Cardiovascular manifestations are an important cause of mortality and morbidity in COVID-19 infections. Conduction system abnormality in the form of symptomatic bradyarrhythmia is underreported in the literature.
AimTo evaluate epidemiological, demographic, laboratory, clinical management, and outcome data of symptomatic bradyarrhythmia in COVID-19 patients.
MethodsThis was a retrospective, observational study including all the adult patients (>18 years) who were diagnosed with COVID-19 infection and had complete heart block (CHB) or symptomatic high-grade Atrio-Ventricular (AV) block requiring a temporary pacemaker insertion (TPI). Epidemiological, demographic, laboratory, clinical management, and outcome data were extracted from all the enrolled patients and studied for the primary clinical composite endpoint of all-cause death.
ResultsThe study population included 15 patients, including 14 patients with CHB and 1 patient with 2:1 AV block. Syncope was the most common presentation. The clinical endpoint in the form of death was seen in 5 patients (33.3%), 3 patients reverted to sinus rhythm, and 7 patients required permanent pacemaker implantation. The markers of inflammation were raised in all patients; however trend toward more inflammation was seen in patients reaching the primary clinical endpoint. 3 out of 7 patients with narrow QRS rhythm reverted to normal sinus rhythm, while all 8 patients with broad complex QRS either died or required a permanent pacemaker insertion.
ConclusionSymptomatic bradyarrhythmia is associated with a high inflammatory state, and high mortality in COVID-19 infection and a transient conduction block in patients with narrow QRS rhythm may suggest local subclinical myocardial inflammation.
Las manifestaciones cardiovasculares son una causa importante de mortalidad y morbilidad en las infecciones por COVID-19. La anomalía del sistema de conducción en forma de bradiarritmia sintomática no se informa en la literatura.
ObjetivoEvaluar datos epidemiológicos, demográficos, de laboratorio, de manejo clínico y de resultado de la bradiarritmia sintomática en pacientes con COVID-19.
MétodosEste fue un estudio observacional retrospectivo que incluyó a todos los pacientes adultos (>18 años) que fueron diagnosticados con infección por COVID-19 y tenían bloqueo cardíaco completo (HBC) o bloqueo auriculoventricular (AV) de alto grado sintomático que requería una inserción de marcapasos (TPI). Los datos epidemiológicos, demográficos, de laboratorio, de manejo clínico y de resultado se extrajeron de todos los pacientes inscritos y se estudiaron para el criterio de valoración clínico primario compuesto de muerte por cualquier causa.
ResultadosLa población del estudio incluyó a 15 pacientes, 14 de los cuales tenían HBC y uno tenía bloqueo AV 2:1. El síncope fue la presentación más común. El criterio de valoración clínico en forma de muerte se observó en 5 pacientes (33,3%), 3 pacientes revirtieron al ritmo sinusal y 7 pacientes requirieron implantación de marcapasos permanente. Los marcadores de inflamación se elevaron en todos los pacientes; sin embargo, se observó una tendencia hacia una mayor inflamación en los pacientes que alcanzaron el criterio de valoración clínico primario. Tres de cada 7 pacientes con QRS estrecho revirtieron al ritmo sinusal normal, mientras que los 8 pacientes con QRS de complejo ancho murieron o requirieron la inserción de un marcapasos permanente.
ConclusiónLa bradiarritmia sintomática se asocia con un estado inflamatorio alto y una alta mortalidad en la infección por COVID-19, y un bloqueo transitorio de la conducción en pacientes con ritmo QRS estrecho puede sugerir una inflamación miocárdica subclínica local.
Cardiovascular disease has been now found to be one of the most important and prevalent complications in a COVID-19 patient affecting the morbidity as well as mortality in such patients.1 Many studies have now described the major cardiovascular manifestations of COVID-19. Evidence of myocardial injury is common among patients hospitalized with COVID-19. Putative causes include hypoxic injury, ischemic injury, stress cardiomyopathy, and systemic inflammatory response syndrome (cytokine storm).2 COVID-19 has been associated with the development of cardiac dysfunction in patients with or without underlying cardiac conditions. Cardiovascular complications due to COVID-19 infection include myocardial infarction (MI), myocarditis, stroke, tachyarrhythmias, bradyarrhythmias, and pulmonary thromboembolism.3 Conduction system disruption in the form of tachyarrhythmia has been adequately documented in patients of COVID-19 disease. However, documentation regarding bradyarrhythmias has been few limited to a couple of case series, which could be found in literature and is likely to be under-reported.4–6 The clinical implications, outcomes, and approach to the management of such a complication are also relatively unknown.
This was a retrospective study to study the demographic, laboratory characteristics and clinical outcomes in symptomatic bradyarrhythmia patients with COVID-19 infection during the 8 month period from 24 March 2020 to 10 November 2020.
MethodsStudy design and participantsThis retrospective, observational study included all the adult patients (>18 years) who were diagnosed with complete heart block (CHB) or symptomatic high-grade Atrio-Ventricular (AV) block requiring a temporary pacemaker insertion (TPI) and presented to the emergency services of our hospital or developed de novo in a COVID-19 positive patient during the duration of his/her hospitalization between 24 March 2020 and 10 November 2020 in a tertiary care center catering COVID-19 patients in north India. The study protocol was approved by the institutional review board.
Data collectionFor the patients presenting to our hospital with such a bradyarrhythmia; epidemiological, demographic, laboratory, clinical management, and outcome data were extracted from all the enrolled patients after due consent from them. The data was checked by two physicians, and a third researcher adjudicated any difference in interpretation between the two primary reviewers
Clinical end-pointsThe enrolled patients were followed for the primary clinical composite endpoint of all-cause death. The patients were also followed-up for the reversal of the CHB and high-grade AV block to sinus rhythm or placement of a permanent pacemaker.
Laboratory proceduresECG was performed in all patients prior to diagnosis and then afterward whenever clinically indicated. Quantitative Troponin T and CK-MB was done initially in all patients at presentation. Routine blood examinations were done, including complete blood count, arterial blood gases, serum biochemical tests (including serum electrolytes, renal and liver function tests), CRP and serum ferritin. Chest radiographs and 2D echo were also done for all patients. Diagnosis of COVID-19 infection was made by rRT-PCR from nasal and oropharyngeal swabs. All epidemiological, clinical and laboratory data were prospectively recorded.
