The measurement of core body temperature in the pulmonary artery (Swan-Ganz catheter) is regarded as the reference standard. Nowadays, new non-invasive methods of measuring core temperature have emerged, such as the 3M SpotOn® and the Dräger Tcore® sensor.
MethodsA cross-sectional descriptive study was conducted on patients undergoing scheduled cardiac surgery. Both devices, and the Swan-Ganz catheter, were simultaneously evaluated. The correlation of temperature values was measured using the intra-class correlation coefficient and Spearman's rank or Pearson correlation analysis. The repeated-measurements version of the Bland and Altman test were used to determine the level of agreement.
ResultsA total of 289 measurements were made. Analysis of Spearman rank correlation between the Swan-Ganz catheter and 3M SpotOn® pair yielded a correlation coefficient (r) of 0.82 [95% confidence interval (95% CI); 0.77–0.85, p<0.001], and the result of ICC was 0.88 (95% CI; 0.85–0.90). Bland–Altman analysis showed a bias (SD) of 0.21°C (0.34) [−2.27 to 2.71]. The Swan-Ganz catheter and Dräger Tcore® pair showed r-values of 0.78 (95% CI; 0.73–0.82 and p<0.001), and ICC of 0.78 (95% CI; 0.74–0.82). This pair showed a bias of 0.48°C (0.42) [−2.02–2.98].
ConclusionsBoth the 3M SpotOn® and the Dräger Tcore® provided a high degree of accuracy and precision in measuring the core temperature, and could be used for the control of normothermia in the patient undergoing cardiac surgery.
La medición de la temperatura corporal central en la arteria pulmonar (catéter de Swan-Ganz) se considera el patrón oro. Hoy en día han surgido nuevos métodos no invasivos para medir la temperatura central, como el 3M SpotOn® y el sensor Dräger Tcore®.
MétodosEste es un estudio descriptivo transversal con pacientes sometidos a cirugía cardíaca programada. Se evaluaron ambos dispositivos y el catéter de Swan-Ganz simultáneamente. La correlación de los valores de temperatura se evaluó mediante el coeficiente de correlación intraclase y el análisis de correlación de Spearman o Pearson. Para determinar el nivel de acuerdo se utilizó la versión de medidas repetidas de la prueba de Bland y Altman.
ResultadosSe realizaron un total de 289 mediciones. El análisis de la correlación de rango de Spearman entre el catéter de Swan-Ganz y el 3M SpotOn® arrojó un coeficiente de correlación (r) de 0,82 (IC 95%: 0,77 a 0,85; p<0,001), y el resultado de ICC fue de 0,88 (IC 95%: 0,85 a 0,90). El análisis de Bland-Altman reveló un sesgo (DE) de 0,21°C (0,34; IC 95%: −2,27 a 2,71). El catéter de Swan-Ganz y Dräger Tcore® mostraron valores de r de 0,78 (IC 95%: 0,73 a 0,82; p<0,001), y el ICC de 0,78 (IC95%: 0,74 a 0,82). El par mostró un sesgo de 0,48°C (0,42; IC95%: −2,02 a 2,98].
ConclusionesTanto el 3M SpotOn® como el Dräger Tcore® proporcionan un alto grado de precisión a la temperatura central y pueden utilizarse para el control de la normotermia en el paciente sometido a cirugía cardíaca.
Question: The authors wonder if perioperative temperature control devices measure accurately.
Findings: There are several devices that are reliable although they may have differences.
Meaning: In patients undergoing cardiac surgery, several devices have been tested with a high degree of accuracy and precision with respect to the gold-standard.
