The aim of this study is to present our experience with three patients who underwent a neonatal Yasui procedure, and to show the new decision-making algorithm.
MethodsThis is a series of three neonates operated on at our hospital between 2017 and 2022.
ResultsAll the patients underwent a primary Yasui. The duration of cardiopulmonary bypass was 275, 249 and 391min, in patient 1, 2 and 3, respectively. After surgery, the Intensive Care Unit stay of patients 1, 2 and 3 was 29, 22 and 24 days, respectively. The patients were discharged in good condition. Subsequent complications during follow-up included the need for percutaneous intervention in patient 1 for the implantation of a stent in the right pulmonary branch (at 6 months postoperatively) and a stent in the right ventricle-pulmonary artery conduit (at 42 months). Patient 2 required right ventricle-pulmonary artery conduit replacement and repair of moderate-severe left ventricular outflow tract obstruction at 16 months postoperatively. Patient 3 needed a reoperation at 3 months postoperatively due to aortic arch stenosis at different levels and a residual ventricular septal defect. Currently, all patients are alive with adequate echocardiographic biventricular function.
ConclusionsIn experienced centers, primary Yasui repair can be performed in the neonatal period with satisfactory results.
El objetivo de este estudio es presentar nuestra experiencia con tres pacientes sometidos a un procedimiento de Yasui neonatal, y mostrar el nuevo algoritmo de toma de decisiones.
MétodosSe trata de una serie de tres neonatos, operados en nuestro hospital entre 2017 y 2022.
ResultadosTodos los pacientes fueron sometidos a un Yasui primario. La duración del bypass cardiopulmonar fue de 275, 249 y 391 minutos, en los pacientes 1, 2 y 3, respectivamente. Tras la cirugía, la estancia en la Unidad de Cuidados Intensivos de los pacientes 1, 2 y 3 fue de 29, 22 y 24 días, respectivamente. Los pacientes fueron dados de alta en buenas condiciones. Las complicaciones posteriores durante el seguimiento incluyeron, la necesidad de intervención percutánea en el paciente 1 para la implantación de un stent en la rama pulmonar derecha (a los seis meses del posoperatorio), y un stent en el conducto ventrículo-pulmonar derecho (a los 42 meses). El paciente 2, requirió la sustitución del conducto ventrículo-pulmonar derecho, y la reparación de la obstrucción moderada-grave del tracto de salida del ventrículo izquierdo, a los 16 meses del posoperatorio. El paciente 3, necesitó una reoperación a los tres meses del posoperatorio, debido a una estenosis del arco aórtico a diferentes niveles, y a una comunicación interventricular residual. Actualmente, todos los pacientes están vivos con una función biventricular ecocardiográfica adecuada.