DefinitionsFever was defined as an axillary temperature of at least 37.5°C. Sepsis and septic shock were defined according to the 2016, Third International Consensus Definition for Sepsis and Septic Shock.7 Secondary infection was diagnosed when patients showed clinical symptoms or signs of pneumonia or bacteremia and a positive culture of a new pathogen was obtained from lower respiratory tract specimens (qualified sputum, endotracheal aspirate, or bronchoalveolar lavage fluid) or blood or urine samples after admission. Acute kidney injury was diagnosed according to the KDIGO clinical practice guidelines8 and acute respiratory distress syndrome (ARDS) was diagnosed as per the Berlin Definition.9 The Sequential Organ Failure Assessment score (SOFA score) was calculated for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation.10 As per WHO, novel coronavirus pneumonia was taken as severe if the patient presented with fever or suspected respiratory infection plus one of the following (1) respiratory rate more than 30 breaths per minute, (2) severe respiratory distress, (3) SpO2≤93%.11 Acute cardiac injury was diagnosed if serum levels of cardiac biomarker, CK-MB was above the 99th percentile upper reference limit, or if new abnormalities were detected on electrocardiography and echocardiography. Hypoalbuminemia was diagnosed when serum albumin was <3.5g/dL. Leucopenia was defined by a total leukocyte count of less than 4000cells per cubic millimeter. Lymphocytopenia was defined as absolute lymphocyte count of less than 1500cells per cubic millimeter. Monocytosis was defined as absolute monocyte count of more than 950 per cubic millimeter. Thrombocytopenia was defined as a platelet count of less than 150,000 per cubic millimeter. Normal ferritin values range 6.4–464ng/mL. Normal CRP values range 0–6mg/L.
Statistical analysisContinuous variables were expressed as mean±SD, normally distributed as median(quartiles), and categorical variables were reported as numbers and percentages. All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 20.0 software (SPSS Inc.).
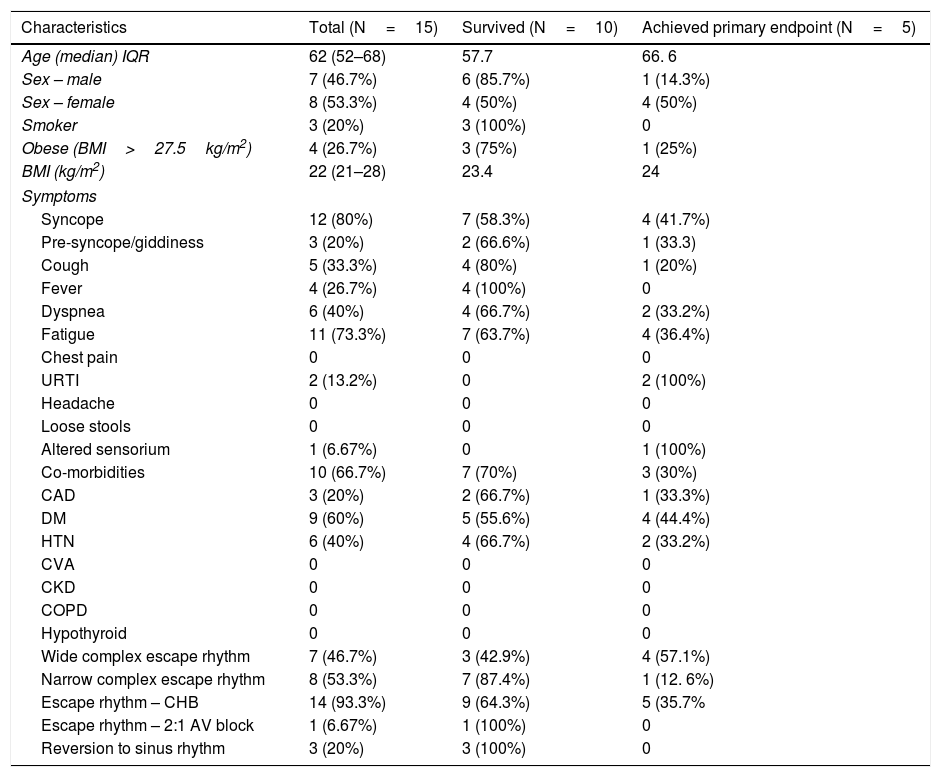
ResultsPatient characteristicsThe demographic characteristics of the patients are as shown in Table 1. The study population included 15 patients with symptomatic complete heart block or high degree AV block requiring TPI insertion. This included 14 patients with complete heart block (CHB) and 1 patient with 2:1 AV block. 13 out of 15 patients had presented with CHB at admission, 1 had a 2:1 AV block at presentation and 1 COVID inpatient had developed a CHB subsequently during hospitalization. The median age was 62 years (IQR, 52–68) and 7 (46.7%) were males. 3 (20%) had a history of smoking and 4 (26.7%) patients were obese with a BMI of >27.5kg/m2. The median BMI was 22kg/m2 (IQR, 21–28).
