The use of a new chemotherapy as adjuvant treatment of colorectal cancer is not free of complications. Monoclonal antibodies are associated with bleeding and intestinal perforations.
ObjectiveTo report the case of a patient who developed a serious complication after treatment with an antiangiogenic drug for colorectal neoplasm.
Clinical caseThe case is presented of a 42-year-old male operated on due to subocclusive rectal cancer with metástasis at the time of diagnosis. Sixteen months after surgery during second-line adjuvant therapy, an intestinal perforation was observed with haemorrhage and intestinal leak to retroperitoneum and left lower extremity. Despite intensive medical and surgical treatment this complication had fatal consequences.
ConclusionsFuture research should be directed at obtaining biomarkers for the specific use of antiangiogenic agents in order to decrease the rate of adverse factors.
La quimioterapia adyuvante en el tratamiento del cáncer colorrectal no está exenta de complicaciones. Los anticuerpos monoclonales se han asociado a sangrado y a perforaciones intestinales.
ObjetivoPresentar el caso de un paciente tratado con un antiangiogénico por una neoplasia colorrectal avanzada, que presentó una grave complicación asociada al tratamiento.
Caso clínicoPaciente de 42 años intervenido de neoplasia rectal en obstrucción con metástasis en el momento del diagnóstico. Dieciséis meses después de la cirugía, durante el tratamiento adyuvante de segunda línea, presentó una perforación intestinal acompañada de rectorragia y fístula intestinal a retroperitoneo y a extremidad inferior izquierda. A pesar del intenso tratamiento quirúrgico y médico, esta complicación tuvo fatales consecuencias.
ConclusionesLas futuras investigaciones deberán estar encaminadas a la obtención de biomarcadores, para adecuar el uso de este tipo de antitumorales con el fin de disminuir el índice de factores adversos.
Deaths from colorectal cancer have reduced dramatically in recent years.1 Screening programmes as well as the development of new chemotherapy treatments have contributed to this. The recent incorporation to treatment of the antiangiogenic therapies, which include aflibercept, has enabled patients with advanced colorectal cancers to improve the time to progression of their disease and overall survival.2 However, these new therapies have toxicities that differ from those of usual chemotherapy and can occasionally be fatal,2 and therefore need to be taken into consideration.
ObjectiveTo present the case of a patient treated with an antiangiogenic for advanced colorectal cancer who presented with a serious complication and to undertake a literature review on the subject.
Clinical caseWe present the case of a 42-year-old man with no relevant history or known drug allergies. In August 2013 he was admitted with intestinal subocclusion symptoms secondary to a stenosing cancer of the rectum and sigmoid colon with liver metastases on the staging study. Surgery was decided as the initial treatment, performing a laparoscopic anterior resection of the rectum, with reconstruction by mechanical colorectal anastomosis (CEEA 28mm) and metastasectomy of liver segments VI, VIII and IV. The anatomopathological study reported intermediate grade (G2) adenocarcinoma of the rectum that was infiltrating serosa with perineural invasion pT4 N1 (GL 2+/12) M1, and 2 of the liver fragments with metastases of 1.5cm and 1.2cm with free margins of 0.5cm and 0.1cm, respectively (segments VI and IV).
During follow-up in June 2014, new liver metastases in segment VIII were detected, and a right regulated hepatectomy performed. Two months later with persistently elevated markers, adjuvant treatment was started with several batches of chemotherapy, despite which the tumour markers remained elevated (CEA, Ca 19-9). Therefore a positron emission tomography was performed revealing uptake at the level of the anterior superior iliac spine, for this reason pelvic radiotherapy treatment was started. In subsequent checks multiple inoperable liver and lung metastases were diagnosed, and after consulting the Tumour Committee, the choice was made to start new cytostatic therapy using aflibercept (dose 4mg/kg bodyweight every 2 weeks).