Inadvertent perioperative hypothermia (IPH) is defined as patient core temperature of below 36.0°C and it is associated with cardiac morbid events,1 blood loss and wound infections.1–3 IPH has a negative impact on operative course, increasing the cost of treatment1,4 and the length of hospital stay.1,5,6 In addition to these effects, IPH decreases drug metabolism and prolongs the duration of post-anaesthetic recovery and the need for care.7,8
Eshraghi et al.9 considered establishing an acceptable agreement between the pulmonary artery (core) and zero-heat-flux to be measured at 0.5°C. This was the first study in which the measurement of thermometry in anesthesiology was introduced, modifying in some cases, the methodology proposed by other authors.10
The measurement of core body temperature in the pulmonary artery via a Swan-Ganz catheter is regarded as the gold standard,11,12 and the rest of the methods currently known they are lower accuracy.13–15 In this sense, the sublingual method is currently regarded as the most reliable16 and it has been demonstrated to be a good measurement both in awake and anaesthetized patients.15 Nasopharyngeal measurement has also been shown to be a reliable method, but it is not applicable in awake or head-injured patients.17
In recent years, new non-invasive methods of measuring core temperature have emerged, such as the 3M SpotOn® and the Dräger Tcore® sensor. The former works by insulating the site of measurement, the forehead, from surrounding influences, and it allows the skin underneath to heat up to body core temperature by creating an isothermal tunnel.15 The latter employs a unique dual-sensor heat flux technology, which, following a short ramp-up time, calculates core body temperature continuously with higher accuracy than the other devices.18 Both devices are placed as a sticker on the patient's forehead.
In spite of the fact that research has been carried out with these devices two by two, no studies have so far applied these two new devices at the same time as the gold standard (Swan-Ganz catheter). Thus, the aim of this study was to compare at the same time the Swan-Ganz catheter with the new non-invasive methods (3M SpotOn® and Dräger Tcore®) in terms of accuracy, precision and correlation in patients undergoing elective surgery.
MethodsEthical aspects and permissionsA favourable report was obtained from the Regional Clinical Research Ethics Committee (Ref 129/18). Requirement for written informed consent was waived by this Committee.
Study design and periodThis is a cross-sectional descriptive study carried out from May to December 2017.
Scope of the studyThis study was conducted at a third-level hospital equipped with 1065 beds and 36 operating rooms.
Inclusion criteriaThe following inclusion criteria were considered:
- -
Patients undergoing programmed valvular surgery.
- -
Placement of the Swan-Ganz catheter to perform the surgery.
- -
Sufficient surface area on the patient's forehead to accommodate both devices (3M SpotOn® and Dräger Tcore®) simultaneously.
Once general anaesthesia was administered and the Swan-Ganz catheter was placed, the non-invasive central temperature measurement devices were placed on the patient's forehead. Analysis was restricted to the intraoperative period, excluding bypass. A recording sheet was used to record the time of the measurement and the temperature of each device at that time. A maximum of 10 measurements per patient were scheduled (depending on the duration of the intervention) with an interval of 30min between each measurement. The first measurement took place 5min after the placement of the three devices. Due to the evaluation of new devices with an unknown reliability and dropout rate, we decided to include a consecutive convenience sample of 41 patients for 7 months.
Study variablesSocio-demographic variables (age, sex, date of intervention) were taken into account, as well as those related to the measurement of the central temperature using the three study devices (Swan-Ganz – gold standard–, 3M SpotOn® and Dräger Tcore®).
Statistical analysisStatistical analyses were performed using statistical software R 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). A p-value of less than 0.05 was considered significant.
The normality of the distribution of continuous variables was determined with the Shapiro–Wilk test. Distribution of temperature data were described as means, medians and standard deviation (SD). Correlation of temperature values was evaluated by intraclass correlation coefficient (ICC) and Spearman's rank or Pearson correlation analysis. Bland–Altman for repeated measures tests19 were used to determine the level of agreement between temperatures, calculating the mean difference between the methods (bias) as a measure of accuracy, the SD of the difference as a measure of precision and 1.96 SD (limits of agreement) with 95% confidence interval. From the literature, we determined that a value of 0.5°C for accuracy and precision is considered as clinically adequate.17
ResultsA total of 289 measurements were made in 41 patients. The mean age of the patients was 69 years (SD±10.8) and 80% were male.
An average of 7 measurements (SD±1.64) was collected per patient. The mean core temperature was 35.51 (SD±1.11), 35.3 (SD±1.11) and 35.02 (SD±1.18) for the Swan-Ganz catheter, the 3M SpotOn® and the Dräger Tcore® sensor, respectively. The mean of measurements per patient every 30min was 7.05 (SD±1.64) with a mean in hours of measuremnts per patient of 3.16 (SD±0.74). Mean (SD) of temperature for Swan-Ganz catheter, 3M SpotOn® and Dräger Tcore® was 35.51 (1.11), 35.30 (1.11) and 35.00 (1.18) centigrades, respectively.