Baseline demographic and clinical profile of the COVID 19 patients.
| Characteristics | Total (N=15) | Survived (N=10) | Achieved primary endpoint (N=5) |
|---|---|---|---|
| Age (median) IQR | 62 (52–68) | 57.7 | 66. 6 |
| Sex – male | 7 (46.7%) | 6 (85.7%) | 1 (14.3%) |
| Sex – female | 8 (53.3%) | 4 (50%) | 4 (50%) |
| Smoker | 3 (20%) | 3 (100%) | 0 |
| Obese (BMI>27.5kg/m2) | 4 (26.7%) | 3 (75%) | 1 (25%) |
| BMI (kg/m2) | 22 (21–28) | 23.4 | 24 |
| Symptoms | |||
| Syncope | 12 (80%) | 7 (58.3%) | 4 (41.7%) |
| Pre-syncope/giddiness | 3 (20%) | 2 (66.6%) | 1 (33.3) |
| Cough | 5 (33.3%) | 4 (80%) | 1 (20%) |
| Fever | 4 (26.7%) | 4 (100%) | 0 |
| Dyspnea | 6 (40%) | 4 (66.7%) | 2 (33.2%) |
| Fatigue | 11 (73.3%) | 7 (63.7%) | 4 (36.4%) |
| Chest pain | 0 | 0 | 0 |
| URTI | 2 (13.2%) | 0 | 2 (100%) |
| Headache | 0 | 0 | 0 |
| Loose stools | 0 | 0 | 0 |
| Altered sensorium | 1 (6.67%) | 0 | 1 (100%) |
| Co-morbidities | 10 (66.7%) | 7 (70%) | 3 (30%) |
| CAD | 3 (20%) | 2 (66.7%) | 1 (33.3%) |
| DM | 9 (60%) | 5 (55.6%) | 4 (44.4%) |
| HTN | 6 (40%) | 4 (66.7%) | 2 (33.2%) |
| CVA | 0 | 0 | 0 |
| CKD | 0 | 0 | 0 |
| COPD | 0 | 0 | 0 |
| Hypothyroid | 0 | 0 | 0 |
| Wide complex escape rhythm | 7 (46.7%) | 3 (42.9%) | 4 (57.1%) |
| Narrow complex escape rhythm | 8 (53.3%) | 7 (87.4%) | 1 (12. 6%) |
| Escape rhythm – CHB | 14 (93.3%) | 9 (64.3%) | 5 (35.7% |
| Escape rhythm – 2:1 AV block | 1 (6.67%) | 1 (100%) | 0 |
| Reversion to sinus rhythm | 3 (20%) | 3 (100%) | 0 |
BMI, body mass index, URTI, upper respiratory tract infection, CAD, coronary artery disease, DM, diabetes mellitus, HTN, hypertension, CVA, cerebrovascular accident, CKD, chronic kidney disease, COPD, chronic obstructive airways disease, CHB, complete heart block, AV, atrioventricular.
Syncope was the most common symptom, which was present in 12 (80%) patients. 3 (20%) patients complained of giddiness and symptoms of pre-syncope. Fatigue was the most common symptom in 11 (73.3%) thereafter, followed by dyspnea 6 (40%), cough 5 (33.3%), and fever 4 (26.7%). Less common symptoms were symptoms suggestive of URTI and altered sensorium. Of the 15 admitted patients, 10 (66.7%) had 1 or more co-morbidities. Diabetes mellitus 9 (60%), hypertension 6 (40%) and CAD 3 (20%) were the most common co-existing illness.
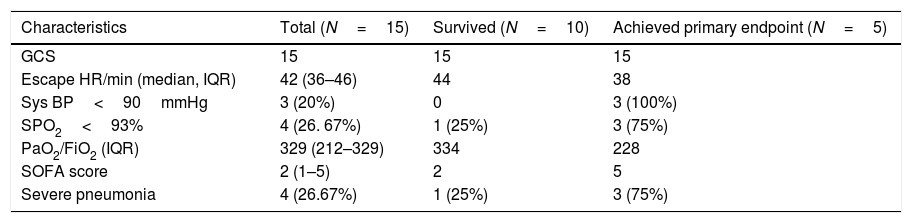
Radiological and laboratory findingsThe laboratory parameters and clinical characteristics of the patients on admission are as shown in Tables 2–4. The most common pattern on chest X-ray was a normal X-ray with no significant findings on admission in 8 patients, although subsequently all of them subsequently developed some opacity during their hospital stay. Bilateral patchy nodular or interstitial shadows were seen in 7 of 15 (45.7%) patients.
Baseline clinical profile of the COVID patients.
| Characteristics | Total (N=15) | Survived (N=10) | Achieved primary endpoint (N=5) |
|---|---|---|---|
| GCS | 15 | 15 | 15 |
| Escape HR/min (median, IQR) | 42 (36–46) | 44 | 38 |
| Sys BP<90mmHg | 3 (20%) | 0 | 3 (100%) |
| SPO2<93% | 4 (26. 67%) | 1 (25%) | 3 (75%) |
| PaO2/FiO2 (IQR) | 329 (212–329) | 334 | 228 |
| SOFA score | 2 (1–5) | 2 | 5 |
| Severe pneumonia | 4 (26.67%) | 1 (25%) | 3 (75%) |
GCS, Glasgow coma scale, HR, heart rate, Sys BP, systolic blood pressure, SOFA, sequential organ failure assessment.
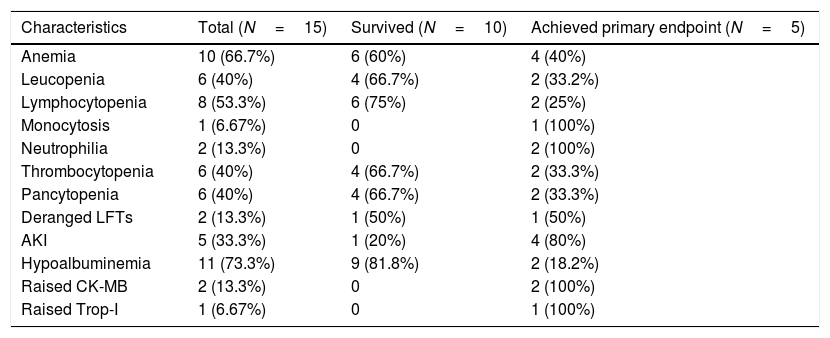
Laboratory profile of the COVID patients.
| Characteristics | Total (N=15) | Survived (N=10) | Achieved primary endpoint (N=5) |
|---|---|---|---|
| Anemia | 10 (66.7%) | 6 (60%) | 4 (40%) |
| Leucopenia | 6 (40%) | 4 (66.7%) | 2 (33.2%) |
| Lymphocytopenia | 8 (53.3%) | 6 (75%) | 2 (25%) |
| Monocytosis | 1 (6.67%) | 0 | 1 (100%) |
| Neutrophilia | 2 (13.3%) | 0 | 2 (100%) |
| Thrombocytopenia | 6 (40%) | 4 (66.7%) | 2 (33.3%) |
| Pancytopenia | 6 (40%) | 4 (66.7%) | 2 (33.3%) |
| Deranged LFTs | 2 (13.3%) | 1 (50%) | 1 (50%) |
| AKI | 5 (33.3%) | 1 (20%) | 4 (80%) |
| Hypoalbuminemia | 11 (73.3%) | 9 (81.8%) | 2 (18.2%) |
| Raised CK-MB | 2 (13.3%) | 0 | 2 (100%) |
| Raised Trop-I | 1 (6.67%) | 0 | 1 (100%) |
LFT, liver function test, AKI, acute kidney injury, CK-MB, creatinine kinase-MB, Trop I, troponin-I.
