The effect of alkali stress on the yield, viscosity, gum structure, and cell ultrastructure of xanthan gum was evaluated at the end of fermentation process of xanthan production by Xanthomonas campestris pv. manihotis 280-95. Although greater xanthan production was observed after a 24h-alkali stress process, a lower viscosity was observed when compared to the alkali stress-free gum, regardless of the alkali stress time. However, this outcome is not conclusive as further studies on gum purification are required to remove excess sodium, verify the efficiency loss and the consequent increase in the polymer viscosity. Alkali stress altered the structure of xanthan gum from a polygon-like shape to a star-like form. At the end of the fermentation, early structural changes in the bacterium were observed. After alkali stress, marked structural differences were observed in the cells. A more vacuolated cytoplasm and discontinuities in the membrane cells evidenced the cell lysis. Xanthan was observed in the form of concentric circles instead of agglomerates as observed prior to the alkali stress.
Xanthan gum is a polysaccharide produced by bacterium species Xanthomonas campestris.1Xanthomonas cells are single straight rods, Gram-negative, 0.4–0.7μm wide and 0.7–1.8μm long.2 Several biological functions, attributed to the exopolysaccharide produced by these pathogenic bacteria, include protection against adverse environmental conditions such as drying, temperature oscillations, radiation, certain chemical products, and adhesion.3
The production and commercialization of xanthan gum as thickener and stabilizer has progressively increased at an annual rate of 5–10%,4 due to its better physicochemical properties as compared to other available polysaccharides. The most significant properties of xanthan gum include its high viscosity at low concentrations and stability for a wide range of temperatures and pH (even in the presence of salts).5,6
Both microorganism growth and xanthan production are influenced by several factors that include the type of bioreactor, operation mode (batch or continuous), medium composition, cultivation conditions (temperature, pH, stirrer speed, air flow rate), fermentation time, strain of the microorganisms and post-fermentation conditions (heat treatment, recovery, purification). Due to the wide application of xanthan gum and its worldwide market, several studies have been performed to optimize xanthan gum production.4,7–13 However, the effect of this polysaccharide on the ultrastructure of cells and behavior of gum at different production stages have not yet been addressed properly, and thus the present study is relevant and may provide greater understanding on this process.
Peter et al.14 observed accumulation of xanthan around the cells cultured on agar using transmission electron microscopy, while Contreras et al.15 have reported plant-bacterial cell interactions during pathogenesis.
The present study aimed to evaluate the effect of alkali stress on yield, viscosity, gum structure, and cell ultrastructure, at the end of fermentation process of xanthan production by X. campestris pv. manihotis 280-95.
Material and methodsMicroorganismThe lyophilized culture X. campestris pv. manihotis 280-95 was kindly gifted from the Culture Collection of the Phytopathological Bacteriology Section of the Biological Institute of Campinas (IBSBF), Campinas SP Brazil, and maintained in solid Yeast-Malt (YM) medium after activation.
Culture mediaFollowing media were used for the culture of the bacterium: standard solid and liquid YM medium,16 medium I7 and nutrient medium.17
The standard YM medium consisted of (w/v) yeast extract 0.3%, malt extract 0.3%, peptone 0.5%, glucose 1.0%, and agar (for the solid medium) 2.5%.
The fermentation medium (Medium MP-I) comprised of (w/v) MgSO4·7H2O 0.02%, KH2PO4 0.5%, H3BO3 0.0006%, (NH4)2SO4 0.2%, FeCl3 0.00024%, CaCl2·2H2O 0.0002%, ZnSO4 0.0002%, citric acid 0.20% and 5% sucrose. The pH of Medium I was adjusted to 7.0 with 0.1M HCl or 0.1M NaOH.
Culture media were sterilized in a vertical autoclave (Phoenix VA-75) at 121°C for 20min. Sucrose was sterilized separately and added to the media prior to use under aseptic conditions.
Inoculum productionAn aliquot of 50mL of standard YM medium was placed in three 250mL-Erlenmeyer flasks with vented caps. Ten standard scoops (platinum 3.0mm diameter-scoop) of culture grown in standard solid YM for 48h at 28°C were added to each flask. The flasks were then incubated on an orbital shaker (New Brunswick Scientific Co. G27) at 28°C and stirred at 250rpm for 24h. Cell suspensions with at least 2.6×109 CFUmL−118 were used as fermentation inoculum.