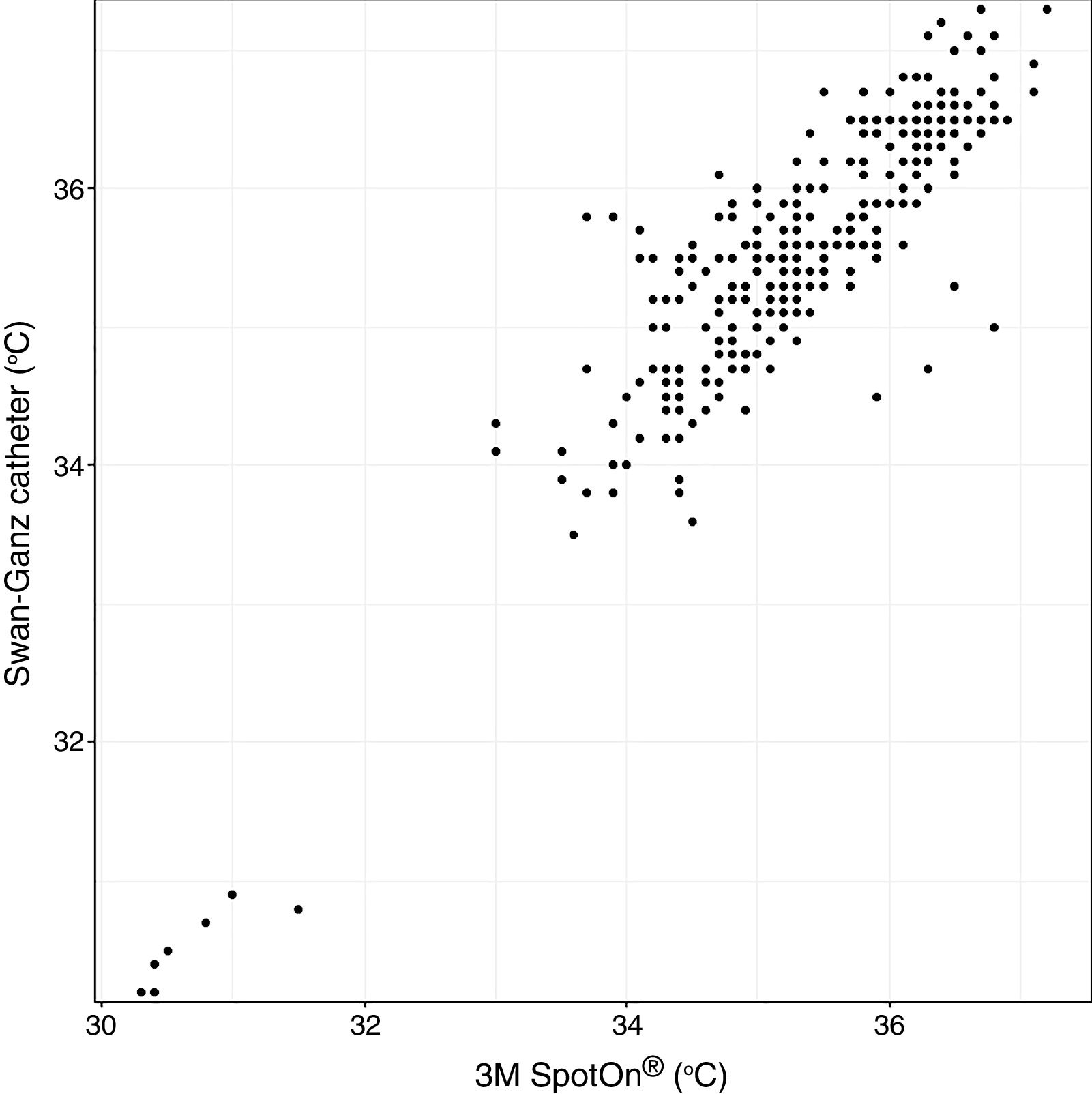
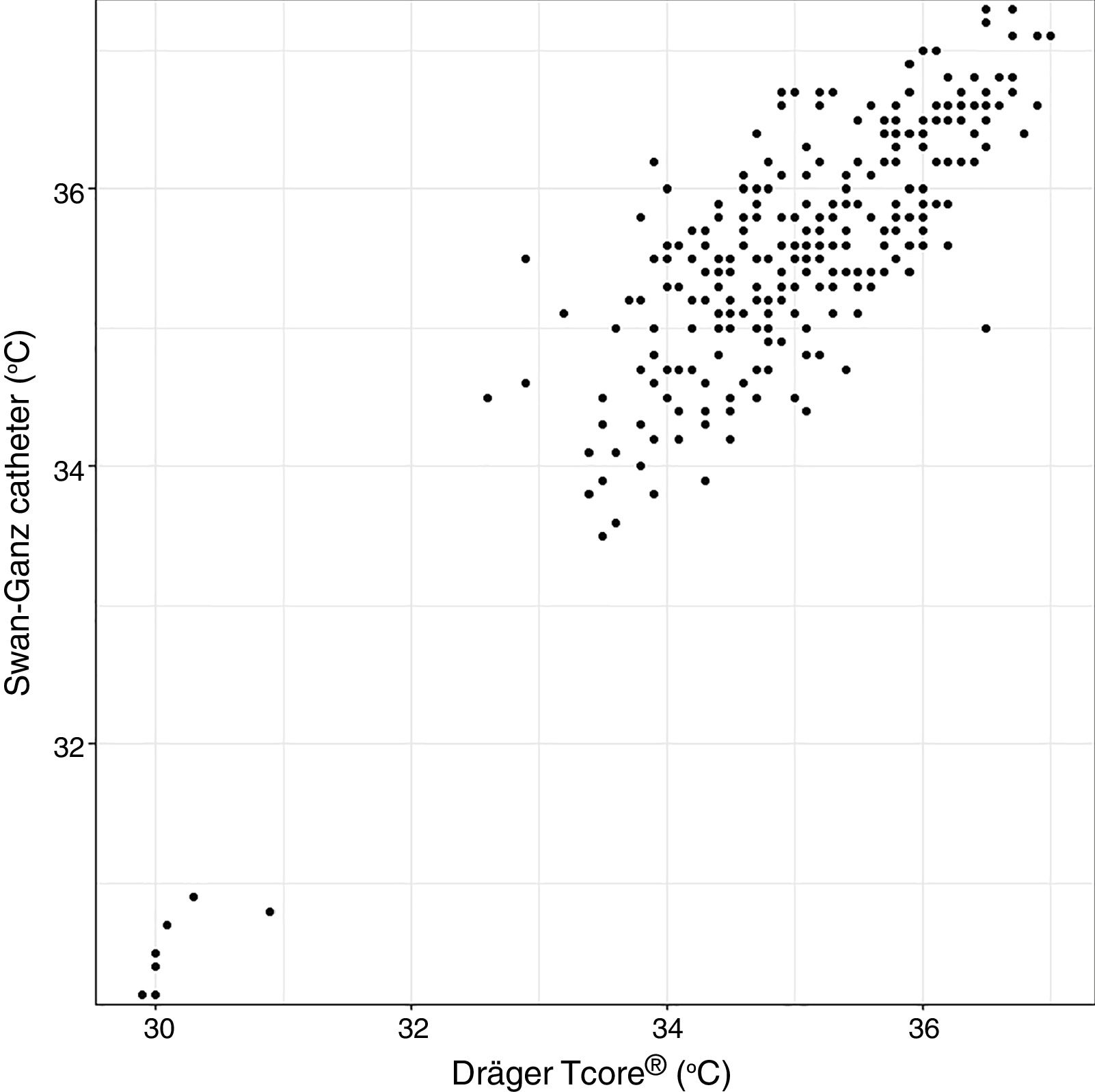
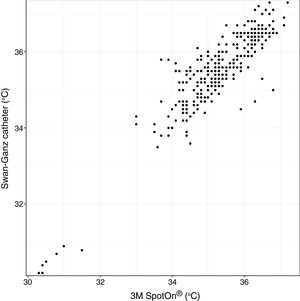
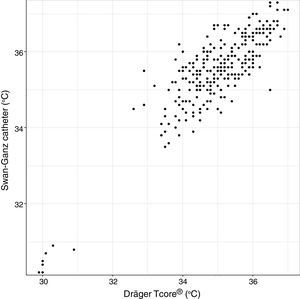
Analysis of Spearman rank correlation between the Swan-Ganz catheter and 3M SpotOn® pair yielded a correlation coefficient (r) of 0.82 [95% confidence interval (95% CI) 0.77–0.85 and p<0.001], and the result of ICC was 0.88 (95% CI 0.85–0.90) (Fig. 1). At the same time, the Swan-Ganz catheter and Dräger Tcore® pair showed r-values of 0.78 (95% CI 0.73–0. 82 and p<0.001), and ICC of 0.78 (95% CI 0.74–0.82) (Fig. 2).