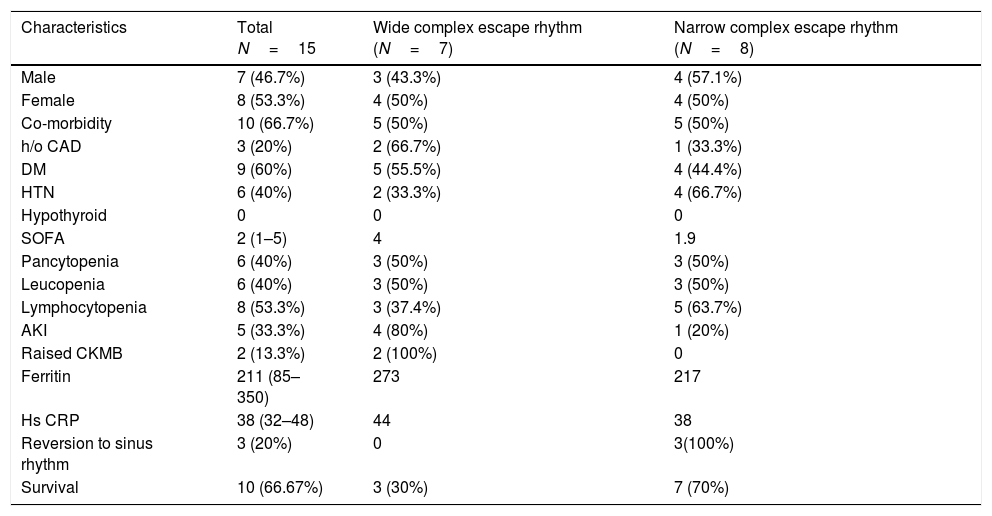
Baseline variables divided according to the type of the patients presenting escape rhythm.
| Characteristics | Total N=15 | Wide complex escape rhythm (N=7) | Narrow complex escape rhythm (N=8) |
|---|---|---|---|
| Male | 7 (46.7%) | 3 (43.3%) | 4 (57.1%) |
| Female | 8 (53.3%) | 4 (50%) | 4 (50%) |
| Co-morbidity | 10 (66.7%) | 5 (50%) | 5 (50%) |
| h/o CAD | 3 (20%) | 2 (66.7%) | 1 (33.3%) |
| DM | 9 (60%) | 5 (55.5%) | 4 (44.4%) |
| HTN | 6 (40%) | 2 (33.3%) | 4 (66.7%) |
| Hypothyroid | 0 | 0 | 0 |
| SOFA | 2 (1–5) | 4 | 1.9 |
| Pancytopenia | 6 (40%) | 3 (50%) | 3 (50%) |
| Leucopenia | 6 (40%) | 3 (50%) | 3 (50%) |
| Lymphocytopenia | 8 (53.3%) | 3 (37.4%) | 5 (63.7%) |
| AKI | 5 (33.3%) | 4 (80%) | 1 (20%) |
| Raised CKMB | 2 (13.3%) | 2 (100%) | 0 |
| Ferritin | 211 (85–350) | 273 | 217 |
| Hs CRP | 38 (32–48) | 44 | 38 |
| Reversion to sinus rhythm | 3 (20%) | 0 | 3(100%) |
| Survival | 10 (66.67%) | 3 (30%) | 7 (70%) |
CAD, coronary artery disease, DM, diabetes mellitus, HTN, hypertension, SOFA, sequential organ failure assessment, AKI, acute kidney injury, CK-MB, creatinine kinase-MB, Hs-CRP, highly sensitive C-reactive protein.
On admission, hypoalbuminemia was present in 11 (73.3%) patients and was the most common finding. Anemia was present in 10 (66.7%) patients. Leucopenia was present in 6 (40%) of the patients, with the majority of patients having lymphocytopenia 8 (53.3%) and 1 (6.67%) patient having monocytosis. Thrombocytopenia was seen in 6 (40%) and pancytopenia in 6 (40%) of the patients. Raised CK-MB was seen in 2 (13.3%), Troponin I was elevated in 1 (6.67%) patient. 5 (33.3%) patients developed AKI and this was relatively more in patients who met the primary outcome, although significance could not be achieved. Alanine aminotransferase, aspartate aminotransferase and serum bilirubin were elevated less commonly.
Markers of inflammation were raised overall in all the patients, although the trend was toward a more inflammatory state in the primary outcome group. Serum ferritin was raised to 211ng/mL (IQR, 85–350) and CRP was raised to a value of 38mg/L (IQR, 32–48).
Vital signsThe vital signs and clinical characteristics of the patients are shown in Table 2. These measures were recorded on the day of hospital admission for all patients, then divided into those who later met the primary composite outcome or not. 4 (26. 67%) patients were diagnosed with severe pneumonia and had more tachypnea and reduced levels of SpO2 and PaO2. The patients who met the primary outcome had significantly more tachypnea, hypotension and reduced levels of SpO2 (<93%). These patients also had more organ injury in the form of acute kidney injury [9 (75%)], raised CKMB, and had increased SOFA scores. The median PaO2/FiO2 was 329 (212–329) and SOFA score was 2 (1–5) (Table 2). The PaO2/FiO2 was lower (228 vs 334) and SOFA scores were higher(5 vs 2) in the patients who expired as compared to those who survived.