ConclusionesEn centros con experiencia, se puede realizar la reparación primaria de Yasui en el periodo neonatal con resultados satisfactorios.
Interrupted aortic arch is a rare, but highly lethal anomaly in early infancy.1 Interruption usually occurs between the left common carotid and the left subclavian arteries (interrupted aortic arch type B), but can occur distal to the left subclavian artery (interrupted aortic arch type A) or between the innominate artery and left common carotid artery (interrupted aortic arch type C), and is usually associated with a large, non-restrictive ventricular septal defect.2
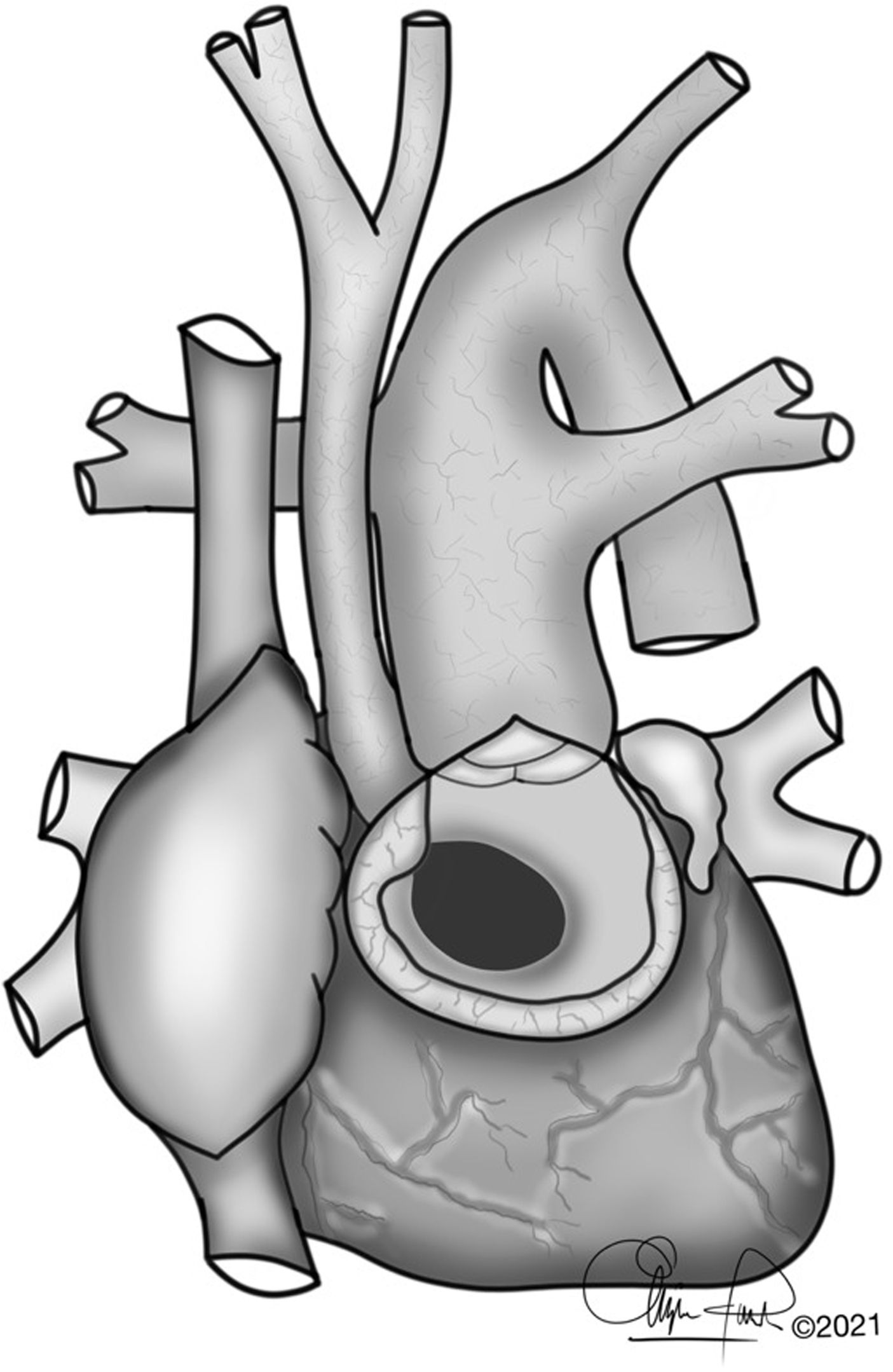
Interrupted aortic arch may be associated with critical aortic atresia or stenosis, and a reasonably well-developed apex-forming left ventricle, due to the presence of the ventricular septal defect.3 The degree of left ventricle hypoplasia and possible dysfunction determines whether neonates with critical left ventricular outflow tract obstruction are managed with univentricular palliation or biventricular repair.4
The first report of primary biventricular repair by Yasui in 2 neonates with interrupted aortic arch and severe aortic stenosis was published in 1987.1,3,5 This procedure consist, in general terms, in that the left ventricular outflow tract is rerouted to the native pulmonary valve through an intra-cardiac baffle arising from the ventricular septal defect; the two great arteries roots are then joined through a Damus–Kaye–Stansel anastomosis; pulmonary blood flow is provided by a right ventricle-pulmonary artery valved conduit.4 This combines a Norwood type arch reconstruction with a Rastelli type operation establishing a biventricular repair.6
The purpose of this study is to present our experience with three patients with very similar anatomical characteristics that underwent a neonatal Yasui procedure, as well as to expose the general management of these patients in our center using a new decision making algorithm.
Materials and methodsMaterials and methodsThis is a series of three neonates who underwent a Yasui operation at “12 de Octubre” Hospital between 2017 and 2022. Patients were identified through the cardiac database, and the medical records were subsequently reviewed.
The first and second patients we present had an interrupted aortic arch type B associated with aortic stenosis and non-restrictive ventricular septal defect. The third patient had aortic atresia, with an ascending aorta of approximately 2mm in diameter and a mild-restrictive ventricular septal defect.
All patients underwent a primary Yasui procedure before 2 weeks of age, at a median weight of 3.1kg.
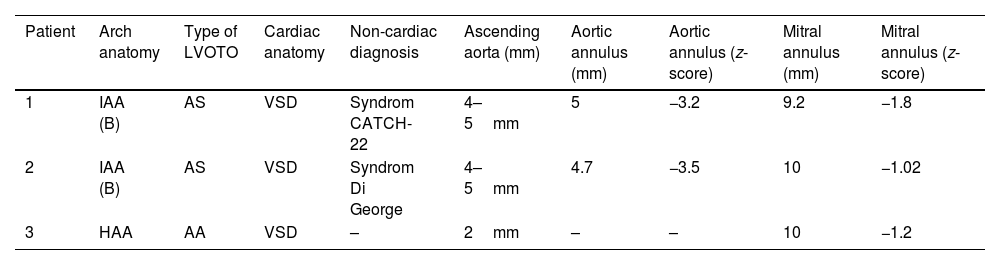
The characteristics and echocardiographic measurements before the Yasui operation are summarized in Table 1.
