Aortic stenosis is a frequent disease in the elderly, and is associated with other systemic pathologies that may contraindicate the surgical procedure. Another option for these patients is percutaneous aortic valve implantation, which is less invasive. We present our initial experience with this procedure.
Material and methodsPatients with aortic stenosis were included once selection criteria were accomplished. Under general anaesthesia and echocardiographic and fluosocopic control, a transcatheter aortic valve was implanted following valvuloplasty. Once concluded the procedure, angiographic and pressure control was realised in order to confirm the valve function.
ResultsBetween November 2014 and May 2015, 6 patients were treated (4 males and 2 females), with a mean age of 78.83±5.66 years-old. The preoperative transvalvular gradient was 90.16±28.53mmHg and posterior to valve implant was 3.33±2.92mmHg (P<0.05). Two patients had concomitant coronary artery disease which had been treated previously. One patient presented with acute right coronary artery occlusion which was immediately treated. However due to previous renal failure, postoperative sepsis and respiratory failure, the patient died one month later.
ConclusionIt was concluded that our preliminary results showed that in selected patients percutaneous aortic valve implantation is a safe procedure with clinical improvement for treated patients.
La estenosis valvular aórtica es frecuente en adultos mayores, y asociada a otras enfermedades puede contraindicar el procedimiento quirúrgico. Otra opción para estos pacientes es un sistema de prótesis valvular aórtica implantado por vía transcateterismo con menor riesgo. Presentamos nuestra experiencia inicial con esta modalidad de tratamiento.
Material y métodosSe seleccionaron pacientes con estenosis aórtica que reunían los criterios de selección. El procedimiento se realizó bajo anestesia general, con apoyo de fluoroscopia y ecocardiografía transesofágica, por acceso femoral. Previa vavuloplastia aórtica, se implantó la prótesis valvular en posición aórtica, se realizó control angiográfico y se midieron presiones para verificar la posición y la funcionalidad.
ResultadosEntre noviembre de 2014 y mayo de 2015 se trataron 6 pacientes (4 varones y 2 mujeres), con una edad promedio de 78.83±5.66 años. El gradiente transvalvular promedio previo al procedimiento fue de 90.16±28.53mmHg, y el posterior al implante, de 3.33±2.92mmHg (p<0.05). Dos pacientes tuvieron además enfermedad arterial coronaria, la cual se trató en un tiempo quirúrgico previo. Un paciente presentó oclusión aguda de la coronaria derecha durante el implante protésico, la cual se resolvió de manera satisfactoria, y debido al antecedente de insuficiencia renal e insuficiencia aórtica posprocedimiento falleció al mes del procedimiento por sepsis y falla respiratoria.
ConclusiónEsta experiencia inicial muestra resultados satisfactorios en la colocación de una prótesis valvular aórtica percutánea y se demuestra la factibilidad del procedimiento para nuestro medio en casos bien seleccionados.
When the correct clinical conditions apply, the standard treatment for aortic valve disease has been the replacement of the valve with a prosthesis that is either mechanical or biological through heart surgery with cardiopulmonary bypass.1,2
Expected mortality for this type of procedure is approximately 3%, although it depends on the patient's concomitant disease which may contraindicate the surgical procedure despite the need to treat the impaired valve. It has thus been the case that high risk patients or those with complications have never been offered surgery by the attending physician and in these conditions up to 33% of patients miss the opportunity to be assessed for surgical treatment.1–3
Less invasive procedures have been developed for this type of patient, aimed at offering them a more favourable recovery, with replacement of the aortic valve through mini sternotomy instead of standard total sternotomy. This approach allows patients to have an earlier recovery.4 There is, however, a group of patients for whom this approach is still risky and they cannot be given any other option except medical treatment to improve the quality of their lives but not survival. Under these circumstances and with the technological advances of recent years, a valvular prosthesis system was designed which could be implanted through the femoral or iliac artery and with fluoroscopic control. The first aortic valve prosthesis implanted with this type of approach was performed by Cribier et al. In 2002.5 A good number of patients have now been implanted with this type of prosthesis worldwide, which has led to a considerable improvement in their survival and quality of life.6–8 It is important to mention that at present aortic valve stenosis is the most frequent valve disorder, especially in elderly patients, who in the majority of cases also present with associated systemic diseases.1 In our environment this therapy modality for treating native aortic valve stenosis is recent. We present our initial experience with this type of prosthesis and the results obtained in a group of patients.