Treatment and main interventionsAll the patients received injectable antibiotics, tab ivermectin and supplemental oxygen therapy initially if indicated clinically. Additional tab azithromycin (500mg once daily for 5 days), low molecular weight heparin (LMWH) according to body weight, dexamethasone and antivirals were given when clinically indicated. A total of 3 (20%) patients received vasopressors in view of hypotension, all of whom expired subsequently. 4 (26.67%) patients were diagnosed with severe pneumonia and required ICU admission due to multi-organ dysfunction. Out of them, 3 patients expired and 1 survived. All five mortalities were considered complications of COVID-19 infection, with hypoxemic respiratory failure and COVID-19 induced or acquired sepsis. The patient specific outcomes were as described in Table 5.
Patient-specific clinical events and outcomes.
| Patient | Presenting complaints | ECG diagnosis | TPI insertion | Escape rhythm | Reversion to sinus rhythm | Decision to implant PPI | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Fever, cough, DyspneaSyncope | CHB | At admission | Narrow complex | No | Day 16 | PPI/VVI |
| 2 | Syncope | CHB | At admission | Wide complex | No | Not done | Expired |
| 3 | Fever, coughDyspneaSyncope | CHB | At admission | Narrow complex | No | Day 18 | PPI/single chamber |
| 4 | Syncope | CHB | At admission | Wide complex | No | Day 16 | PPI/double chamber |