Patient characteristics and echocardiographic measurements before the Yasui operation.
| Patient | Arch anatomy | Type of LVOTO | Cardiac anatomy | Non-cardiac diagnosis | Ascending aorta (mm) | Aortic annulus (mm) | Aortic annulus (z-score) | Mitral annulus (mm) | Mitral annulus (z-score) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | IAA (B) | AS | VSD | Syndrom CATCH-22 | 4–5mm | 5 | −3.2 | 9.2 | −1.8 |
| 2 | IAA (B) | AS | VSD | Syndrom Di George | 4–5mm | 4.7 | −3.5 | 10 | −1.02 |
| 3 | HAA | AA | VSD | – | 2mm | – | – | 10 | −1.2 |
Abbreviations: IAA, interrupted aortic arch; AS, aortic stenosis; AA, aortic atresia; HAA, hypoplasia of ascending aorta; VSD, ventricular septal defect.
Patients with this congenital anomaly, who are born in our hospital or are referred from other centers, are discussed in a multidisciplinary session to decide the management and therapeutic strategy.
Maintaining ductal patency by infusion of prostaglandin E1 represents the first step in the medical resuscitation of the neonate with interrupted aortic arch. Because the lower half of the body is dependent on perfusion through the ductus, and because blood in the ductus can also pass into the pulmonary circulation, it is important that pulmonary and systemic circulations are well balanced. This can be achieved by avoiding a high level of inspired oxygen (usually room air is appropriate), as well as avoiding respiratory alkalosis caused by hyperventilation.7 On the other hand, systemic vasodilators are used to optimize systemic pressures. Metabolic acidosis should be aggressively treated. Because myocardial function is likely to be depressed somewhat at the time of presentation, and it may be necessary for the heart to handle a moderate volume load (depending on the balance between pulmonary and systemic circulations), an inotropic agent is used on certain occasions. Intensive medical treatment is usually maintained for a few days before surgery. It should be avoided to take a child to the operating room with any abnormalities of acid–base, renal, or hepatic indices.7
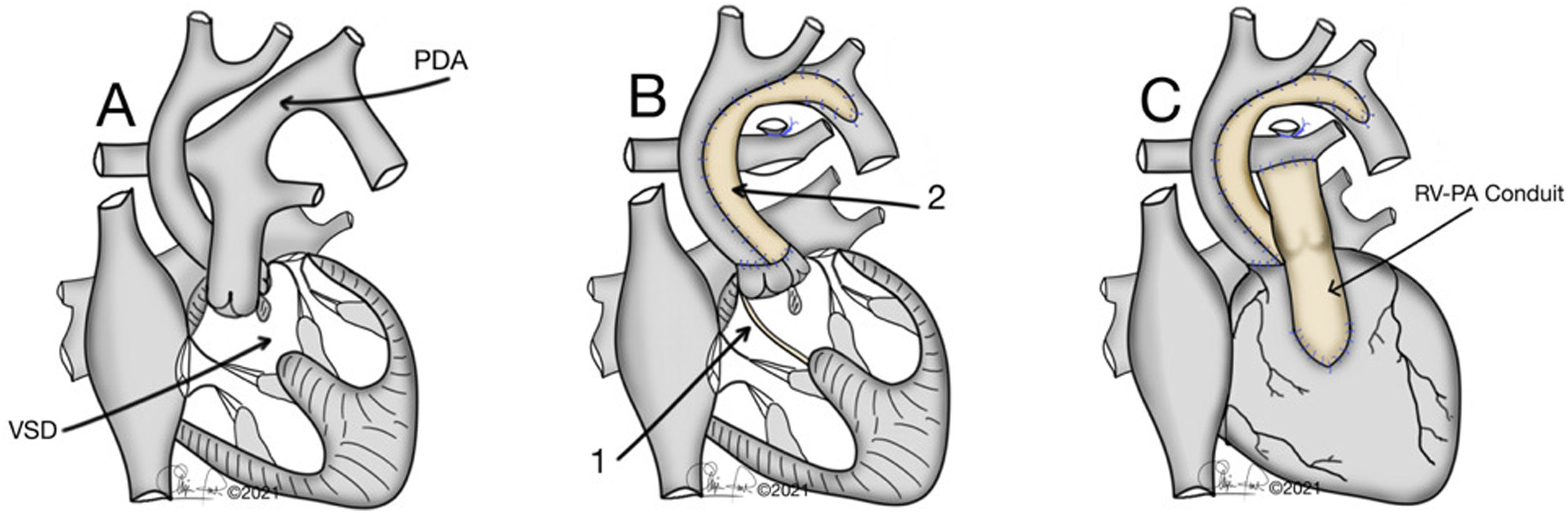
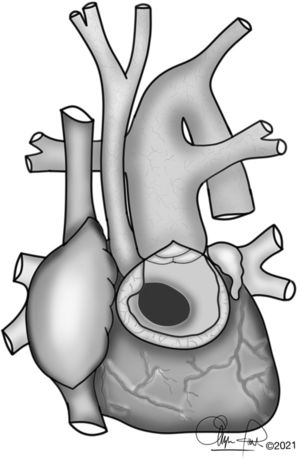
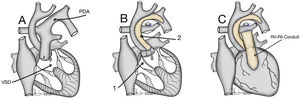
The typical anatomy of patients who underwent a Yasui procedure is shown in Figs. 1 and 2.
