biomass increase for R. oryzae and C. tropicalis on certain agrowastes. The obtained data about the microbial growth of both R. oryzae and C. tropicalis in OMW showed both microorganisms growths were different for shake cultures and bioreactor cultures unexpectedly. These differences could be attributed to the interaction of microorganism with OMW nutrients in fermentation conditions and strain of microorganism.2 In our previous study41 on OMW, we observed the microbial growth of Trichoderma atroviride in shake cultures was higher than bioreactor cultures, whereas the yeast Torulaspora delbrueckii acts adverse growth behavior from T. atroviride at the same conditions. Jin et al.42 investigated the growth of different Rhizopus strains in potato, corn, wheat starch and pineapple processing waste water streams for lactic acid production. Similar to our results, an increase in fungal biomass was found to be about 2 and 4.5 fold for R. oryzae and R. arrhizus in the fermentation of all studied agrowaste. The researchers also indicated that sharp increase in biomass formation of R. oryzae 2062 was higher than R. arrhizus 36017 at 30°C and 150rpm in all agrowastes. Saraçoğlu and Çavuşoğlu43 found that biomass of C. tropicalis’ Kuen 1022 in sunflower hull hydrolysate medium (66g/L) increased in 6–7 fold until 20h fermentation at 30°C; stirring at 140rpm. Oberoi et al.44 investigated enhanced ethanol production via fermentation of rice straw hydrolysate by C. tropicalis ATCC 13803 which adapted and non-adapted for a rice straw hydrolysate medium. They found that the biomass of adapted and non-adapted C. tropicalis ATCC 13803 increased about 3 and 2 folds during 6–18h fermentation at 35°C and 120rpm, respectively and both microorganisms biomass remained stationary during 18–24h of the fermentation.
Flavor production characteristics by Rhizopus oryzae and Candida tropicalis in OMWAroma active compounds of 5 days (∼135h) fermented and unfermented OMW were shown in Table 2.
Aroma-active compounds of unfermented and fermented OMW with R. oryzae and C. tropicalis (n=2).
| RIa | Volatile compound | Aroma quality | Identification methods | Aroma intensityb (mean±SE) | |||
|---|---|---|---|---|---|---|---|
| Control | R. oryzae | Control | C. tropicalis | ||||
| 598 | Diacetyl | Butter | RI,O | 1.25±0.25 | 0.60±0.10 | 4.75±0.75 | 4.50±1.50 |
| 734 | 2-Pentanone | Oily | RI,MS,O | ND | 1.50±0.50 | ND | ND |
| 802 | Hexanal | Green grass | RI,MS,O | 3.0±1.0 | ND | 2.0±0.10 | ND |
| 856 | Unknown 1 | Dirty, acid | RI,O | 5.50±0.50 | 5.25±1.25 | 0.75±0.75 | ND |
| 862 | Isovaleric acid | Sour, fruity | RI,O | 5.50±0.50 | 4.75±0.25 | 1.75±0.25 | 1.50±1.50 |
| 869 | Sytrene | Acid, dirty | RI,MS,O | ND | ND | 2.75±0.25 | ND |
| 898 | Methional | Boiled potato | RI,O | 4.50±0.50 | ND | 4.50±1.50 | 3.75±0.25 |
| 928 | 2-Acetyl-1-pyroline | Popcorn | RI,O | ND | 0.50±0.50 | ND | ND |
| 975 | Unknown 2 | Metallic | RI,O | 5.75±0.25 | 3.0±0.10 | 4.50±1.50 | 3.75±0.25 |
| 999 | Hexyl acetate | Cologne | RI,O | 4.50±0.50 | 0.75±0.25 | 1.50±0.50 | 0.75±0.75 |
| 1040 | d-Limonene | Citrus | RI,MS,O | 1.75±0.25 | 3.00±1.0 | 1.5±0.50. | 2.50±0.10 |
| 1050 | Benzeneacetaldehyde | Rose, flower | RI,MS,O | 2.50±0.50 | 2.0±2.0 | ND | ND |
| 1058 | Unknown 3 | Vegetable oil | RI,O | 2.0±1.0 | 2.0±0.10 | ND | ND |
| 1077 | o-Cresol | Wet towel | RI,MS,O | 1.0±1.0 | 1.50±0.50 | 3.0±1.0 | 2.25±0.25 |
| 1095 | Guaiacol | Burn sugar | RI,MS,O | ND | ND | 3.50±1.50 | ND |
| 1120 | Unknown 4 | Sour | RI,O | 3.0±0.10 | 0.50±0.50 | ND | ND |
| 1138 | (E)-2-Nonenal | Hay | RI,MS,O | 5.0±0.10 | 2.0±1.0 | 2.50±0.50 | ND |
| 1144 | 2-Phenylethanol | Rose | RI,MS,O | 2.0±0.10 | 3.0±0.10 | 1.0±0.50 | 1.75±0.25 |