Biopolymer productionAfter 24h, the inoculum was transferred aseptically to a 2L-reactor vessel containing 1.35L of fermentation medium (Medium MP-I) and 3 drops of pure Tween 80. New Brunswick's MULTIGEN fermenter monitored to provide an airflow at 1.5vvm, at 28.5±0.5°C and 500rpm for 72h was used. The pH was initially adjusted to 7.0, and a change in pH was not followed throughout 72h.
Alkali stressAfter 72h of fermentation, 15mL of 10M sodium hydroxide was added to the fermentation medium until an alkali stress at pH 12 was reached. The fermented medium was sampled after 1h, 24h, and 48h. The inoculum production, fermentation process, and alkali stress were performed in triplicate.
Biopolymer separationThe culture medium was centrifuged at 22,300×g and 20°C for 15min to separate the biomass. Ethanol (92.8° GL) was added to the supernatant at a ratio of 4:1 to precipitate the biopolymer. The culture medium was stirred for 5min, and the gum was collected on a wire mesh, placed on a glass Petri dish, frozen, and lyophilized. Then, the material was weighed and ground.
Xanthan yieldThe lyophilized gum was placed in a desiccator and weighed on an analytical balance, and the gum yield was expressed by gum weight per volume of precipitated broth (gL−1).
ViscosityThe rheological behavior of the samples was evaluated by a HAAK rheometer RS 150, using coaxial cylinder type sensor DG 41. The viscosity of the samples was determined at 25°C. For that, 1.0% (w/v) xanthan solutions were prepared using distilled water, the mixture was stirred for 2h, and then heated at 60°C for 20min.19 The rheological analysis was performed in triplicate, using shear rate from 0.01s−1 to 60s−1, with 5100mm slot for 300s.
Scanning electron microscopyThe structure of xanthan gum powder produced without alkali stress (W/AS) and with alkali stress (AS) at pH 12 for 1h was assessed by scanning electron microscopy.
Gum samples were first fixed overnight in a solution containing 0.1M sodium cacodylate buffer pH 7.4, 25% glutaraldehyde and tannic acid, and then post-fixed in osmium tetroxide. Pellets were washed thrice with sodium cacodylate buffer pH 7.4, between the first and second fixation procedure, and at the end of the second fixation. The samples were then subjected to a dehydration series in 30%, 50%, 70%, and 90% (v/v) ethanol solutions for about 20min each. Critical point drying of the samples was accomplished via Balzers CPD 030. The samples were fixed to metal stubs and gold coated with a sputter coater (Balzers CD 050 Sputter 5), followed by measurements at 15kV in a scanning electron microscope JSM 5800 LV JEOL.20,21
Transmission electron microscopyThe samples subjected to transmission electron microscopy were: pure inoculum (PI), initial fermentation (IF), final fermentation (W/AS) (after 72h fermentation), and alkali stress for 1h (AS1h), 24h (AS24h) and 48h (AS48h).