(A) Interrupted aortic arch type B associated with aortic stenosis and non-restrictive ventricular septal defect. VSD: ventricular septal defect; PDA: Patent ductus arteriosus. (B) 1: Redirection of left ventricle outflow through the ventricular septal defect to both semilunar valves (Damus–Kaye–Stansel anastomosis); 2: Aortic arch reconstruction. (C) Establishment of right ventricle – pulmonary artery continuity using a valved conduit. RV-PA: right ventricle-pulmonary artery.
After discharge from the hospital, the patients continued with the corresponding follow-up adapted to their evolution.
OperationThe concept of the Yasui operation is a combination of aortic arch reconstruction, redirection of left ventricle outflow through the ventricular septal defect to both semilunar valves (joined by a Damus–Kaye–Stansel anastomosis) and establishment of right ventricle-pulmonary artery continuity using a valved conduit5 (Fig. 2).
Cannulation strategiesIn patient 1 (interrupted aortic arch), arterial perfusion was established with a 3.5mm polytetrafluoroethylene graft (Gore-Tex®) anastomosed to the origin of the right carotid artery. The graft was cannulated with an 8-French cannula (cerebral perfusion). The descending aorta was cannulated directly through the proximal ductus arteriosus also with an 8-French cannula (systemic perfusion) and the 2 cannulae were connected to the arterial perfusion line.
In patient 2 (interrupted aortic arch), arterial perfusion was established with a 6-French cannula in the ascending aorta (cerebral perfusion). The descending aorta was cannulated directly through the proximal ductus arteriosus with an 8-French cannula (systemic perfusion) and the 2 cannulae were connected to the arterial perfusion line.
In patient 3 (aortic atresia and hypoplasia of ascending aorta), arterial perfusion was established with a 6-French cannula in the aortic arch, proximal to the right carotid artery (cerebral perfusion). The descending aorta was cannulated directly through the proximal ductus arteriosus with an 8-French cannula (systemic perfusion) and the 2 cannulae were connected to the arterial perfusion line.
In all patients venous return was established with bicaval cannulation, and extracorporeal circulation was performed in hypothermia at 26° C.
Aortic reconstructionIn recent years in our center, during aortic arch surgery, we use myocardial perfusion in addition to selective cerebral perfusion, thus reducing cardiac ischemia times.
Reconstruction of the aortic arch, in the first two patients, was performed by end-to-end anastomosis of the posterior wall of the descending thoracic aorta to the ascending aorta, and anterior enlargement with an autologous pericardium patch in patient 1 and a pulmonary
homograft patch in patient 2. With adequate mobilization of the ascending and descending aortas, this is easily performed without tension.
The third patient had no interruption of the aortic arch, so reconstruction of the aortic arch and ascending aorta consisted of an anterior enlargement with a CardioCel® 3D patch.
Subsequently, the Damus–Kaye–Stansel connection was made between the proximal pulmonary trunk and the ascending aorta.
Intraventricular reroutingA vertical right ventricular outflow tract incision was made just below the pulmonary valve to the right of and parallel to the left anterior descending coronary artery. This ventriculotomy exposed the perimembranous ventricular septal defect. In all cases, a bovine pericardium patch was used to baffle the left ventricle outflow through the ventricular septal defect to the pulmonary artery.
In patient 3, ventricular septal defect enlargement was performed (due to an original restrictive defect), achieving a diameter of approximately 8mm.
Right ventricle to pulmonary artery connectionA right ventricle to pulmonary artery connection was established using a Hancock conduit (14mm) in patient 1, and a Contegra conduit (12mm) in patients 2 and 3, according to surgeon preference.