| 5 | Syncope | CHB | At admission | Narrow complex | No | Day 17 | PPI/single chamber |
| 6 | Fever, Dyspnea, syncope | CHB | COVID+ve ICU, day 7 | Wide complex | No | Not done | Expired |
| 7 | Pre-Syncope | CHB | At admission | Narrow complex | YesDay 8 | Not done | Discharged |
| 8 | Syncope | CHB | At admission | Narrow complex | YesDay 14 | Not done | Discharged |
| 9 | Cough, dyspnea, pre-syncope | CHB | At admission | Narrow complex | No | Day 28 | PPI/double chamber |
| 10 | Syncope | 2:1 AV block | At admission | Narrow complex | YesDay 5 | Not done | Discharged |
| 11 | Pre-syncope | CHB | At admission | Wide complex | No | Not done | Expired |
| 12 | Fever, Syncope | CHB | At admission | Wide complex | No | Day 21 | PPI/single chamber |
| 13 | Syncope | CHB | At admission | Wide complex | No | Day 19 | PPI/single chamber |
| 14 | Dyspnea, Syncope | CHB | At admission | Narrow complex | No | Not done | Expired |
| 15 | Cough, Dyspnea, syncope | CHB | At admission | Wide complex | No | Not done | Expired |
CHB, complete heart block, PPI, permanent pacemaker implantation.
All 15 patients were symptomatic on presentation with 12 (80%) having syncope and 3 (20%) having a history of pre-syncope or giddiness. In 14 patients, a TPI was instituted via a femoral vein approach under fluoroscopic guidance in the cath lab on presentation to the hospital emergency. 