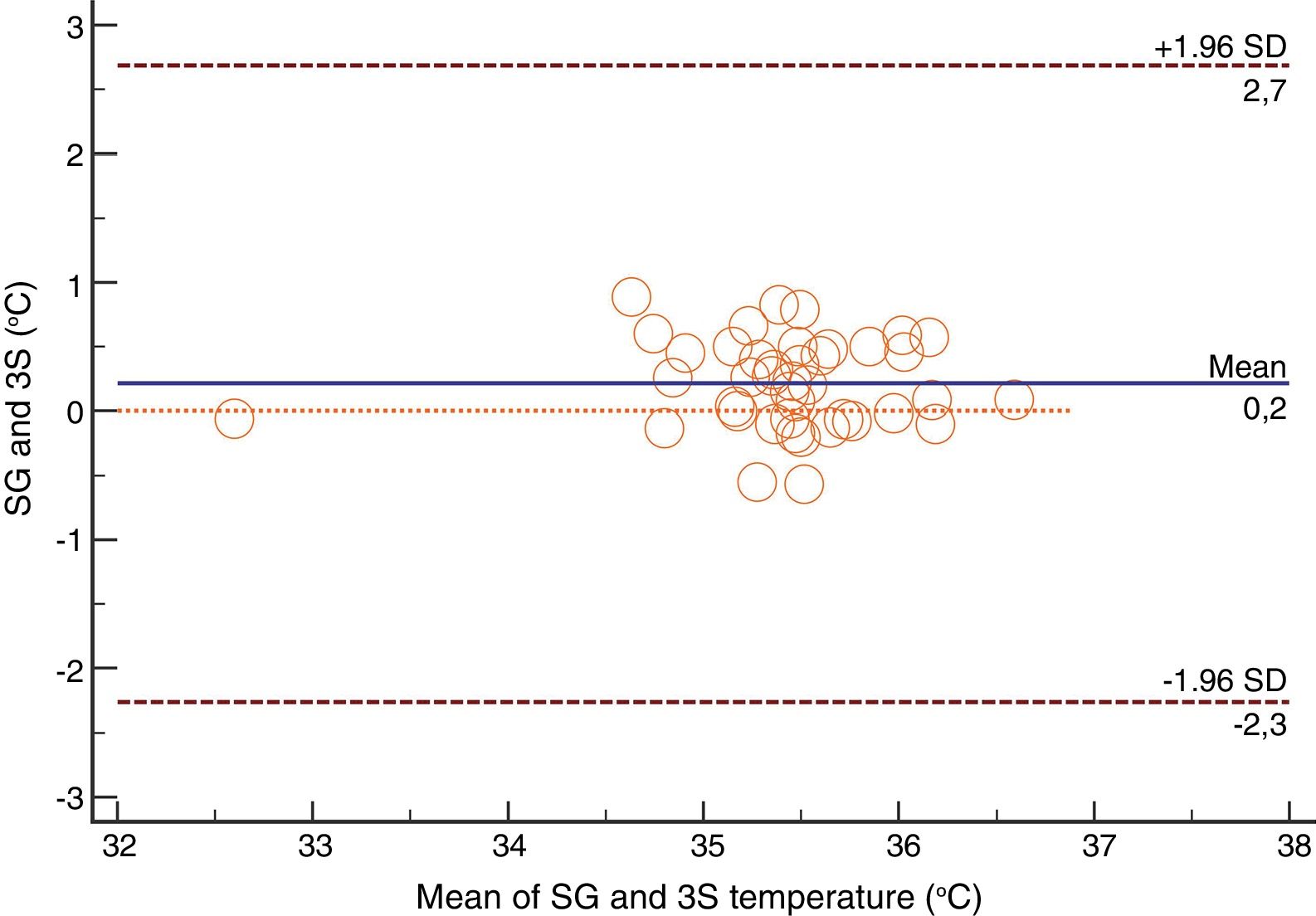
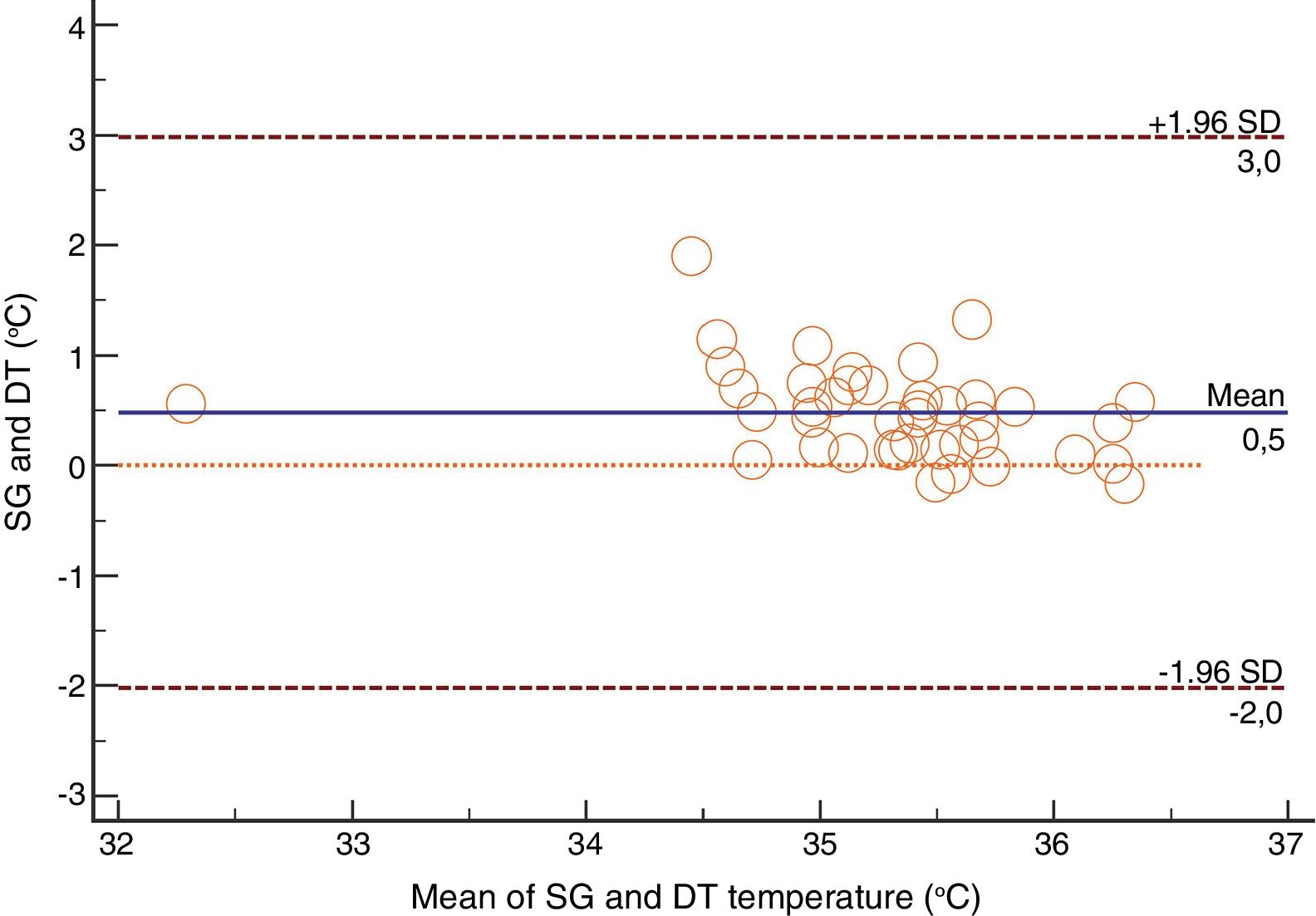
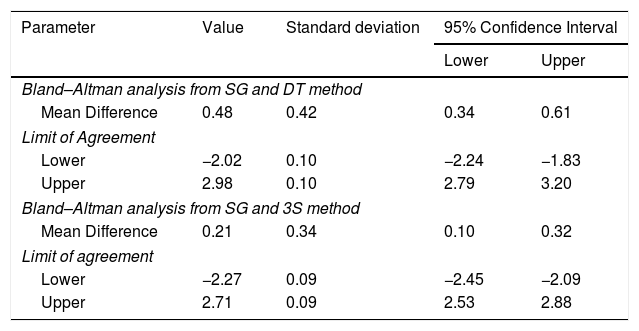
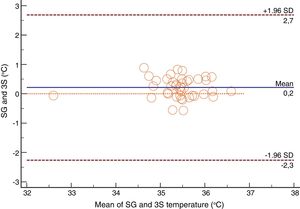
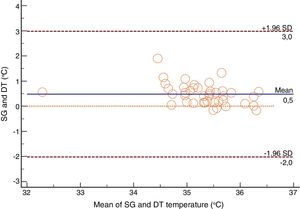
For the Swan-Ganz catheter and 3M SpotOn®, Bland–Altman for repeated measures analysis revealed a bias (SD) [95% limits of agreement] of 0.21°C (0.34) [−2.27 to 2.71] (Fig. 3). The Swan-Ganz catheter and Dräger Tcore® pair showed a bias of 0.48° C (0.42) [−2.02 to 2.98] (Fig. 4) (Table 1).