Ethical considerationsGiven the characteristics of the article, approval by the Ethics Committee or Institutional Review Board is not required, nor is the request of informed consent for publication. Written informed consent for surgery was obtained from the patient’s parents.
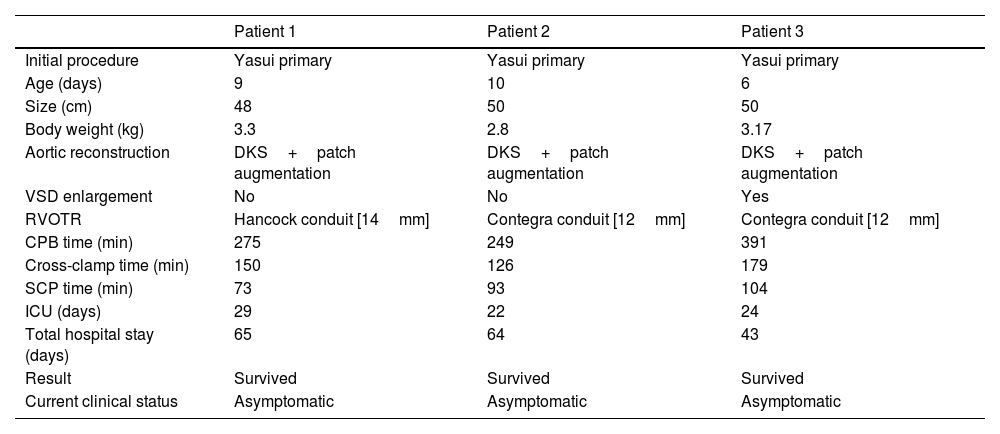
ResultsOperative details are presented in Table 2.
Perioperative data of the Yasui operation.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Initial procedure | Yasui primary | Yasui primary | Yasui primary |
| Age (days) | 9 | 10 | 6 |
| Size (cm) | 48 | 50 | 50 |
| Body weight (kg) | 3.3 | 2.8 | 3.17 |
| Aortic reconstruction | DKS+patch augmentation | DKS+patch augmentation | DKS+patch augmentation |
| VSD enlargement | No | No | Yes |
| RVOTR | Hancock conduit [14mm] | Contegra conduit [12mm] | Contegra conduit [12mm] |
| CPB time (min) | 275 | 249 | 391 |
| Cross-clamp time (min) | 150 | 126 | 179 |
| SCP time (min) | 73 | 93 | 104 |
| ICU (days) | 29 | 22 | 24 |
| Total hospital stay (days) | 65 | 64 | 43 |
| Result | Survived | Survived | Survived |
| Current clinical status | Asymptomatic | Asymptomatic | Asymptomatic |
Abbreviations: DKS, Damus–Kaye Stansel; VSD, ventricular septal defect; RVOTR, right ventricular outflow tract reconstruction; CPB, cardiopulmonary bypass; SCP, selective cerebral perfusion.
In the first two patients, concomitant myocardial perfusion was used during 26 and 25min respectively. In the third patient, due to the characteristics of the diminutive aorta, myocardial perfusion was not possible during reconstruction of the aortic arch.
The duration of cardiopulmonary bypass was 275, 249 and 391min, and myocardial ischemia was 150, 126 and 179min, in patient 1, 2 and 3, respectively.
Patient 1 presented the right subclavian artery originating from the right pulmonary branch, and for this reason this artery was reimplanted in the ascending aorta during the intervention. This justifies the longer surgical times compared to the second patient (similar anatomy).
The sternum was left open in all three cases and subsequently closed in the Intensive Care Unit, as is usual practice in complex neonatal cases.
Patient 1, during his stay in the Intensive Care Unit, required surgical revision due to postoperative cardiac tamponade. The thorax could be closed 5 days after surgery. He presented episodes of junctional tachycardia that required antiarrhythmic treatment with amiodarone. Peritoneal dialysis was required during the first postoperative days. Extubation could be performed 11 days postoperatively.
In patient 2, the thorax was closed 24h after admission to the Intensive Care Unit. Five days after surgery she was extubated, but a few hours later she had to be intubated again due to inspiratory stridor associated with respiratory work. Treatment with dexamethasone was started, allowing definitive extubation on the seventh postoperative day. Fibrobronchoscopy was performed, showing a left vocal cord paresis with partial collapse of the right intermediate bronchus. She developed acute renal insufficiency which resolved progressively with the optimization of the treatment. She needed antibiotic treatment due to a sepsis caused by Enterococcus faecalis.