1 patient was being managed as severe COVID-19 pneumonia and had developed a complete heart block during hospital admission in which the TPI was instituted bedside via a subclavian vein approach. Echocardiography performed during hospitalization or prior to PPI, revealed normal left ventricular systolic function in the majority of patients (left ventricular ejection fraction 55±14.3%). The three patients who had a previous history of CAD had reduced LVEF (45%, 45% and 50%). No significant QT prolongation was noted at baseline or during hospitalizations in any patient. 2 patients with a narrow complex CHB had an RBBB pattern ventricular escape rhythm.
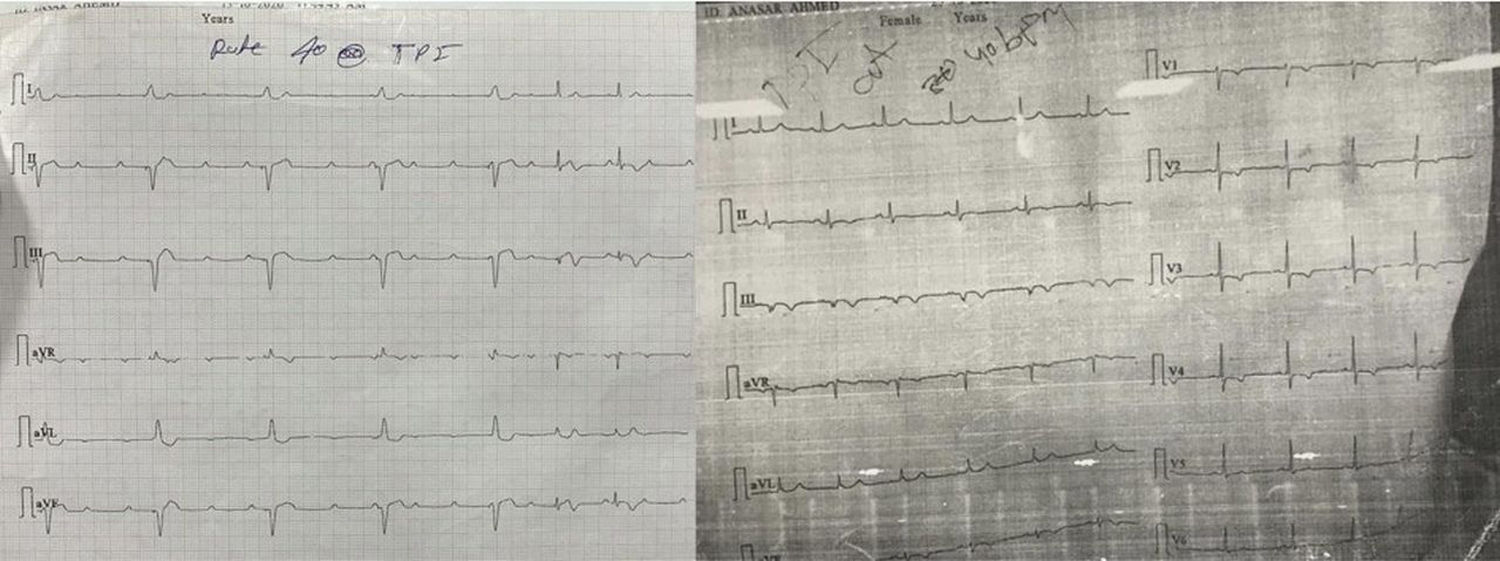
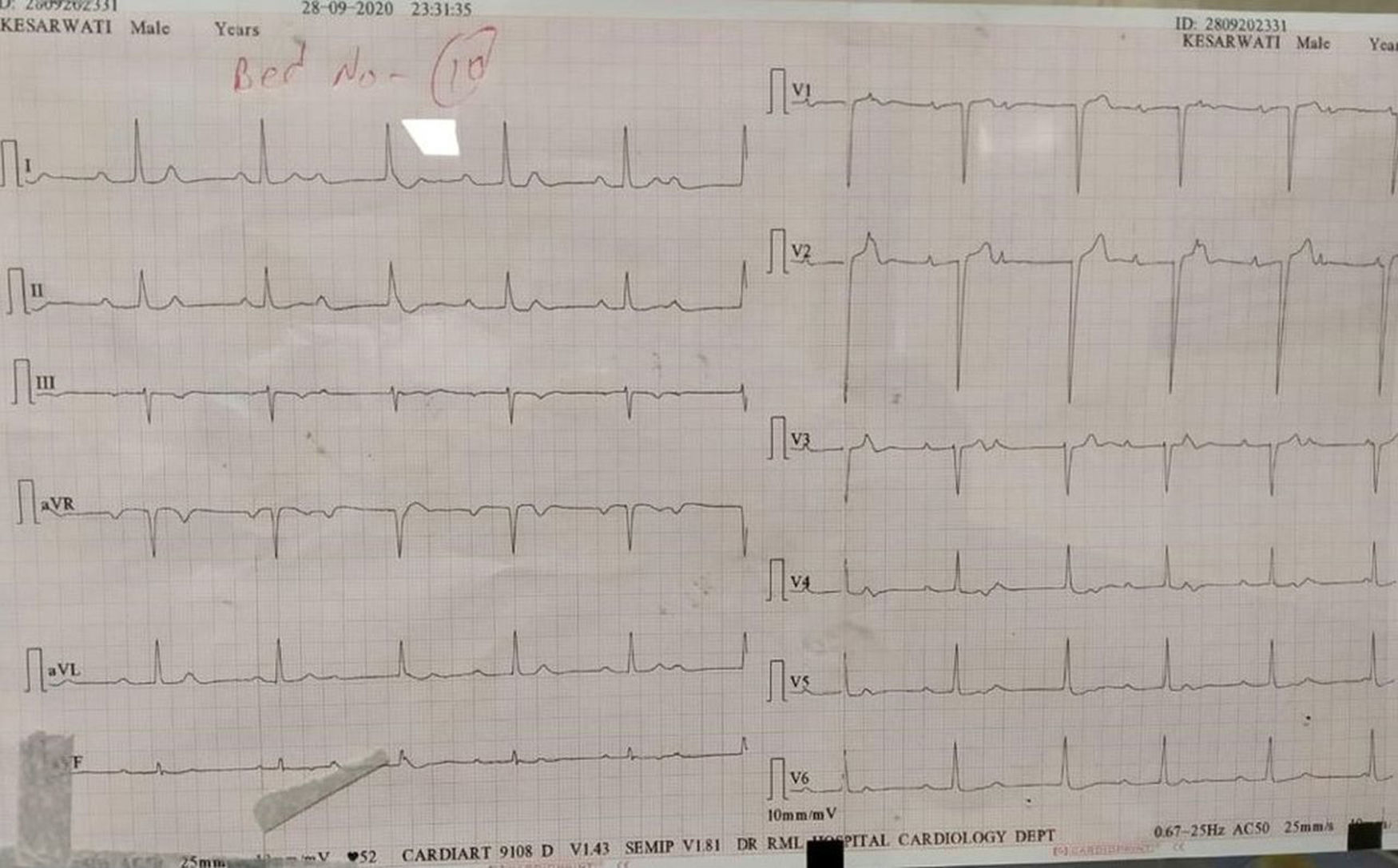
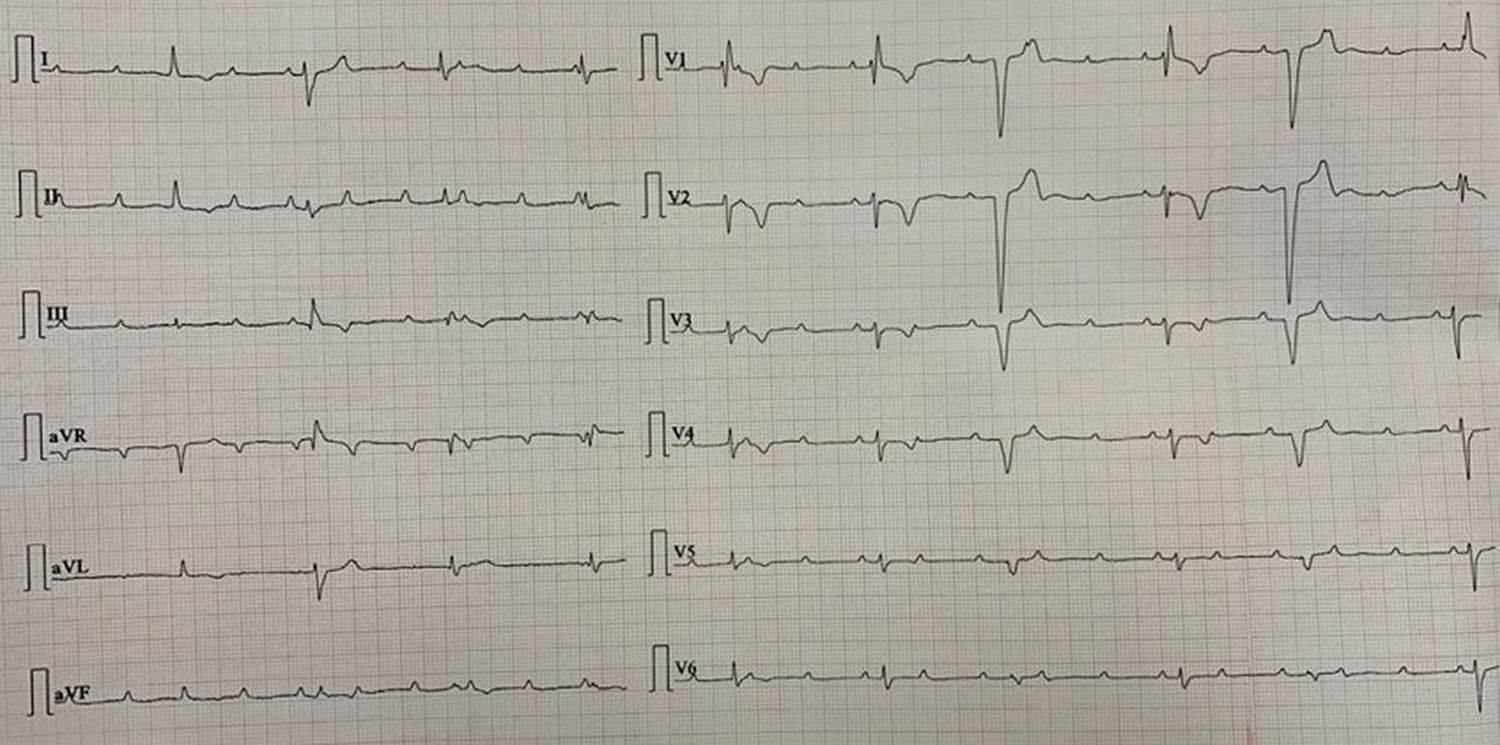
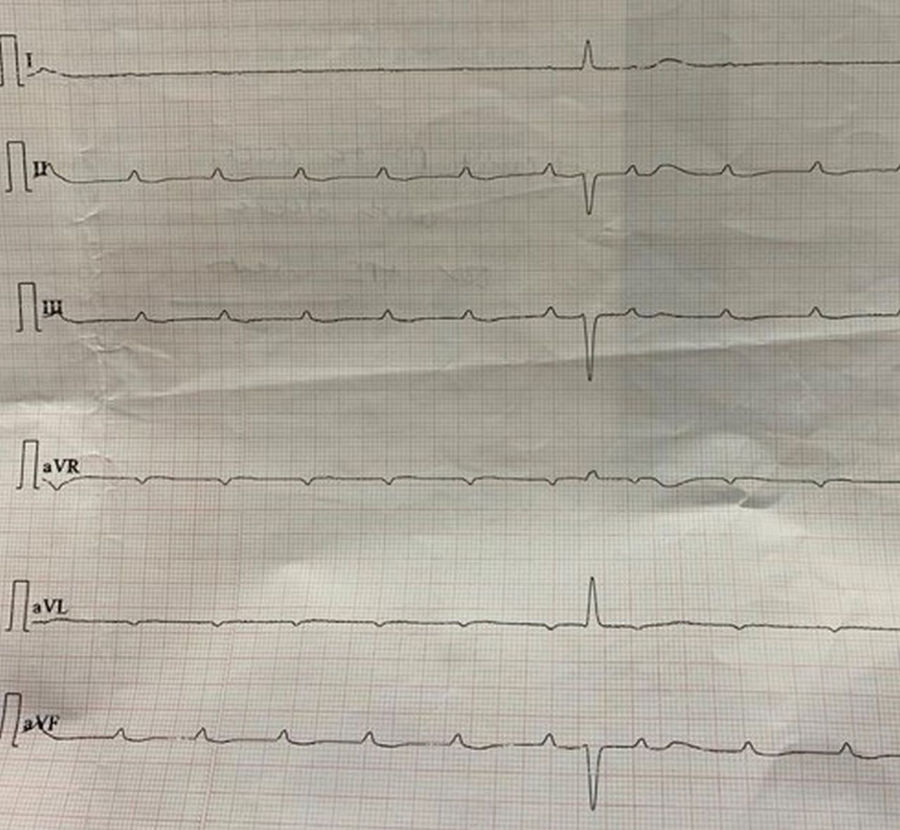
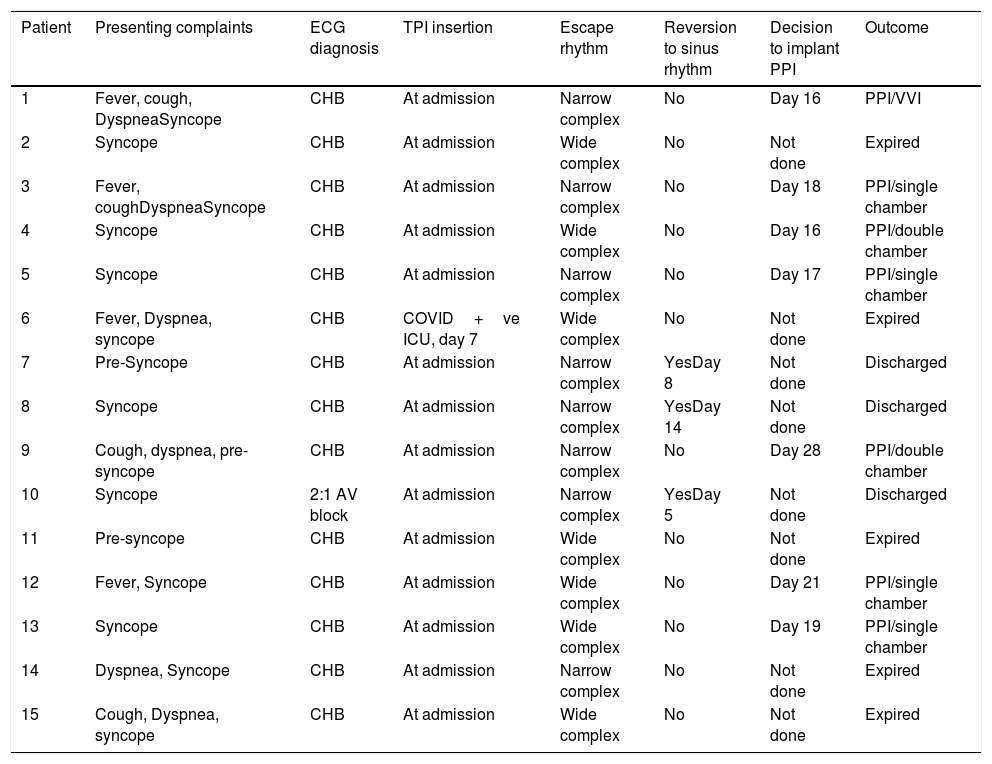
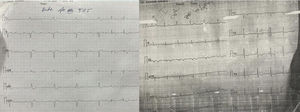
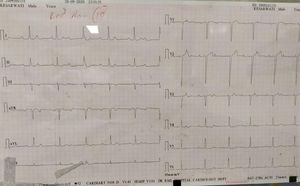
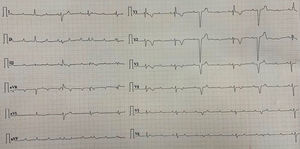
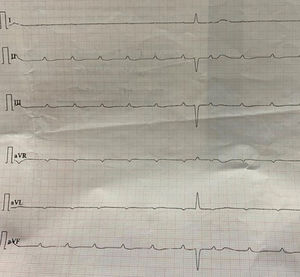
The majority of the patients had a narrow complex escape rhythm which was seen in 8 (53.3%) patients, while a broad complex escape rhythm was seen in 7 patients. Of the patients having a narrow complex escape rhythm, 3 patients self-reverted to a sinus rhythm after a few days (Figs. 1–3), while none of the patients with a broad complex escape rhythm reverted back to a sinus rhythm (Fig. 4).