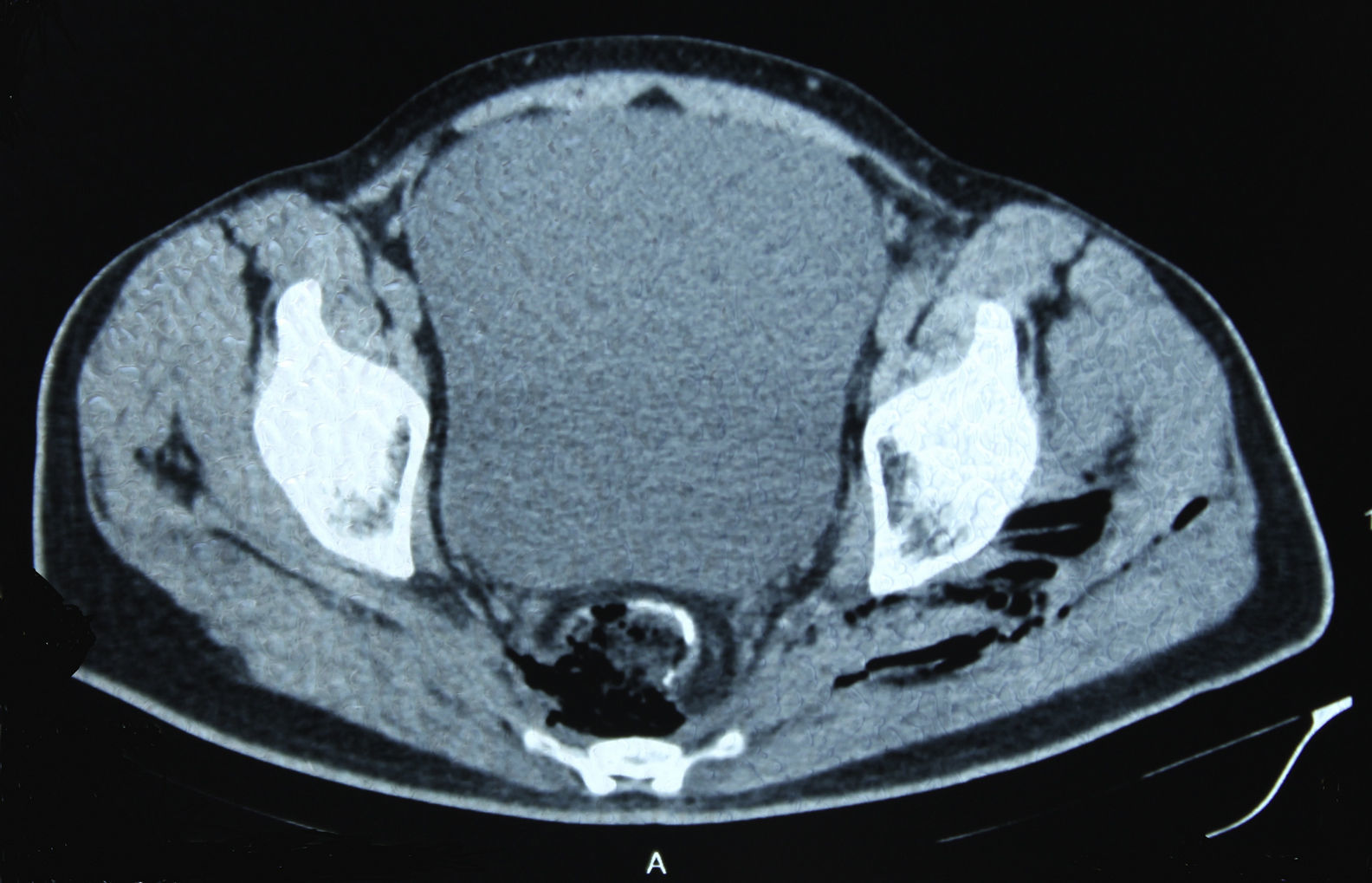
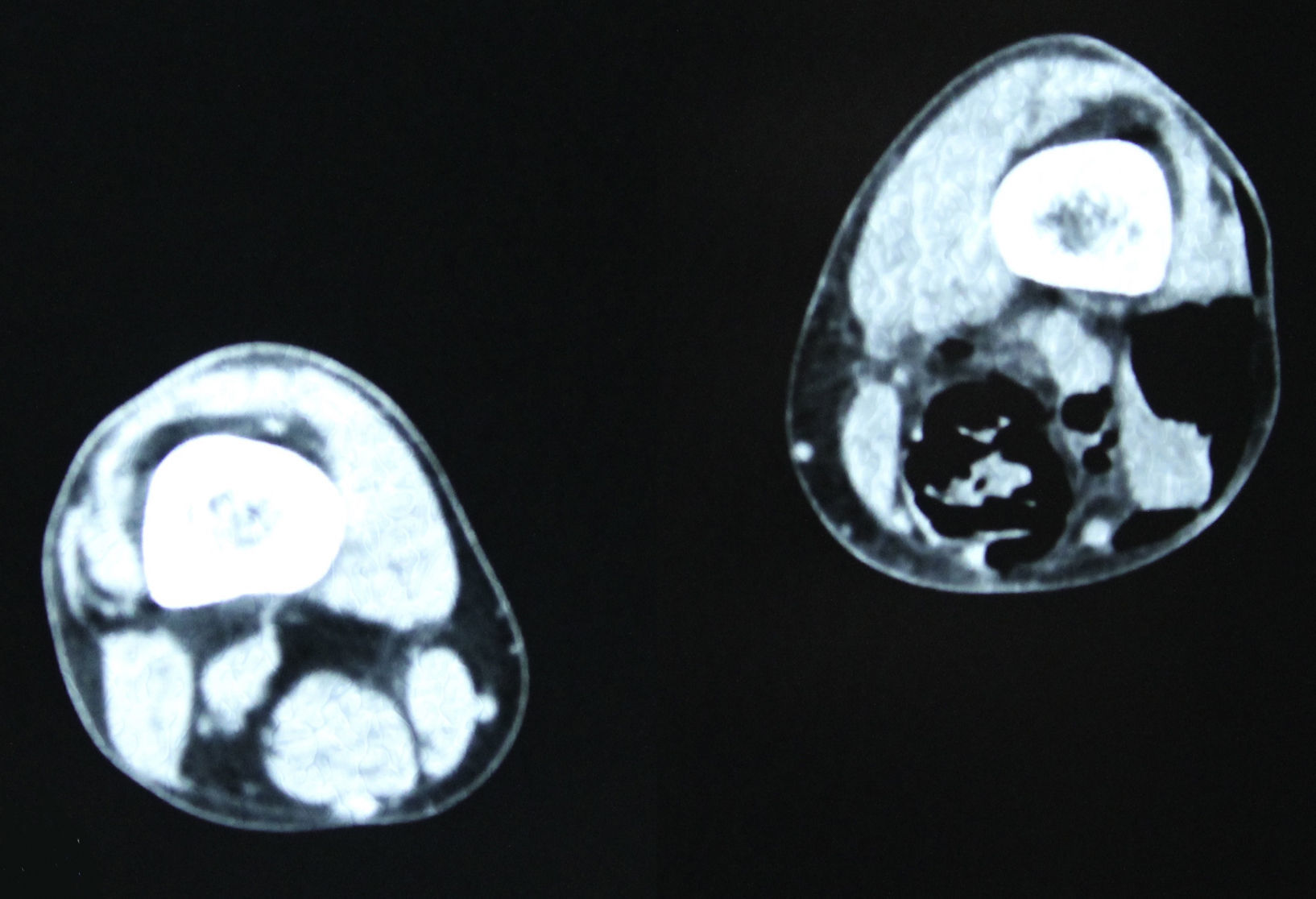
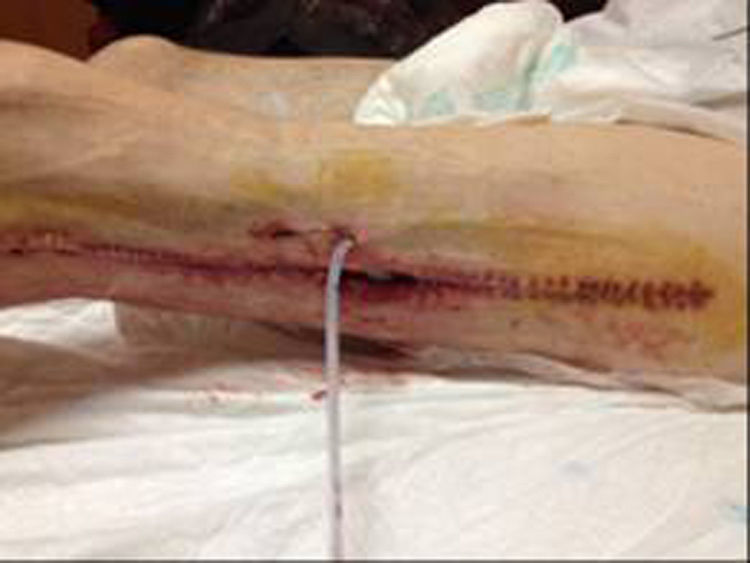
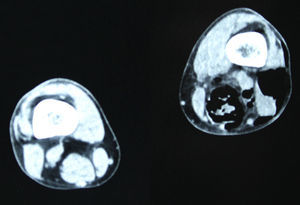
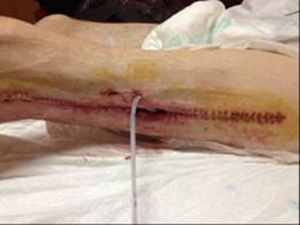
In December 2014 during treatment with aflibercept (4 cycles), the patient attended the Emergency Department with pain in the lower left limb, associated with increased calibre and crepitus on the lateral external face, from the thigh root to the patellar area. Computed tomography (Figs. 1 and 2) revealed air spaces between the muscle planes the length of the lower left limb. The probable cause was spontaneous perforation at the level of the rectal anastomosis, with retrocutaneous fistulation to the lower extremity. Emergency surgical revision was proposed and the following performed: loop colostomy at the level of the left iliac fossa, wide fasciotomy of the extremity, flushing and drainage of a large abscess located the length of the entire lower left limb (Fig. 3).
Twelve days later, with the persistent ileum and the abundant purulent drainage through the fasciotomy despite monitoring and daily treatments, a definitive terminal colostomy was made in the left iliac fossa. During the postoperative period, the patient presented symptoms of paralytic ileus, which was resolved conservatively, and a self-limiting episode of rectorrhagia accompanied by bleeding through the surgical wound of the lower extremity, which triggered haemodynamic repercussions and required transfusion of 4 packed red blood cell units. When the patient had stabilised, the symptoms of bleeding stopped but abundant purulent drainage persisted from the lower limb wounds, and therefore Vacuum Assisted Closure™ was commenced. The patient gradually showed clinical improvement, his wounds progressed favourably and granulated by second intention enabling him to be discharged a month after his admission to hospital. Forty-eight hours later he was readmitted with further symptoms of massive rectorrhagia through the colostomy and abundant bleeding through the left thigh wounds, with major haemodynamic repercussions requiring a further transfusion of packed red blood cells. Over the following hours, despite the therapeutic measures to stabilise the patient, incoercible bleeding persisted which eventually caused his death due to hypovolemic shock after catastrophic haemorrhage.