The samples were collected at their appropriate time point according to fermentation pathway, and stored at 4°C. Then they were centrifuged at 12,200×g for 5min at room temperature and re-suspended in ultra-pure water, stirred, and centrifuged again under the same conditions. The procedure was repeated thrice. The pellets were fixed overnight in a solution containing 0.1M sodium cacodylate buffer pH 7.4, 25% glutaraldehyde, and tannic acid, and then post-fixed in osmium tetroxide for 2h. The pellets were washed thrice with sodium cacodylate buffer between the first and second fixation procedures and at the end of the second fixation. The samples were then subjected to a dehydration series in ethanol solutions, absolute ethanol, a mixture of propylene oxide and absolute ethanol (1:1), and propylene oxide alone, for approximately 20min each, with the exception of propylene oxide. The resin and propylene oxide (1:1) remained on the rotator apparatus for 2h. The oxide was removed and blended with the pure resin. The Eppendorf tubes remained inside the rotator overnight. After inclusion, polymerization was carried out at 60°C for 48h. The material was sectioned using ultramicrotome Leica Ultra Cut, and observed under a Zeiss LEO 906 Transmission Electron Microscope, at a magnification between 6000 and 27800×.20,22,23
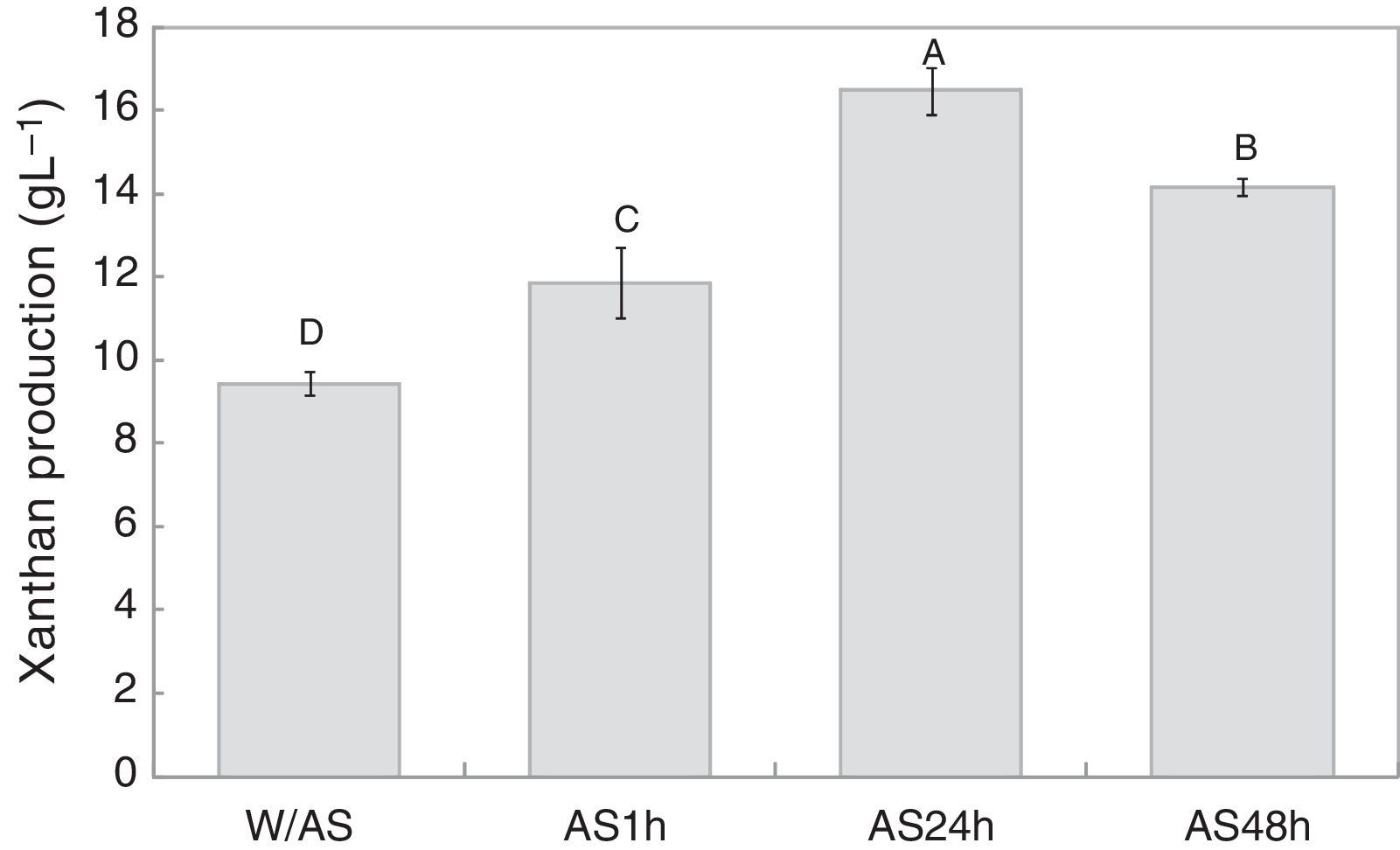
Results and discussionFermentation and alkali stress processAs shown in Fig. 1, 9.4gL−1 of xanthan gum was obtained after 72h of fermentation while 11.8gL−1 of xanthan gum was obtained after 1h of alkali stress, which was 25.7% higher than the amount obtained at the end of the fermentation. After 24h of alkali stress, the amount of biopolymer per liter of fermentation medium increased to 16.5gL−1, translating to a 74.8% increase in xanthan production as compared to the conventional process without alkali stress, which after 48h decreased to 14.2gL−1.
The present results indicate that the alkali stress might have induced the cells to produce a greater amount of exopolysaccharides as a protective mechanism against adverse conditions of the medium, while the lower yield after 48h of alkali stress could be due to the alkaline hydrolysis of the polymer.
Low production rates were observed when pH was not controlled during processing. Papagianni et al.11 evaluated xanthan production by X. campestris ATCC 1395 under non-controlled pH conditions at different stirring speeds. The highest yield obtained by them was approximately 6.5gL−1 at 600rpm, which is lower than that obtained in the current study. In contrast, Borges et al.24 studied the xanthan production by Xanthomonas arboricola pv. pruni 106 under non-controlled pH conditions, and found slightly higher production rate of 10.2gL−1 at 400rpm.