Out of the 15 patients, 5 patients expired and 3 patients rhythm had self-reverted. A permanent pacemaker was inserted in the remaining 7 patients. Permanent pacemaker implantation was done after clinical recovery of the patient or after 14 days of COVID-19 infection; whichever is maximum. 2 patients had a dual chamber pacemaker implanted and in the remaining five, a single chamber pacemaker was implanted.
DiscussionCOVID-19 has been associated with the development of cardiac dysfunction in patients with or without underlying cardiac condition. The exact mechanism of cardiac involvement during COVID-19 remains unknown and is open to debate. Acute bradycardia in a COVID-19 patient may be either due to direct SARS-CoV-2 infiltration of myocardial cells and the dedicated conduction system, aggravation of preexisting conduction disease during acute illness, or maybe as a result of collateral damage from systemic inflammatory reaction and cytokine storm.2 Also, as myocarditis has been extensively reported in patients with COVID-19, local myocardial inflammation and injury may affect the conduction system resulting in such conduction blocks. Post mortem evidence in patients has shown the presence of SARS-CoV-2 RNA in cardiac myocytes and endothelial cells, suggesting a direct involvement of the myocardium in this COVID-19 disease.2 SARS-CoV-2 may also activate the angiotensin-converting enzyme 2 (ACE2) receptor. ACE2 expression has been demonstrated specifically in Sino-atrial (SA) nodal cells, and ACE2 overexpression has previously been associated with conduction disturbances.12 Also, the SARS-CoV causing the 2002 outbreak was associated with sinus bradycardia in up to 15% of patients.13 This may partly explain the affinity of the virus to the conduction system, although it has yet to be established conclusively.
In our study, short-term in-hospital mortality was high at 33.3%, despite prompt management of bradycardia with pacing support. All patients had an elevation of inflammatory markers. However, the trend was of more evidence of inflammation in the primary outcome group. Also, four patients had severe COVID-19 pneumonia which itself has a poor prognosis. Out of these 3 patients expired and only 1 survived. Also, if we take into account only the non-severe pneumonia patients requiring TPI insertion, the mortality comes down to 2 out of 11 that is 18%. One of the case series previously published has reported poor prognosis of patients with a conduction system disease with short-term mortality as high as 57%.6 However, the case series published by Gupta et al. reported a good short-term outcome, although the patient clinical profile may not have been that severe in the group.5 Overall the presence of a conduction system disease seems to be a harbinger of a state of intense inflammation in the body despite lack of coexistent cardiomyopathy or acute cardiac injury. However, no significant difference was seen in the laboratory parameters between the two subgroups, although it might be due to the small sample size. None of the patients were on calcium channel blockers, beta-blockers, digoxin or anti-arrhythmic drugs at baseline. None of the patients had any reversible cause of the conduction defects.
In some patients, bradyarrhythmias presented prior to the onset of respiratory symptoms. Fever, cough and dyspnea were seen in only 26%, 33% and 40%, respectively. This has important implications for health care worker safety in the initial management of these patients and treating all patients as potentially infected and due precautions to be taken during management.
Majority of patients with symptomatic bradycardia presented with a narrow complex escape rhythm [(8 (53.3%)]. These patients had a good outcome [7 (87.4%) vs 3 (42.9%)] in our study when compared to the patients who had a wide complex escape rhythm. Also, the evidence of inflammation in the form of elevated CRP, ferritin and organ injury in the form of raised CKMB and AKI was higher in the subgroup of patients who had a wide complex escape rhythm. These have important prognostic implications in the management of patients with symptomatic bradycardia. No evidence of myocarditis or cardiac injury was present in the majority of patients. Also 3 out of 8 patients of the narrow complex escape rhythm group reverted back to the sinus rhythm while none of the 7 patients of a broad complex tachycardia did so. This has an implication suggesting a transient nature of the conduction system damage in some of these patients which most probably according to the previous evidence is due to local subclinical myocardial inflammation and subsequent damage to the conduction system. Also the patients with a broad QRS complex escape rhythm seem to have a more severe inflammatory state and poor recovery of rhythm as well as overall morbidity as against those with a narrow complex escape rhythm.
ConclusionSymptomatic bradyarrhythmia is associated with high inflammatory state and high mortality in COVID-19 infection despite management with temporary pacemaker, especially in patients with broad QRS complex and a transient conduction block in patients with narrow QRS rhythm may suggest local subclinical myocardial inflammation.
FundingNone.
Conflict of interestNone.