Patient 3, during his stay in the Intensive Care Unit, in the context of right ventricular failure, required peritoneal dialysis associated with diuretic treatment. Thoracic closure was performed 3 days postoperatively. He developed complete atrioventricular block requiring definitive epicardial pacemaker implantation two weeks after surgery. Thirty-six hours after pacemaker implantation, the patient was extubated.
Intensive Care Unit stay of patient 1, 2 and 3 was 29, 22 and 24 days, respectively; total hospital stay was 65, 64 and 43 days, respectively.
The follow-up time has been 59 months in patient 1, 49 months in patient 2 and 10 months in patient 3.
At 6 months of follow-up, patient 1 required a stent implantation in the right pulmonary branch, and at 42 months he underwent angioplasty of the previously implanted stent, and a stent implantation in the right ventricle-pulmonary artery conduit was performed.
Patient 2 progressively developed stenosis of the right ventricle-pulmonary artery conduit during follow-up, so 16 months after the first surgery, a 14mm Hancock valved conduit was implanted. In addition, during this intervention a moderate-severe obstruction of the left ventricular outflow tract was observed and repaired in the same surgical act.
In the out-patient postoperative cardiac examinations of the third patient, a residual ventricular septal defect of approximately 3mm was observed. Moreover, at the level of the aortic arch there was an area of coarctation distal to the take-off of the supra-aortic trunks with a minimum diameter of 2.8mm and a maximum gradient of 90–100mmHg, without clear diastolic extension. In addition, stenosis was observed at the junction of the ascending aorta with the arch, with a maximum gradient of 40mmHg. The first therapeutic option was balloon angioplasty, which was performed 3 months after initial surgery, without significant changes. In view of this situation, elective priority surgery was indicated to repair the arch and close the ventricular septal defect. During the surgery, we found a severe endovascular reaction to the aortic arch patch, with formation of a 2mm thick fibrous tissue covering circumferentially the entire portion of the previously augmented aortic arch (from Damus–Kaye–Stansel anastomosis to post-ductal aorta), leaving only a 4–5mm patent lumen. This reaction was also observed on the external surface of the patch. Contegra's conduit was normofunctioning. The residual ventricular septal defect was localized at the level of the septal leaflet of the tricuspid valve. We performed an aortic arch augmentation with heterologous bovine pericardial patch and we closed the residual ventricular septal defect with simple U stitches. The intraoperative epicardial echocardiography showed an adequate arch enlargement, and no residual intracardiac lesions. The thorax was closed primarily, and the patient was transferred to the Intensive Care Unit. During his stay in the Intensive Care Unit there was a tendency to arterial hypertension which required aggressive intravenous treatment. The patient was extubated at 48h from admission to the Intensive Care Unit. On the fourth postoperative day, he was discharged from Intensive Care Unit to the hospital ward, and on postoperative day 16 he was discharged home.
All patients are alive and doing well at last follow-up, with adequate objective echocardiographic biventricular function.
DiscussionThe Yasui operation is a useful approach for the management of patients with interrupted aortic arch and critical left ventricular outflow tract obstruction.6 Since its publication in 1987, several case reports or small series have described the utility of this operation.6,8–21
The Yasui procedure does have an advantage compared with a Norwood single-ventricle approach for two important physiologic reasons. First, a two-ventricle repair results in normalization of the circulation. Secondly, the Yasui repair also results in fully saturated blood going to the systemic circulation. In contradistinction, the Norwood procedure creates a “parallel circulation,” which has proven to be a far more tenuous physiology.3
Biventricular repair in this spectrum of patients can be performed in a single stage (primary Yasui procedure) or in different stages, and both procedures have advantages and disadvantages.
