Aortoesophageal fistula (AEF) is a rare but life-threatening complication of esophageal stent placement characterized by the formation of a communication between the aorta and the esophagus, which can result in catastrophic hemorrhage and sepsis, making it a potentially fatal complication that requires prompt diagnosis and management. The surgical treatment strategy for each case should be designed based on the patient's clinical presentation and the availability of endovascular or open-surgical expertise and resources.
The aim of this study is to show the first latinoamerican experience in the treatment of AEF secondary to self-expandable partially covered metal stents (PCSEMS) with a retrospective case series study between January 2018 and April 2023 aimed at evaluating the surgical approach and postoperative outcomes of patients with AEF secondary to PCSEMS placement in 2 centers in Bogota, Colombia. A study with a sample of 2 patients was performed. The two patients were treated using an open surgical approach with laparotomy and left anterolateral thoracotomy.
La fístula aortoesofágica es una complicación rara pero potencialmente mortal de la inserción de un stent esofágico. Está caracterizada por la formación de una comunicación entre la aorta y el esófago, lo cual puede resultar en hemorragia masiva y sepsis, convirtiéndola en una complicación potencialmente mortal que requiere un diagnóstico y manejo oportuno. La estrategia de tratamiento quirúrgico para cada caso debe diseñarse en función de la presentación clínica del paciente y la disponibilidad de recursos endovasculares o de cirugía abierta según la experticia.
El objetivo de este estudio es mostrar la primera experiencia latinoamericana en el tratamiento de la fístula aortoesofágica secundaria a stents metálicos autoexpandibles parcialmente cubiertos (PCSEMS) con un estudio de serie de casos retrospectivo entre enero de 2018 y abril de 2023 con el objetivo de evaluar el abordaje quirúrgico y los resultados postoperatorios de pacientes con FAE secundaria a colocación de PCSEMS en 2 centros de Bogotá, Colombia. Se realizó un estudio con una muestra de 2 pacientes. Ambos pacientes fueron tratados mediante abordaje quirúrgico abierto con laparotomía y toracotomía anterolateral izquierda.
Aortoesophageal fistula (AEF) is a rare but life-threatening complication of esophageal stent placement. AEF is characterized by the formation of a communication between the aorta and the esophagus, which can result in catastrophic hemorrhage and sepsis. Self-expandable partially covered esophageal stents (PCSEMS) are commonly used to manage malignant and benign esophageal structures. Although PCSEMS effectively improves dysphagia and quality of life in patients with esophageal cancer, the risk of AEF associated with PCSEMS placement remains a concern.1–3
The exact incidence of AEF secondary to PCSEMS placement is unknown, but several case reports and small case series have documented this complication. In addition, the risk factors and pathophysiology of AEF secondary to PCSEMS placement are poorly understood.1–3 This study aims to show the first Latin American experience in the treatment of AEF secondary to PCSEMS.
MethodsThis retrospective case series study evaluates the surgical approach and postoperative outcomes of patients with AEF secondary to PCSEMS placement in 2 centers in Bogota, Colombia.
Patient selectionAll patients diagnosed with AEF secondary to PCSEMS placement between January 2018 and April 2023 who underwent surgical intervention at two major academic medical centers in Bogota. Patient data was obtained from electronic medical records, intraoperative photos, and radiological imaging studies.
Data collectionPatient demographic data, comorbidities, details of the PCSEMS placement procedure, time to development of AEF, symptoms at presentation, imaging findings, surgical approach, operative elements, intraoperative complications, length of hospital stay, and postoperative outcomes were collected for each patient.
Surgical approachAll patients underwent surgical intervention for the management of AEF secondary to PCSEMS placement. The surgical approach was individualized based on the patient's clinical presentation, hemodynamic stability, and imaging findings. Possible surgical approaches included thoracotomy and thoracoabdominal approach.
Statistical analysisDescriptive statistics were used to summarize the data. Continuous variables were reported as mean and standard deviation or median and interquartile range, depending on their distribution. Categorical variables were reported as frequencies and percentages.
ResultsDuring this study that covered a period of five years in two major academic medical centers, we found only 2 cases of AEF secondary to PCSEMS. Demographic and clinical data are summarized in Table 1. The two patients were treated using an open surgical approach with laparotomy and left anterolateral thoracotomy due to the unavailability of endovascular management. The incidence of this study was 1.6%.