| 1157 | (Z)-2-Nonenal | Cucumber | RI,MS,O | 2.0±2.0 | 1.0±0.10 | 2.50±0.50 | ND |
| 1158 | (E,Z)-2,6-Nonadien-1-ol | Hay | RI,O | 3.50±0.50 | ND | 2.75±0.75 | 1.0±1.0 |
| 1186 | Naphthalene | Dirty | RI,MS,O | ND | ND | 0.75±0.75 | 1.0±1.0 |
| 1191 | Carveol | Minty | RI,MS,O | 0.50±0.50 | ND | ND | ND |
| 1216 | 2,4-Nonadienal | Burnt oil | RI,MS,O | 5.75±0.25 | 1.50±0.50 | 3.25±0.75 | 2.0±2.0 |
| 1268 | Geraniol | Sweet, flower | RI,O | 0.50±0.50 | 1.0±1.0 | 2.0±0.10 | 1.0±1.0 |
| 1322 | (E,E)-2,4-Decadienal | Burnt oil | RI,MS,O | ND | ND | 4.50±0.50 | 1.0±0.50 |
| 1372 | α-Cubebene | Sweet | RI,MS,O | 3.0±1.0 | ND | ND | ND |
Intensity of aroma compounds marked bold increased in fermented OMW.
In total, 17 and 13 aroma active compounds were identified in fermented OMW by R. oryzae and C. tropicalis respectively. Identified aroma-active compounds included acids, alcohols, aldehydes, esters, ketones, terpene, and 4 unknown compounds. Among these aroma-active compounds, unknown 1 associated with dirty-acid aroma, isovaleric acid, hexyl acetate methional, (E)-2-nonenal and 2,4-nonadienal were detected at higher intensities in unfermented OMW. Moreover, it was determined that there were some differences in aroma profile of unfermented OMW. These differences might be related in pH value of the OMV solution; absorption behavior of SPME fiber and sensitivity of perception of panelists during GC-O analysis
OMW fermented with R. oryzae had higher intensities of 2-pentanone, d-limonene and 2-phenylethanol than unfermented OMW whereas d-limonene was only found to be at higher intensity in OMW fermented with C. tropicalis. These GCO results revealed that 2-pentanone, d-limonene and 2-phenylethanol can be produced from fermented OMW.
Limonene is monoterpen which is one of the main compounds of citrus essential oils and 2-phenylethanol is aromatic alcohol with rose like flavor. d-Limonene and 2-phenylethanol can be found in many plant sources. Both flavor compounds are greatly used in food, perfume and cosmetic industry. 2-Phenylethanol and d-limonene are naturally produced by distillation of citrus peel and rose petals, respectively. From aspect of yeast and fungal metabolism, there are many biochemical pathways for flavor compounds. Among these pathways, production of alcohols and ester type flavor compounds was achieved by yeast via and Acetyl Coenzyme A/Alcohol Acetyl Transferase reaction and Ehrlich pathway which covered transamination, decarboxylation, oxidation and reduction of branched chain amino acids. Moreover, some methyl ketones and lactones were produced by β-oxidation of long-chain hydroxy fatty acids (e.g. ricinoleic acid) by yeast and mold. It was also known that several fungi produced most of the terpene by the Mevalonate pathway as found in higher plant and biotransformation reactions.45–49 Therefore, it was concluded that 2-pentanone, d-limonene and 2-phenylethanol from OMW were produced by R. oryzae and C. tropicalis through aforementioned reactions. Several researchers have been pointed out similar approaches for the production of flavor compounds from agrowaste.7,11,41,50 In most recently, Mantzouridou and Paraskevopoulou50 demonstrated de novo synthesis of fruity esters as isoamyl acetate, ethyl dodecanoate, decanoate, octanoate and phenyl ethyl acetate from orange peel waste was achieved by Saccharomyces cerevisiae at high level. In our previous study41 on OMW, we observed that the filamentous fungus Trichoderma atroviride produces 1-octen-3-ol and 2-octenol at high level, and 2-phenylethanol and menthol also can be produced by using Torulaspora delbrueckii.