Mean Difference and Limits of Agreement between methods.
| Parameter | Value | Standard deviation | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Bland–Altman analysis from SG and DT method | ||||
| Mean Difference | 0.48 | 0.42 | 0.34 | 0.61 |
| Limit of Agreement | ||||
| Lower | −2.02 | 0.10 | −2.24 | −1.83 |
| Upper | 2.98 | 0.10 | 2.79 | 3.20 |
| Bland–Altman analysis from SG and 3S method | ||||
| Mean Difference | 0.21 | 0.34 | 0.10 | 0.32 |
| Limit of agreement | ||||
| Lower | −2.27 | 0.09 | −2.45 | −2.09 |
| Upper | 2.71 | 0.09 | 2.53 | 2.88 |
SG: Swan-Ganz catheter; 3S: 3M SpotOn®; DT: Dräger Tcore®.
These results allowed us to fulfil the objective of the study through the methodology described above. On the one hand, like authors such as Iden (2015),14 the correlation coefficient was calculated to determine the statistical relationship of the two measurements. On the other hand, this parameter was complemented with the intraclass correlation coefficient (ICC) and the Bland–Altman graphics, like other authors have done11,15,20 in order to provide the accuracy (mean difference of all the pairs for each non-invasive site) and precision (standard deviation) data to the above.
Both the 3M SpotOn® and the Dräger Tcore® were compared to the gold standard (Swan-Ganz catheter) and they showed a strong correlation in terms of core temperature measurement from a non-invasive point of view, under the same conditions. However, it seems that, while it is true that they both have a correlation coefficient and an ICC above 0.70, according to the study sample, the results seem to be more favourable for the 3M SpotOn®. The scatter plot in Fig. 2 shows a greater amplitude at the points than in Fig. 1. The same thing happens in Fig. 4 with respect to Fig. 3: for each temperature in degrees, more dispersion is observed in Dräger Tcore® than in 3M SpotOn®, obtaining greater standard deviation in the Bland–Altman test. In comparison with the Swan-Ganz catheter, the 3M SpotOn® sensor showed a bias of no more than 0.21°C, which can be interpreted as negligible. The Dräger Tcore® showed a bias of up to 0.48°C, with similar accuracy and precision between the two devices and the Swan-Ganz catheter, and, although this falls within the parameters established as normal by the most experienced authors in the field of normothermia, 0.5°C is the limit allowed for correct accuracy and precision.17,21 In further analysis, the sensitivity and specificity values were 94% and 74% for 3M SpotOn® and 95% and 65% Dräger Tcore® respectively (Table 2). For the validity of the devices, we calculated the sensitivity and specificity in comparison with the gold-standard, defining hypothermia as temperature values below 35.5°C. Regarding sensitivity and specificity parameters, a high sensitivity is observed for both devices (greater than 90% in both cases), however, there is more than a 10% difference between the specificity results. Only 6 and 5% respectively of the hypothermic values (<35.5°C) would not be detected with these devices (sensitivity). However, 3M SpotOn® obtained 26% false positives compared to 35% for Dräger Tcore®, with temperatures <35.5°C that would require unnecessary heating and additional costs. These false positive values would be reduced by lowering the level of what we consider hypothermia to 35°C.