Initial palliation for staged patients consists of a Norwood type aortic arch reconstruction, which includes a Damus–Kaye–Stansel anastomosis. Pulmonary blood flow is provided by a modified Blalock–Taussig shunt or a right ventricle-pulmonary artery shunt. The following stage would consist in channeling the ventricular septal defect to the semilunar valves (joined by a Damus–Kaye–Stansel anastomosis) with enlargement of the ventricular septal defect (if necessary) and creation of right ventricle-pulmonary artery continuity with a valved conduit (and removal of the previous shunt).2
The approach of performing a multistage repair has certain advantages: the intracardiac part of the surgery (ventricular septal defect closure) would be performed in older (and bigger) patients, away from the fragile neonatal period. It also gives us the possibility of inserting a larger conduit between the Right ventricle and the pulmonary artery.22 This strategy avoids the initial surgical complexity of primary corrective surgery, and allows a period of growth prior to final biventricular repair, which may facilitate better selection of candidates, as there may be some patients who will benefit from a univentricular physiology and who could not otherwise have been identified, which is especially important in the case of impaired left ventricular function.21 On the other side, we can identify a few disadvantages. First of all, this physiology is associated with significant operative and short-medium term mortality, which reflects that these patients present a more complex postoperative period; secondly, we leave them longer time with a “parallel circulation”, with desaturated blood reaching the systemic circulation. There is no evidence in the literature to support the idea that this physiology is better tolerated in patients with two adequately formed ventricles.22
The advantage of the single-stage approach is the early normalization of the circulation, creating a two-ventricle system with fully saturated blood going to the systemic circulation. This approach also increases the interval to the next reoperation compared with an initial Norwood procedure. Disadvantages of the single-stage approach include a more technically demanding operation, with prolongation of the crossclamp and bypass times, insertion of small conduit sizes, and possibly a higher risk for surgically induced heart block.3
The placement of a neonatal conduit implies the replacement of the conduit within 3–4 years. Therefore, with either approach, these patients are likely to undergo at least two operations in their first 3 years of life.22
For the above reasons, in recent years we have chosen to perform the Yasui procedure in a single stage. In addition, different series have been published evaluating the results of a primary Yasui procedure, with excellent results.6,21,23,24
Another surgical option that could be used for patients with critical aortic stenosis/atresia, interrupted or hypoplastic aortic arch, ventricular septal defect, and a normal-sized left ventricle, is the neonatal Ross–Konno operation with aortic arch repair. This option would result in a two-ventricle system, and thus confer the same physiologic advantages as the Yasui with respect to normalization of the circulation. But the Ross–Konno option does not circumvent the issues associated with right ventricular outflow tract conduits.3 A point in favor of the Yasui operation, is the added technical difficulty of coronary harvesting and reimplantation in the Ross–Konno operation in neonates with diminutive ascending aorta. When there is a large size discrepancy between the pulmonary and aortic annulus, reimplanted coronary arteries may end-up in an unusual position, which can result in coronary insufficiency.5
In our opinion, the arterial translocation that must be performed in these patients to perform a Ross procedure involves a very short distance mobilization, which, together with the great difficulty of translocating the coronary ostium in a patient with a diminutive aorta, does not justify the complexity of performing such an operation.