Christen et al.7 indicated that R. oryzae can produce acetaldehyde, ethanol (sweet), 1-propanol, ethyl acetate, ethyl propionate and 3-methyl butanol from Amaranth grain supplemented with mineral salt solution. In a study by Chatterjee and Bhattacharyya,51 production of α-terpineol (floral) from α-pinene (herbal) by microbial oxidation of C. tropicalis MTCC 230 with a yield of 77% was achieved. We did not observe the production of acetaldehyde, ethanol, 1-propanol, ethyl acetate, ethyl propionate and 3-methyl butanol from OMW by fermentation of R. oryzae and α-terpineol by C. tropicalis by compared with the findings of previous studies.7,51 This could be related to composition of agrowaste and fermentation conditions including aeration, temperature and fermentation scale.52
In fermented OMW, methoxy phenyl oxime and methyl butanoate could not be identified by GCO analysis, but we determined these compounds by GC–MS. These results can be attributed to “odor threshold” and “odor recognition threshold”. Because, the concentration of volatile compounds in matrix has to be higher than both threshold values in order to identify the volatile compound by GCO technique.53 Methoxy phenyl oxime is N-containing compound with both phenyl and methoxy groups. There is little information on flavor characteristics of methoxy phenyl oxime.54 Some researchers identified it as contaminant come from SPME fiber. They point out that is originated from the glue that is used for SPME fiber.55 However, the compound has been found naturally in some food products,56–58 especially bamboo shoots and secondary metabolites of myxobacteria59 by some researchers. Methyl butanoate which is associated fruity flavor is the ester of butyric acid. Likewise most of ester compounds, it was biosynthesized with Ehrlich pathway and reactions of lipase enzyme by microorganism.
Table 3 shows the changes in concentration of flavor compounds produced by R. oryzae and C. tropicalis from OMW during shake cultures.
Changes in concentration of flavor compounds in OMW during shake cultures of R. oryzae and C. topicalis.
| Volatile compound | RIa | Aroma quality | Mean (μg/kgOMW)b±SE | ||
|---|---|---|---|---|---|
| Rhizopus oryzae | |||||
| Controlc | 72h | 288h | |||
| 2-Pentanone | 682 | Sweet | 12.29±8.72 | 14.16±10.04 | 24.28±3.30 |
| Methoxyphenyloxime | 926 | – | 61.74±29.17 | 64.95±29.73 | 92.26±3.32 |
| d-Limonene | 1032 | Lemon, citrus | 40.13±1.05B | 185.56±17.47A | 17.22±1.60B |
| 2-Phenylethanol | 1118 | Rose | 1.35±0.96B | 13.91±6.57A | 11.71±8.30A |
| Volatile compound | RIa | Aroma quality | Mean (μg/kgOMW)b±SE | ||
|---|---|---|---|---|---|
| Candida tropicalis | |||||
| Controlc | 72h | 288h | |||
| Methyl butanoate | 717 | Fruity | Nd | 4.67±0.20A | 0.40±0.20B |
| d-Limonene | 1032 | Lemon, citrus | 119.64±77.04AB | 249.54±30.82A | 8.93±6.26B |
A,B Means followed by different superscript letter represent significant differences in the same flavor compound through the fermentation time.