Different behaviors have been observed under non-controlled pH conditions, as the medium pH might either decrease from neutral to near pH 5 or even increase, depending on several factors including medium composition, strain used and processing conditions.11,24,25
However, the present study demonstrates that the gum production tends to increase with alkali stress or pH control. Gupte and Kamat26 evaluated xanthan production by X. campestris ICA-125 at different pH values (4–9), and observed the highest yield at pH 6 with a production rate of 18.6gL−1, which decreased and reached 3.1gL−1 at pH 9. In contrast, Borges et al.24 found a slight reduction in xanthan production at pH 9 when compared to pH 7, with values of 16.05 and 16.9gL−1, respectively. Liakopoulou-Kyriakides et al.27 investigated xanthan production by X. campestris XLM1521 and found an increase from pH 6 to 8, reaching 17.3gL−1 at pH 8. These values were close to those obtained in the current study using alkali stress.
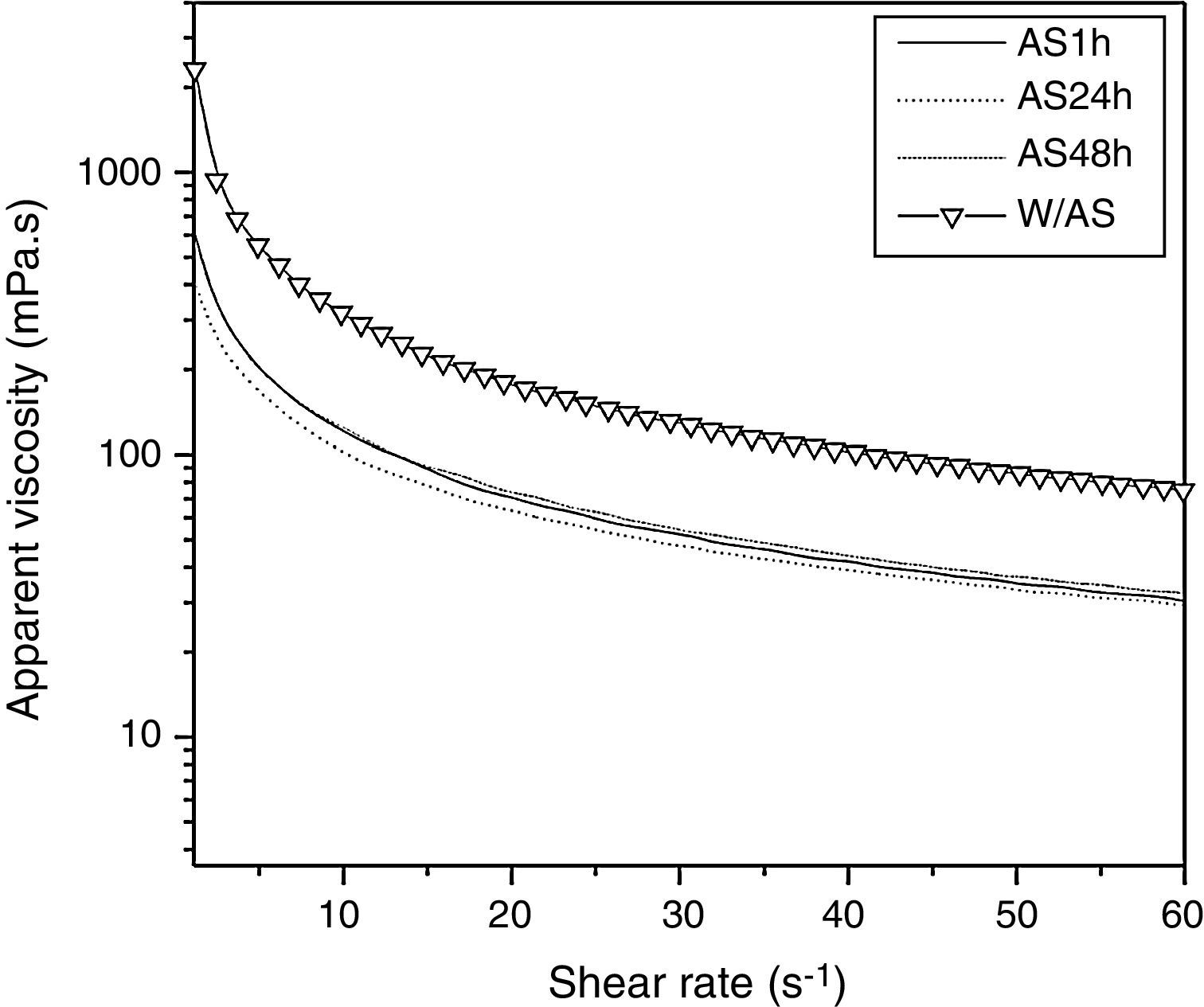
As shown in Fig. 2, the 1% solutions prepared from xanthan gums obtained from the conventional fermentation alkali stress free (W/AS) and with alkali stress process for 1h (AS1h), 24h (AS24h), and 48h (AS48h) exhibited a pseudoplastic fluid behavior, as also observed by other authors.6,28–31 The samples not subjected to alkali stress (W/AS) exhibited apparent viscosity of 317mPas at a shear rate of 10s−1, while the samples subjected to AS for 1h, 24h, and 48h yielded viscosity values of approximately 117mPas, under the same shear rate. It is worth noting that the quality of the gum obtained after the alkali stress was 64% lower as compared to the gum obtained without the alkali stress process while the time of alkali stress did not affect the quality of the gum obtained in the present study.