DiscussionColorectal cancer is the third most frequently diagnosed cancer in men and second in women. Its mortality rate has decreased progressively since 1980, which might be explained by early detection of the disease and the emergence of new, more effective and individualised adjuvant and neoadjuvant therapies. However, in cases that have metastasised by the time they are diagnosed, about 20%, survival at 5 years is no more than 7%.3
The monoclonal antibodies should be mentioned as part of the therapies targeting these patients. These include bevacizumab which, associated with fluorouracil, leucovorin and oxaliplatin (FOLFOX), currently constitutes the first line therapy for these patients. Its mechanism of action is based on the control of angiogenesis. Other antiangiogenic antibodies with different mechanisms of action such as the vascular endothelial growth factor inhibitor or anti-VEGF (aflibercept) and tyrosine kinase inhibitor (sorafenib, sunitinib, vandetanib, pazopanib, etc.) act to inhibit angiogenesis by blocking the vascular endothelial growth factors (VEGF) and have equally demonstrated a clear clinical benefit in the management of various solid tumours.4
Within these new therapies we shall focus on aflibercept, since this is the drug we used to treat the patient in this case. It is a recombinant human protein with antiangiogenic effect, which behaves as a decoy receptor to block VEGF A and B and the placental growth factors. According to various studies, it has shown better ubiquitous efficacy in tumours whose growth depends on pathologic angiogenesis.5 Therefore the current recommendation is that they should be used as second line treatment for patients with colorectal carcinoma metastases in combination with 5-fluorouracil, leucovorin and irinotecan (FOLFIRI) and for resistant patients or those who have presented progression after treatment with oxaliplatin.5 However, although studies have demonstrated that this combination improves survival and disease-free time in this group of metastatic patients, there is also sufficient evidence to confirm that the use of aflibercept in the treatment of patients with solid tumours is associated with a higher risk of fatal adverse events,6 a greater incidence of major haemorrhagic events4 and a statistically significantly greater risk of gastrointestinal perforation.5 Haemorrhagic events associated with aflibercept have their origin in the anti-VEGF mechanism.
VEGF has many actions on the vascular wall to regulate permeability and proliferation, and their inhibition, and therefore causes changes to the wall which predispose to haemorrhagic phenomena.4
However, the pathogenesis of perforations associated with antiangiogenics is not so well known. Various mechanisms have been proposed such as the prior existence of damage to the intestinal wall due to post-chemotherapy colitis, diverticulitis, gastric ulcer or tumour necrosis, or due to thromboembolic phenomena that cause intestinal ischaemia and subsequent perforation. In research mice, it has been confirmed that antiangiogenic drugs cause regression of the intestinal villi, which might encourage the development of microperforations.7 Another significant complication related to aflibercept is the onset of infections, including high grade infections that compromise survival. In the patient in this case this was manifested by a major soft tissue infection in the lower extremity secondary to retrorectal progression of the anastomotic perforation. The mechanism that triggers infections associated with aflibercept remains unknown. The most accepted theories proposed are the development of aflibercept-related neutropenia or that inhibition of the VEGF receptor could block haematopoietic stem-cell cycling.8
On the other hand, it is also known that the combination of stereotaxic radiotherapy and antiangiogenic drugs significantly increases severe intestinal damage, compared to the independent use of either therapy.9
The patient in this case presented all of the described factors at once: a solid metastatic tumour treated with previous chemotherapy regimens and pelvic radiotherapy at high doses before rescue therapy with aflibercept due to progression of the disease. After reviewing different meta-analyses, the greater incidence of haemorrhage, infection and perforation associated with the use of this drug, considering the type of patient, contributed to these side effects and triggered a torrent of processes that proved fatal, as we present. However, and despite all of this, in the current clinical scenario and after our literature review, its use remains clearly justified provided the approved indications are followed.7 In the knowledge that aflibercept is effective in a specific type of patients and that it is highly toxic,10 since it presents a 46% infection rate, 4.2% major haemorrhage rate, and 1.9% rate of gastrointestinal problems, it is essential that its use is optimised. Studies should be geared towards discovering predictive biomarkers, avoiding use of the drug in patients with known risk of bleeding from gastroduodenal ulcer, haemoptysis, etc., and promptly managing intestinal perforations in order to minimise morbidity and mortality.11
ConclusionsFuture studies should be aimed at discovering predictive biomarkers to enable the optimal use of aflibercept in patients with solid tumours, in order to reduce the incidence of fatal adverse events such as those presented in this case.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interest to declare.
Please cite this article as: Echazarreta-Gallego E, Elía-Guedea M, Córdoba-Díaz de Laspra E. Devastadora complicación tras tratamiento con aflibercept. Cir Cir. 2017;85:260–263.