Fermentation time had a significant effect on the concentration of volatile compounds which were produced by both microorganisms (p<0.05). It was determined that concentrations of d-limonene (citrus) and 2-phenylethanol (rose) significantly increased in OMW during 72h of fermentation of R. oryzae. The maximum concentrations of d-limonene and 2-phenylethanol were determined as 185.56 and 13.91μg/kg OMW, respectively. The concentration of 2-petanone and methoxy phenyl oxime gradually increased during fermentation of R. oryzae. However, no significant changes were determined in the concentration of both compounds (Table 3). It was thought that this observation is related to a high standard error of results for the compounds. During the fermentation of C. tropicalis, the concentrations of d-limonene and methyl butanoate (fruity) significantly increased similar to the fermentation of R. oryzae. The maximum concentration of d-limonene and methyl butanoate was 249.54 and 4.67μg/kg OMW at 72h of fermentation. The concentrations of flavor compounds produced by both microorganisms decreased after 72h fermentation.
Fig. 1 shows changes in concentration of flavor compounds produced from in OMW by R. oryzae and C. tropicalis in bioreactor cultures.
In bioreactor cultures, fermentation time had also significant effect on the amount of flavor compounds in batch fermentation (p<0.05). As seen from Fig. 1A, the concentration of 2-pentanone and methoxy phenyl oxime increased in OMW during 72h of fermentation by R. oryzae, while the concentration of d-limonene and 2-phenylethanol increased during 180h of fermentation. After these times, a significant decrease in the concentration of all flavor compounds was observed throughout the fermentation (p<0.05). The maximum concentration of 2-pentanone, methoxy phenyl oxime, d-limonene and 2-phenylethanol were 19.46, 34.44, 87.73 and 11.25μg/kg OMW, respectively. In the case of fermentation by C. tropicalis (Fig. 1B), concentration of methyl butanoate and d-limonene were reached at maximum level in the fermentation time of 72h and 180h and the maximum concentration of these compounds were determined as 7.26 and 11.95μg/kg OMW, respectively. Moreover, a significant decrease was also observed in the amounts of all volatile compounds produced by C. tropicalis throughout the fermentation similar to the fermentation of R. oryzae (p<0.05).
Mantzouridou and Paraskevopoulou50 investigated the usage potential of orange pulp for microbial flavor production by S. cerevisiae in under semi-anaerobic and micro aerobic conditions. The researchers observed that acetate esters as isoamyl acetate and phenyl ethyl acetate concentrations in culture media decreased after 48h the fermentation time in microaerobic conditions whereas concentrations of ethyl hexanoate, ethyl octanoate and ethyl decanoate decreased after 24h in the same fermentation conditions. Rossi et al.60 investigated the producing fruity flavor by Ceratocystis fimbriata in solid state fermentation using citric pulp as culture media. They found that isoamyl acetate and ethyl acetate were produced as fruity flavor by selected microorganism, and both flavor concentrations have been increased during 48h fermentation time. In our previous study30 on microbial flavor production from tomato pomace by Kluyveromyces marxianus, we determined that the concentrations of isoamyl alcohol, isovaleric acid and phenyl ethyl alcohol have been gradually increased during 24, 48 and 72h fermentation time, respectively. After these times, significant decreases in the concentrations of all volatiles were observed through 120h fermentation. However, Moradi et al.61 observed that a gradual increase in γ-decalactone concentration in synthetic media during 75h fermentation time when they fed bioreactor with castor oil at a rate of 5mL/h and used atmospheric air for aeration. When Table 3 and Fig. 1 taken into consideration, general decrease was observed in the concentration of all volatile compounds after a particular time in shake cultures and bioreactor cultures similarly the results of previous studies.41,50,60 This observation might be related to stripping effect which is unavoidably discharge of the flavor compounds in the shake cultures and bioreactor cultures due to flavor compounds volatilities and their dissolving behavior in culture media during the fermentation period.62 For instance, this stripping process was modeled and investigated experimentally for the production of ethyl acetate from whey by K. marxianus. It was found that the stripping rate of the flavor compounds nearly independent of the stirring and was proportional to the gas flow. They pointed out the stripping rate was governed by the absorption capacity of the exhaust gas rather than the phase transfer in the bioreactor.