Similar results were observed by Gupte and Kamat26 and Borges et al.,24 who also evaluated the viscosity of the gum produced at different pH values.
As reported by Gupte and Kamat,26 the viscosity of the xanthan solution increased by 1% when produced at pH 4–7 (153mPas to 559mPas, respectively), with a decrease at pH 8 and pH 9 (280mPas to 124mPas, respectively).
Borges et al.24 have shown that the gum produced under non-controlled pH conditions and at pH 5 (76.3mPas and 77.7mPas, respectively) had higher viscosity rates when compared to that produced at pH 7 and pH 9 (52.8mPas and 50.4mPas, respectively), at a shear rate of 19s−1, using 0.5% solution. According to these authors, this phenomenon could be due to the lower molecular weight of the xanthan produced at higher pH values.
Besides molecular weight, the concentration of xanthan might affect the viscosity when associated with salts. Zhong et al.32 demonstrated that at low xanthan concentration, addition of salt (Na+ and Ca2+) lowered the solution viscosity, while at higher xanthan concentration (5000mg/L), the salt addition increased the viscosity.
Thus, a possible explanation for the lower viscosity of xanthan produced under alkali stress conditions might be the hydrolysis of gum under these conditions and electrostatic repulsion in the polymer, as shown by the microscopy results.
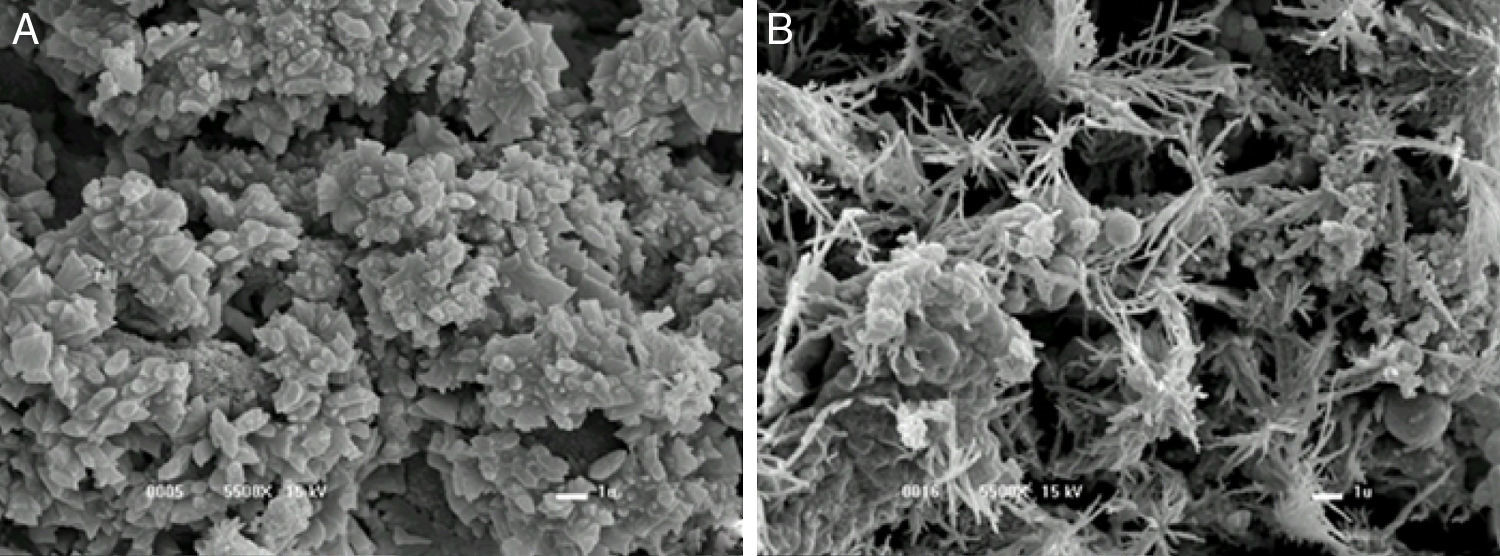
Gum structure with and without alkali stressAs depicted in Fig. 3A and B, the gum without alkali stress showed a polygonal structure, whereas the gum subjected to alkali stress had star-shaped structure. While comparing these results with those found by Borges et al.,24 it was assumed that no reduction in molecular weight occurred. It has also been suggested that the high sodium concentration due to the alkali might lead to the structure compaction due to the repulsion caused by the positively charged ions. The present results corroborate with those obtained by Carrington et al.33 on the effect of salts on the extension flow properties. In fact, high salt concentration may change xanthan molecule from an expanded to a compact conformation. The changes in the molecule hydrodynamic size can lead to a decrease in viscosity, as observed in the current study.