Demographic and clinical data.
| Aortoesophageal fistula demographic and clinical data | ||
|---|---|---|
| Patient | ||
| 1 | 2 | |
| Age | 69 | 73 |
| Gender | Male | Male |
| Cancer type | Distal esophageal adenocarcinoma | Distal esophageal adenocarcinoma |
| Associated diseases | Arterial hypertension | Arterial hypertension, type 2 diabetes mellitus |
| Surgical approach | Left anterolateral thoracotomy plus vertical esophagostomy plus primary repair with polytetrafluoroethylene and primary closure of distal esophagus | Laparotomy plus total gastrectomy plus distal esophagectomy plus thoracic descending aorta primary repair with polytetrafluoroethylene patch and extraction of the PCSEMS |
| Bleeding | 1500cc | 2500cc |
| Intensive care unit (days) | 7 | 0 |
| Hospital stay (days) | 0 | 0 |
| Mortality during surgery | No | No |
| Mortality after surgery | Yes | Yes |
| Aortic endograft infection | No | No |
| Reintervention | No | No |
| Final surgical treatment | No | No |
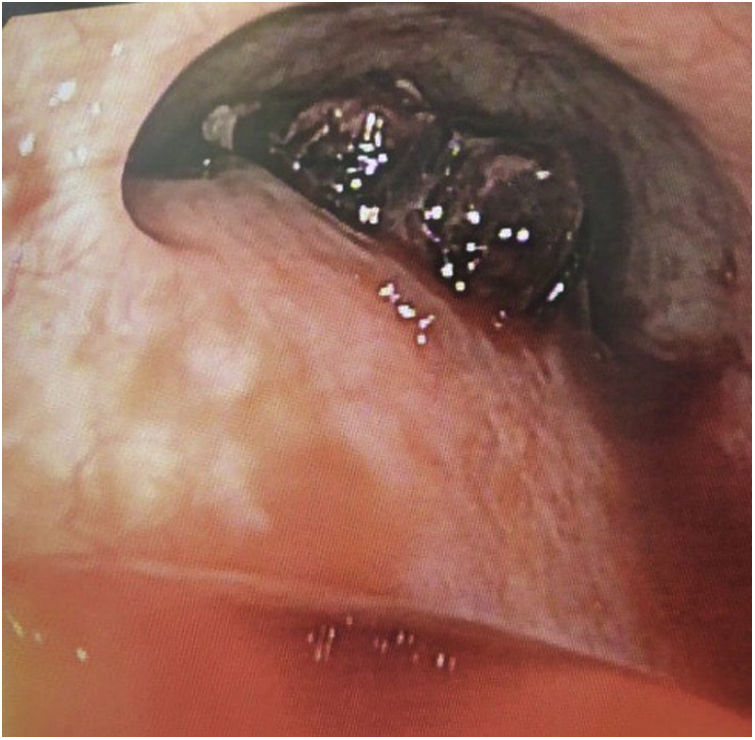
The first patient was a 69-year-old man with a diagnosis of stage 4 cancer of the gastroesophageal junction with hepatic metastases in neoadjuvant treatment with chemotherapy and an esophageal stent in order to make the feeding process easier. Three weeks after placing the stent, the patient returns to the emergency room due to a massive upper digestive tract bleeding and hypovolemic shock stage three. This patient went under an endoscopic procedure before the surgical approach that showed a distal aortoesophageal fistula associated with the distal part of the stent (Fig. 1) that required a left anterolateral thoracotomy with a vertical esophagostomy. This procedure was converted into a clamshell due to the massive bleeding, finally, requiring aorta primary repair with polytetrafluoroethylene (PTFE) patch and primary closure of the distal esophagus over the PCSEMS.