The productivities of flavor compounds produced both strains in bioreactor cultures have varied (Table 4). It was determined that productivity of d-limonene produced by R. oryaze was higher than those of C. tropicalis. So, it can be said the production of d-limonene from OMW by R. oryzae were higher than C. tropicalis. Moreover, 2-phentylethanol has the lowest productivity mean while similar productivity values were observed for d-limonene and methoxy-phenyl-oxime in the case of R. oryzae fermentation. By comparing the productivity results of this study with previous studies, different productivity values were reported for various flavor compounds.30,63 Mantzourido et al.63 investigated the production of some esters from orange peel waste by solid state fermentation of S. cerevisiae. They found that the productivity values varied between 0.10 and 3.23mg/kgh for some ethyl esters including ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl dodecanoate. In a study by Guneser et al.30 1.41 and 5.27μg/kgh of productivity values for isoamyl alcohol produced from tomato pomace by K. marxianus and D. hansenii, respectively. Moreover, Guneser et al.41 reported that 8.81μg/kgh of productivity value for 1-octen-3-ol produced from OMW by T. atroviride.
The productivity of flavor compounds produced by R. oryzae and C. tropicalis in bireactor cultures.
| Volatile compounds | Productivitya Mean (μg/kgh)±S.E. | |
|---|---|---|
| R. oryzae | C. tropicalis | |
| 2-Pentanone | 0.26±0.01 | Nc |
| Methoxyphenyloxime | 0.45±0.01 | Nc |
| d-Limonene | 0.47±0.01 | 0.06±0.01 |
| 2-Phenylethanol | 0.06±0.01 | Nc |
| Methyl butanoate | Nc | 0.1±0.01 |
Interpretation of relationship between flavor compounds which are produced by biotechnological process and their sensory properties in fermented culture matrix has not been discussed in detail in the previous studies. However, this interpretation is essential to determine the sensory quality of flavor compounds. Here, we conducted sensory analysis to evaluate the relationship between quantity of produced flavor compound and its sensory impact. Eleven aroma terms were developed by panelists for fermented OMW. Fig. 2 shows sensory characteristics of fermented and unfermented OMW.
There were significant differences between fermented and unfermented OMW in terms of oily, unripe olive and woody/herbal, wet towel, sweet aromatic (p<0.05). OMW fermented with R. oryzae has a higher intensity in terms of unripe olive, wet towel and sweet aromatic than unfermented OMW. Woody/herbal, rancid and fruity aromas were at high intensity in unfermented OMW (Fig. 2A). Significant differences were also observed for unfermented and fermented OMW in the fermentation of C. tropicalis. Unripe olive and oily aromas were found to be at high intensities in fermented OMW and unfermented OMW has only a high intensity of wet bulgur aroma. Other sensory characteristics score were found to be a similar in fermented and unfermented OMW (Fig. 2B). It can be said that sweet aromatic is related to 2-pentanone, 2-phentylethanol and d-limonene while methoxy phenyl oxime are associated wet towel flavor. Moreover, unripe olive and oily flavors seem to relate to methyl butanoate and d-limonene.
ConclusionThe results show that OMW can be considered as a raw material for biotechnological production of flavors. The microbial production of 2-pentanone, d-limonene and 2-phenylethanol were achieved from OMW by fermentation of R. oryzae, while C. tropicalis produced d-limonene and methyl butanoate. In the study, the stripping effect was observed in bioreactor fermentation. Therefore, modeling of stripping effect is required for produced flavor compounds by taking into account temperature, flow rate, agitation speed and volatility in the next step for improved the productivity. Further studies should be conducted to evaluate the production potential of natural flavors from OMW by using other microorganism with biotechnological approaches.
Conflict of interestThe authors declare that they have no conflict of interest.
This study was funded by The Scientific and Technological Council of Turkey (TUBITAK, Ankara Turkey; Project No. 110O903 COST). The authors would like to thank Bioflavour COST Action FA0907 for technical supporting of this scientific work.