However, opposite behavior was observed by Savi-Junior et al.,34 who found that the viscosity decreased at low salt concentration as the polymer coil became contracted, providing a smaller hydrodynamic volume.
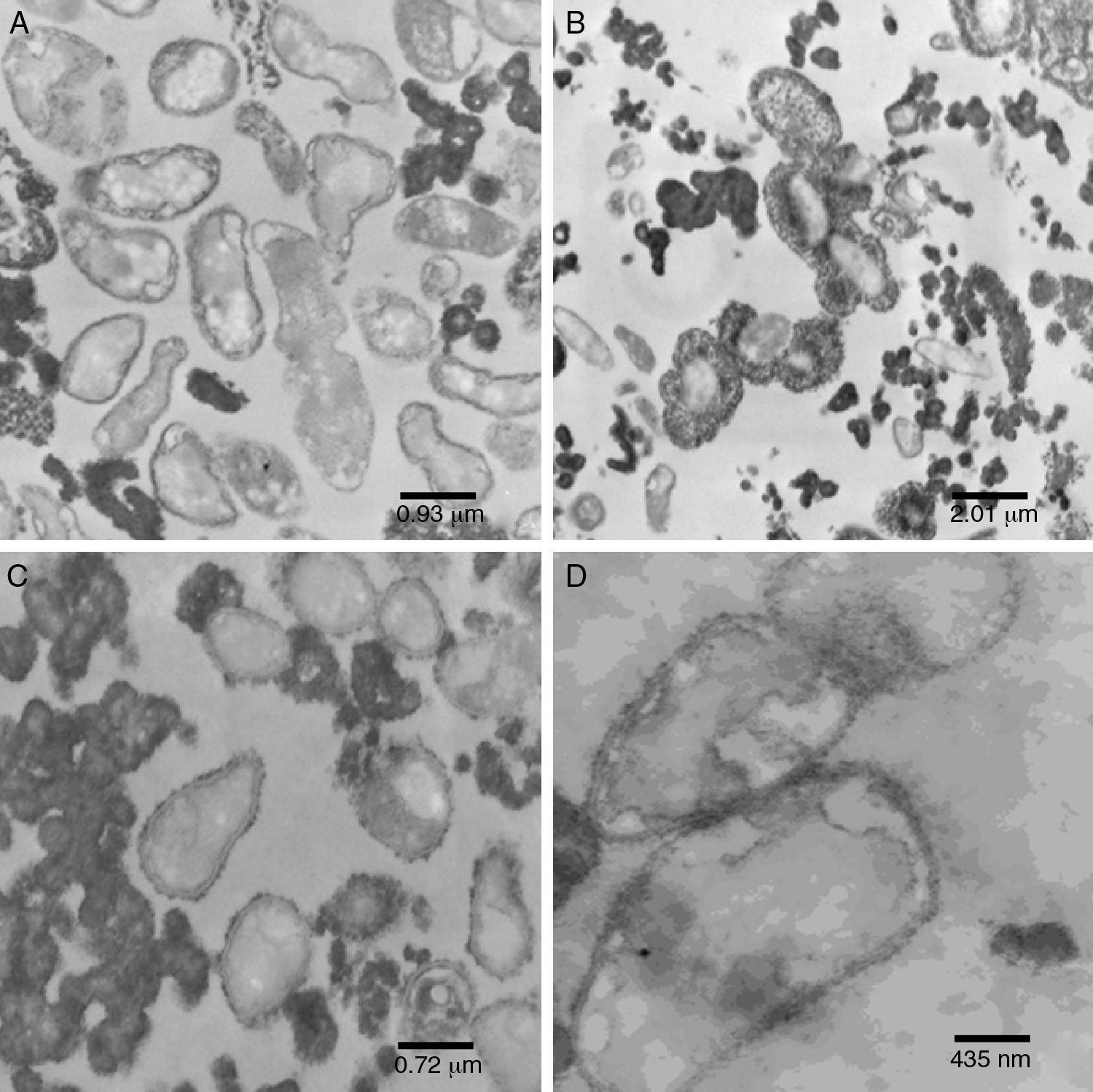
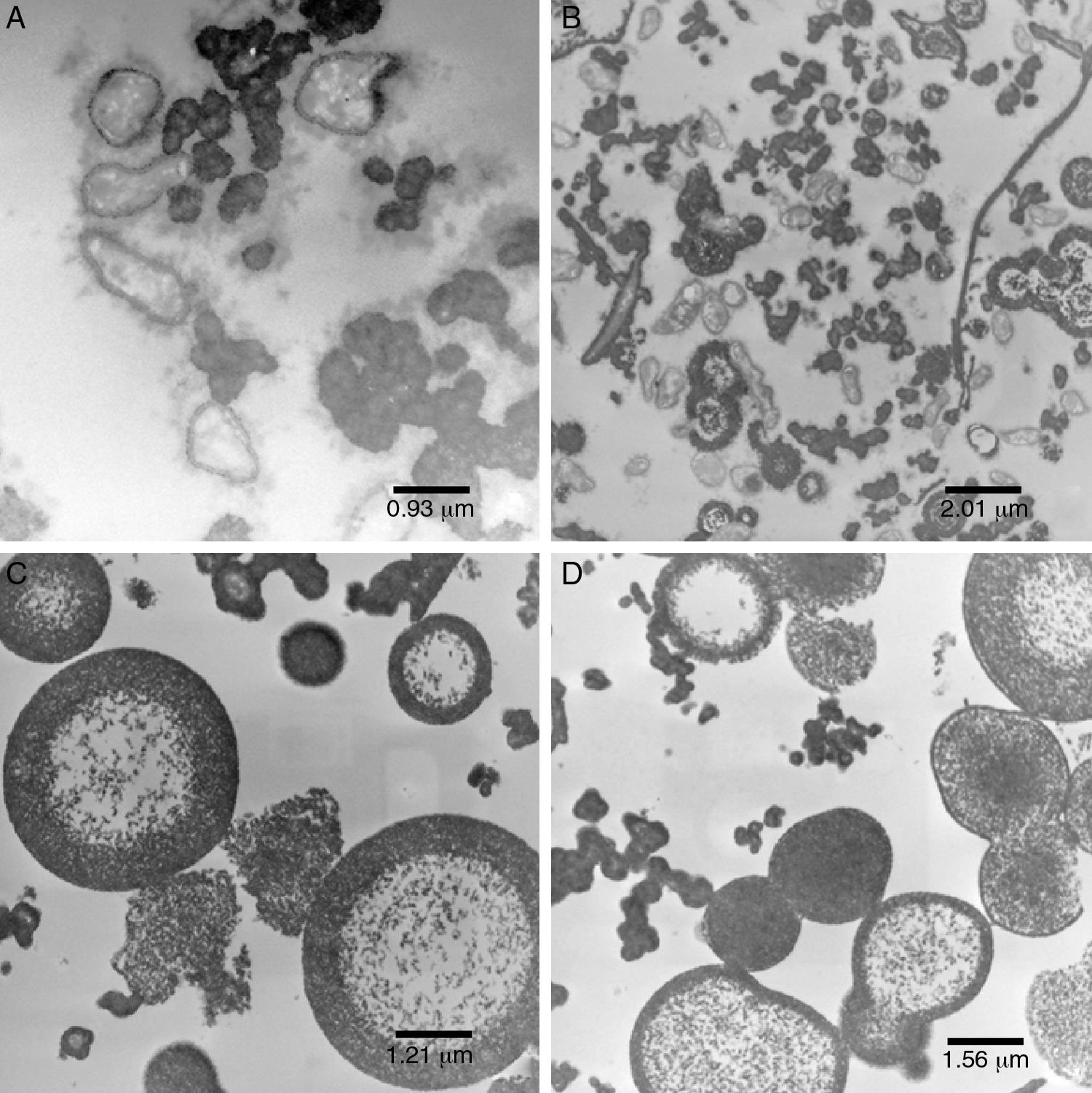
Ultrastructure analysisPure inoculum (PI)The cells showed presence of the features typical for Xanthomonas (Fig. 4A). Although the bacterium appeared as rods, the histological ultracuts by ultramicrotome revealed that the bacterium might appear in longitudinal, sagittal, and transversal cuts. In fact, Fig. 4A revealed that the outer membrane and cytoplasmic structures of the bacterial cells were intact. No bacterial lysis was observed, thus indicating that cells from the pure inoculum were viable. Cells division can also be observed through a bifurcation followed by stretching in the middle of the cell.