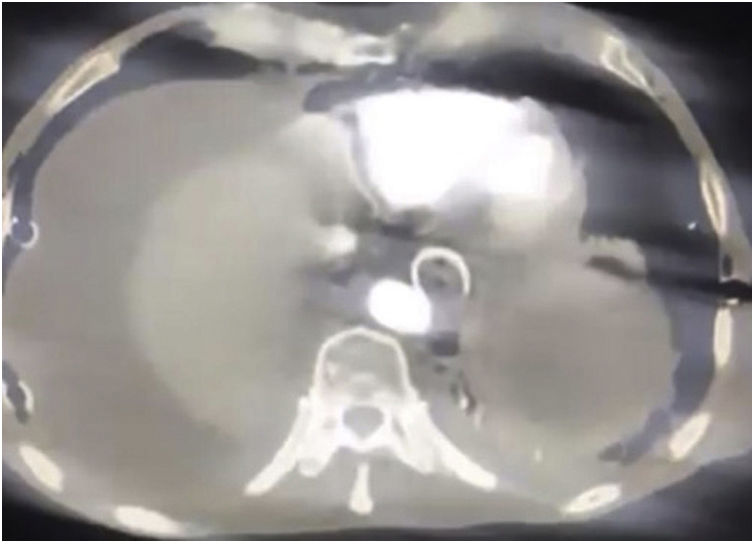
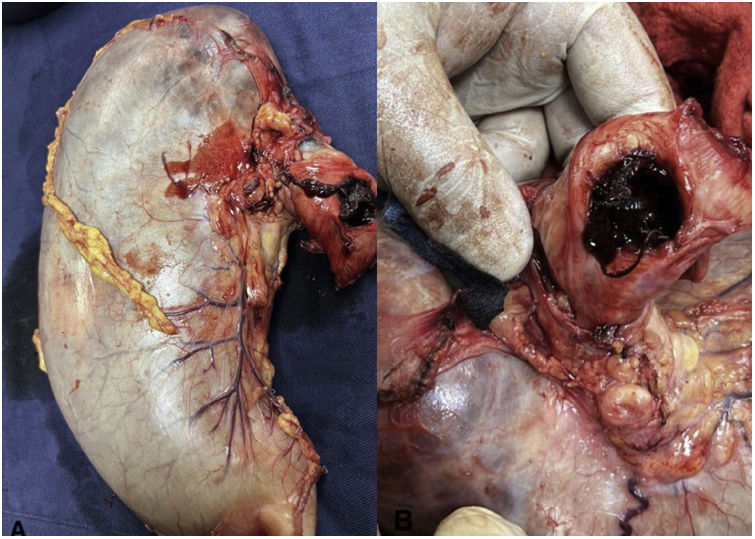
The second patient was a 73-year-old man diagnosed with stage 3 carcinoma of the gastroesophageal union that received neoadjuvant therapy with chemotherapy and radiotherapy. The stent was placed, but 20 days later he returned with hemodynamic instability stage 4 due to upper digestive tract bleeding, with non-response to fluid resuscitation. CT scan showed the image of the stent having direct contact with the aortic wall (Fig. 2) and went under open surgical procedure since vascular surgery was not available. The patient required a laparotomy with distal esophagectomy and total gastrectomy (Fig. 3 (A, B)) plus thoracic descending aorta primary repair with polytetrafluoroethylene (PTFE) patch and extraction of the PCSEMS (Fig. 4). These previous two patients were hemodynamically unstable with no response to volume, additionally, the institutions did not have the availability of thoracic aortic endograft at the moment of the AEF massive bleeding. Both patients died, the first one in the first 24h after the open surgical procedure and the second one in the 7 postoperative days in the intensive care unit due to a ventilator-associated pneumonia.