The benefits of the Yasui procedure compared with alternative treatment strategies are difficult to evaluate because of the relative rarity and heterogeneity of this heart defect.3
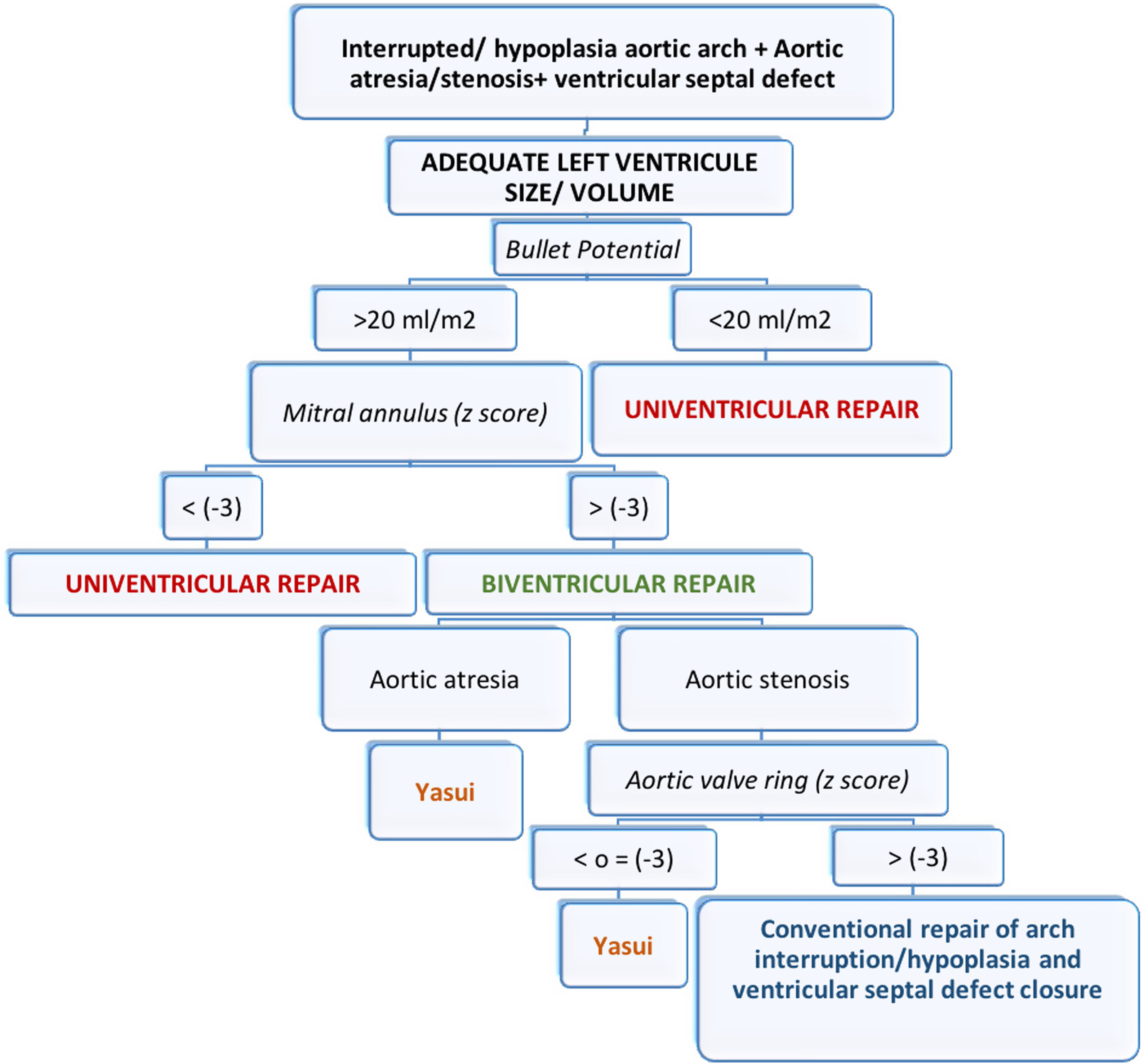
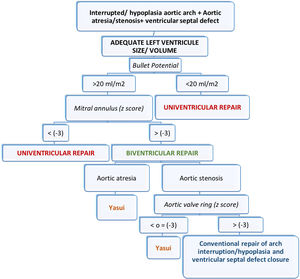
In certain circumstances the left ventricle instead of being small, globular and underdeveloped, is rather flattened or even crescentic, compressed by a dilated right ventricle with overloaded pressure and volume. In such conditions of distorted left ventricle geometry, small preoperative left ventricle volumes may not accurately predict the feasibility of biventricular repair.25 The septum should adopt a normal position after the operation normalizes the loading conditions. Therefore, the postoperative capacity can best be predicted by the preoperative left ventricle potential volume, which is the volume if the septal position were normal,25 and this is what we consider as the “potential Bullet”. Several studies have now demonstrated that 15–20ml/m2 is the minimal left ventricle volume necessary to support the systemic circulation.25,26
If we obtain a potential Bullet volume greater than 20ml/m2 and the mitral annulus has a z-score greater than −3, we consider a biventricular repair in our patient. Like us, other authors set a mitral annulus with a z-score greater than −3 as the limit for considering a biventricular repair.22
After assessing the possibility of biventricular repair, the next step would be to decide on the best surgical strategy. If the patient has aortic atresia, the indication is to perform a Yasui procedure (this would be the case of our third patient). If the patient has aortic stenosis, we must assess the size of the aortic annulus. If the aortic annulus has a z-score of less than −3, we perform a Yasui procedure. If the z-score is greater than −3, we consider conventional repair of arch interruption/hypoplasia and ventricular septal defect closure (z-scores were calculated according to the data from of Pettersen et al.).27
The size of the aortic annulus to consider performing a Yasui procedure varies from one author to another. Carrillo et al. perform this procedure when the subaortic and valvular area (measured in millimeters) is less than the patient's weight (measured in kilograms), or when the aortic valve has a z-score of less than −3.5 Kanter et al. perform Yasui when the diameter of the left ventricular outflow tract (valvular or subaortic region) is less than 4mm; when it is between 4–4.5mm, both a Yasui procedure and conventional repair are considered.2
Fig. 3 shows a schematic representation of our above mentioned protocol.
ConclusionIn patients with critical aortic stenosis/atresia, interrupted or hypoplastic aortic arch, ventricular septal defect, and a normal-sized left ventricle, we advocate for primary neonatal repair with the Yasui operation, as we believe that the sooner the infant reaches a normal physiologic state, the better will be the long-term outcome. In addition, the immediate postoperative course of patients with a complete repair is less complicated than in those with an initial palliative procedure.
We can conclude that in experienced centers, primary Yasui repair can be performed in neonatal period with satisfactory results, low mortality and good left ventricular function in the short-medium term. Current series with a larger number of patients and long-term follow-up are needed to demonstrate the superiority of the Yasui procedure over other treatment alternatives.
Financial supportThis research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interestNone.
To the entire team of Pediatric Congenital Cardiac Surgery and Pediatric Cardiology of the 12 de Octubre University Hospital, for their support, advice and fundamental help in the preparation of this article.