The production of xanthan gum around some cells in very low quantity was also observed. During the preparation of inoculum, an increase in cell concentration was observed, and only a small amount of gum was produced. The gum surrounding the cell prevented the transport of nutrients and extended the growth phase.35,36
Initial fermentation (IF)The bacterial cells were intact at the beginning of the fermentation (Fig. 4B), which suggests that the initial stages of the fermentation process occurred as expected. As shown in Fig. 4B, a large amount of gum was found around the bacterial cells.
Since the fermentation medium (medium I) contained various salts, it was believed that xanthan gum surrounding the bacteria moved away during fermentation (Fig. 4B), probably due to the changes in osmolality.
The extracellular polysaccharides may be classified into two types: (I) polymers physically attached to the microbial cell in a capsule form, and (II) polymers excreted by the microbial cells in the medium in the form of loose slime.37 The present results suggest a revision of such classification, since xanthan gum can be classified either as type (I) or type (II) depending on the medium conditions.
Final fermentation without alkali stress (W/AS)Early structural changes in bacteria, such as cytoplasmic vacuolization and membrane discontinuity indicated an early stage of bacterial lysis (Fig. 4C).
The prolonged fermentation time of 72h at this stage led to different gum behavior, since xanthan gum was present as agglomerates rather than around the bacteria, possibly due to the different osmolality than that is ideal for bacterial growth.
Alkali stress after 1h (AS1h)The alkali stress conditions of this study consisted in raising pH to 12.0. After 1h (AS1h), marked structural differences in the bacterial cells were observed (Figs. 4D, 5A and B). A more vacuolated cytoplasm and the discontinuity in the cell membranes as evidence of cell lysis were observed (Fig. 4D).
Despite the considerable decrease in cells number, the image at lowest magnification (Fig. 5B) revealed the presence of certain bacterial cells.
Xanthan gum was found in an organized shape (Fig. 5B) rather than agglomerates as reported earlier. This was quite similar to water–oil emulsion system with a hydrophobic interior and a polysaccharide xanthan gum on the outer layer. Such an organized conformation, in the form of concentric circles, had never been reported in earlier studies.
Alkali stress after 24h (AS24h)After the alkali stress of 24h, no bacterial cells were observed, which indicates a possibility of complete disintegration of the bacterial membranes (Fig. 5C). A more organized shape with concentric circles was observed in this case (Fig. 5C) in contrast to that obtained after 1h of alkali stress.
Co-occurrence of acetic and pyruvic acid molecules with xanthan molecules results in anion-type of polysaccharides.5,37,38 It is believed that when this biopolymer is subjected to strong alkaline conditions, the charges are neutralized prior to the electrostatic repulsion. In the absence of repulsion, there might be some changes in the conformation of the molecule (Fig. 3B) and in the way the gum is deposited (Fig. 5C).
Alkali stress after 48h (AS48h)We did not see much difference between the results obtained after 48h and 24h of alkali stress (Fig. 5D).
ConclusionXanthan production was enhanced by a 24h-alkali stress process in X. campestris pv. manihotis 280-95 strain while grown in a 2L bioreactor. However, regardless the alkali stress time, xanthan gum was observed to be of lower viscosity as compared to the alkali stress-free gum. Nevertheless, this result is not conclusive as further studies are required such as gum purification to remove the excess sodium, and verification of the efficiency loss and consequent increase in the polymer viscosity.
Alkali stress altered the structure of xanthan from polygon-like shape to a star-like form. At the end of the fermentation, early structural changes in the cells were observed. Whereas, marked structural differences were observed in the cells after the alkali stress process. A more vacuolated cytoplasm and discontinuities in the cell membranes indicated occurrence of the cell lysis. The gum was seen to be in the form of concentric circles after the alkali stress instead of the agglomerates as seen prior to the alkali stress.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank CNPq for the financial support and the Biological Institute of Campinas for providing the bacterial strain.