The initial report on AEF dates to 1818 when a fragment of beef bone was discovered. In 1914, Chiari described for the first time AEF as a triad of midthoracic pain or dysphagia, followed by sentinel hemorrhage and fatal exsanguination after a symptom-free interval of hours to days.1,2 Aortoesophageal fistula (AEF) is a rare but devastating complication of esophageal self-expandable partially covered metal stent (PCSEMS) placement.3–5
In this retrospective case series study, we report two cases of AEF secondary to PCSEMS in Latin America, data that is consistent with the unusual presentation of this complication. Both patients were men with a mean age of 71 years with gastroesophageal adenocarcinoma cancer. Indications for stent placement are palliative, and include the reduction of dysphagia and the improvement of oral intake in patients with partial or total obstruction of the esophageal light secondary to cancer.6
In 1983, Frimberger1 treated a patient with an esophageal stricture by placing a stent. Nowadays, esophageal stents (ESs) have gained popularity for managing dysphagia in patients.1 A previous study by Li Y. et al. reported that out of 153 patients who experienced SEMS-related adverse events, 43 died, accounting for 28.1% of all adverse events. Among these deaths, 14 were due to massive bleeding, representing 32.6% of deaths and 9.15% of all adverse events.8 According to our findings, patients with gastroesophageal adenocarcinoma cancer who require SEMS placement should be closely monitored for the development of AEF.1,4,5
The causes of SEMS-related AEF include repeated mechanical actions during interventional operations leading to injury, tearing, or rupture; high pressure from the ES on the esophageal wall affecting the blood supply to the nourishing vessels; increased swelling at both ends of the SEMS causing localized ischemia, necrosis, or ulceration resulting in AEF; tumor growth and invasion; and placement of the SEMS at an angle with the esophageal wall causing friction between the SEMS and the esophageal wall that combined with vessel pulsation and respiratory movement, leads to AEF.1,8 The main risk factors for SEMS-related AEF development includes previously repeated dilations, previous radiotherapy, proximal stricture location, and inappropriate stent choice.5,7 The incidence of AEF can be reduced by shortening the duration of retrievable SEMS placement or using a biodegradable fully covered stent.
Aryaie et al. retrospectively reported the use of SEMS in treating 20 patients with anastomotic leaks following foregut surgery. Among them, AEF formation complicated the treatment in 2 patients (10%). It is crucial to be attentive to SEMS-related AEF, as the development of AEF after ES implantation can occur between 18 days and 11 months.8 The surgical approach for AEF secondary to SEMS has been controversial, with no clear consensus on the optimal treatment. In our study, the two patients were treated using an open surgical approach with laparotomy and left anterolateral thoracotomy. These two open surgical cases had a high intraoperative bleeding volume.
However, other approaches such as the endovascular approach have been associated with a higher risk of recurrence and mortality, especially in cases of severe infection. Notwithstanding, the choice of surgical approach should be individualized based on the patient's clinical presentation and the availability of endovascular or open surgical expertise and resources.3,4,7
Computed tomography angiography is a valuable diagnostic tool for ES-related AEF, with a sensitivity ranging from 40% to 90% and specificity from 33% to 100%.1 In this report, AEF was further confirmed through angiography of the aorta, considered the “diagnostic criterion standard.” Patients who underwent TEVAR (endovascular repair of the thoracic aorta) had significantly longer survival compared to those who did not receive treatment for aortic rupture and succumbed to hemorrhage shortly after hematemesis.9,10 The mortality rate of AEF is reported to be approximately 77% with intervention and 100% with no treatment.1 Conservative management approaches include the administration of broad-spectrum antibiotics and proton pump inhibitors, along with potential enteral feeding via a percutaneous endoscopic gastrostomy or esophageal fistula bypass. However, these measures often result in fatal outcomes due to recurrent hemorrhage, chronic infection, and mediastinitis.5 Since 1994, when endovascular treatment for aortic lesions associated with AEF was first reported, TEVAR has emerged as a rapid, minimally invasive, and a very effective alternative to surgical intervention for urgent and emergent management of AEF patients. It provides rapid stabilization of hemodynamics by controlling bleeding from the fistula site.1 However, the prognosis varies depending on the underlying cause.
Our study had several limitations, including its retrospective design and small sample size. Additionally, we did not have the availability of an endovascular approach. Despite these limitations, our study adds to the limited literature on AEF secondary to PCSEMS and highlights the challenges in the management of this devastating complication.7
ConclusionAEF secondary to PCSEMS is a rare but potentially fatal complication that requires prompt diagnosis and management. Our study suggests that patients with gastroesophageal adenocarcinoma cancer who require SEMS placement should be closely monitored for the development of AEF. The choice of surgical approach should be individualized based on the patient's clinical presentation and the availability of endovascular or open surgical expertise and resources. Our findings emphasize the need for further research to improve the management and outcomes of AEF secondary to PCSEMS. Future studies should aim to identify risk factors for AEF development, evaluate the optimal surgical approach, and assess long-term outcomes.
Ethical considerationsInformed consent was obtained from the relatives of both patients for the publication of this article.
Conflict of interestsThe authors declare that they have no conflict of interest.