Sensitivity and specificity calculation for both devices.
| Hypothermiaa | Swan-Ganz | Hypothermiaa | Swan-Ganz | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||
| Dräger Tcore® | Positive | 117 | 58 | 3M SpotOn® | Positive | 116 | 42 |
| Negative | 6 | 108 | Negative | 7 | 124 | ||
Dräger Tcore® sensitivity: 95% 3M SpotOn® sensitivity: 94%.
Dräger Tcore® specificity: 65% 3M SpotOn® specificity: 74%.
In recent years, numerous investigations have tested these types of non-invasive devices for the measurement of central temperature,14,20 and this has important advantages in the prevention of surgical wound4,9,13 and patient recovery time,5,8 even more so in the intra-operative field. Until now, adequate control of the core temperature in the surgical patient was performed through invasive techniques with an unstable balance between risk and benefit to the patient.1 As the most accurate and reliable methods of core temperature measurement were often invasive,21–23 normothermia control was limited to interventions where the placement of these devices was strictly required, leaving temperature control aside in interventions where insertion was not necessary.
The high precision and accuracy of these non-invasive devices is an advantage for the correct practice related to the control of normothermia and an indication recommended in clinical practice guidelines for the National Institute of Health and Clinical Excellence (NICE),1,24 the Centers for Disease Control and Prevention (CDC),2 the World Health Organization,3 the Canadian Patient Safety Institute25 or the National Health Service Scotland.26 This measure is also recommended in Spain, where the nationwide Zero Surgical Infection Project (2014) is being carried out,27,28 which includes 5 preventive measures on the basis of the available evidence29: antibiotic prophylaxis, skin antisepsis, no hair elimination, normothermia and normoglycemia.
Another fact to take into account is the facility of use of these devices and, in particular, the Dräger Tcore® has an advantage: while the 3M SpotOn® has a separate monitor and no adapters to operating room monitors, the Dräger Tcore® has an adaptor to connect to these monitors, adding the data graph to the constant monitor itself, facilitating the work of the anaesthesiologists and avoiding additional disinfection of the devices in the operating room as a possible vector for the transmission of microorganisms.
Finally, although the main strength of this paper is that it is the first research that tests these two non-invasive devices simultaneously with the gold-standard core temperature measurement device, it has some limitations. Firstly, the number of measurements could have been higher, although other similar studies have used similar samples.20,30 This may bias the measurements somewhat so that they may proceed from the same subjects. Therefore, the researchers have tried to achieve 7 average measurements per patient to reduce this possibility and increase the sample size of the temperature measurement study. Secondly, this research has been carried out only in patients undergoing cardiac surgery, so it would be interesting to perform other investigations in other types of surgeries in order to expand and ratify these results. Thirdly, sensitivity and specificity may not be accurate since the number of measurements should be higher. However, these results could be understood as an estimate of these parameters for evaluating diagnostic tests that should be complemented with more extensive studies. Finally, it may be that some of the differences could be explained by the method of placing the devices on the patient's forehead by the researchers, but to avoid this possible bias it can be said that all of them were specifically instructed in the devices placement methodology.
ConclusionsBoth the 3M SpotOn® and the Dräger Tcore® provide a high degree of accuracy, precision and sensitivity to the core temperature measurement using a non-invasive, easy and comfortable methods and they could be used for the control of normothermia in the patient undergoing cardiac surgery.
Clinical trial number and registry urlNot applicable.
Prior presentationsNot applicable.
Author's individual contributionAll authors have read and agree and accept the content of the manuscript.
Gómez-Romero FJ: This author performed the analyzes and drafted the initial version of the manuscript.
Fernández-Prada M: This author carried out the design of the study and the procedures with the Research Ethics Committee, collaborated in the initial drafting of the document and organized the collection of data.
Fernández-Suárez FE: This author was responsible for the data collection in the operating room.
Gutiérrez-González C: This author collected the data in the operating room.
Estrada-Martínez M: This author collected the data in the operating room.
Cachero-Martínez D: This author collected the data in the operating room.
Suárez-Fernández S: This author collected the data in the operating room.
García-González N: This author collected the data in the operating room.
Picatto-Hernández MD: This author contributed to the critical reading of the manuscript.
Martínez-Ortega C: This author contributed to the critical reading of the manuscript.
Navarro-Gracia JF: This author collaborated with the analysis of the data and the critical reading of the manuscript.
FundingThe authors have no sources of funding to declare for this manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
Not applicable.