Material and methodsBetween 1st November 2014 and 31st May 2015 were selected with the already established inclusion criteria accepted in international medical literature, and were offered the option of treatment with an endovascular device for aortic valve replacement to be implanted with peripheral venous access.
Criteria for referral to this procedure were: patients with a diagnosis of critical aortic valve stenosis (area of aortic valve <1cm2 measured by echocardiography), with a mean gradient of aortic pressure of >40mmHg, elevated surgical risk (EuroSCORE>20%) and/or rating >10%1 from the Society of Thoracic Surgeons. In the case of coronary arterial disease, this was to be previously treated using coronary angioplasty.
Preparation for the procedure included measuring the aortic valve ring using computed tomography with slices gated at aortic valve level and using transthoracic and transesophageal echocardiography.
An echocardiogram, computed angiography and cardiac catheterisation with aortogram and coronariography in addition to pressure measurement was performed on all patients.
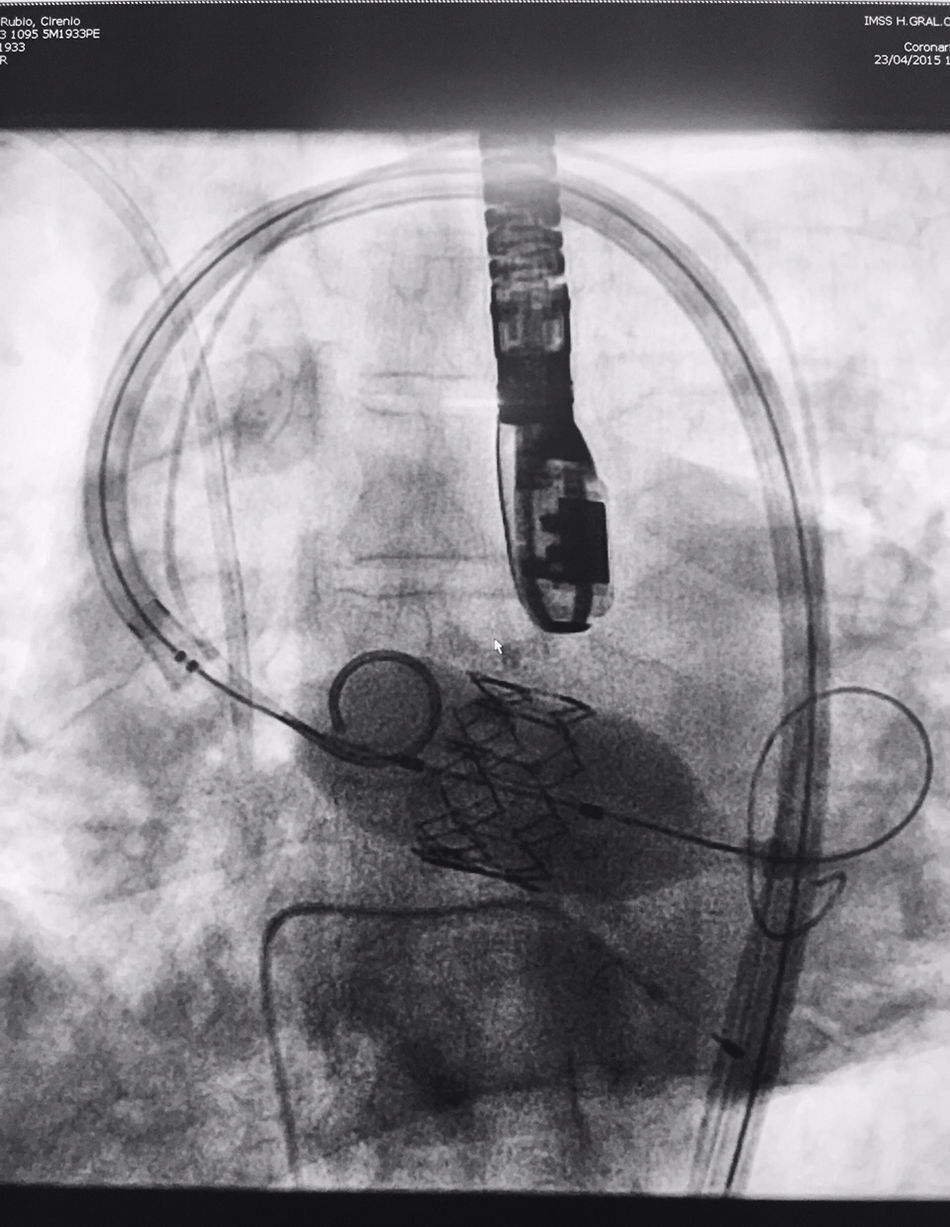
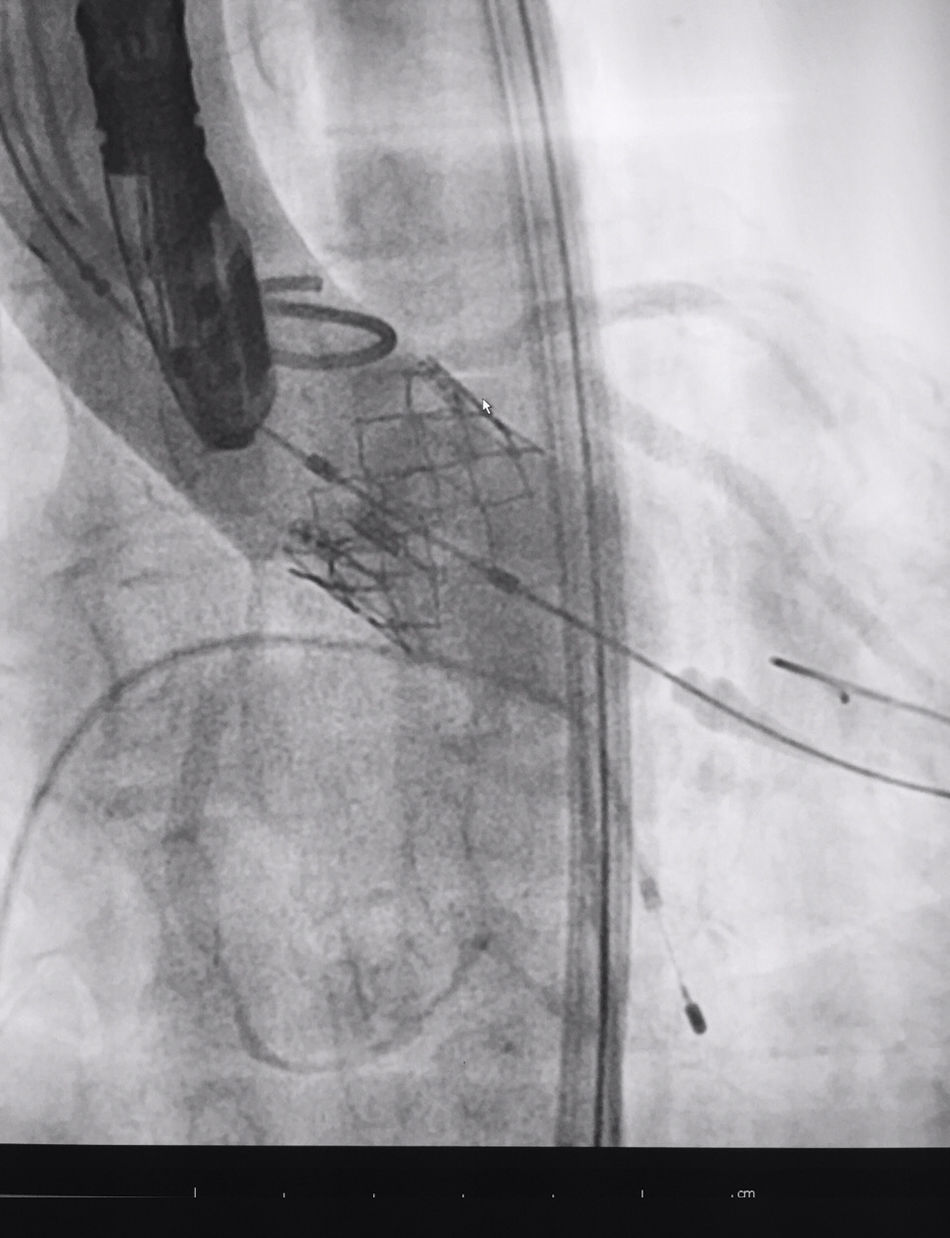
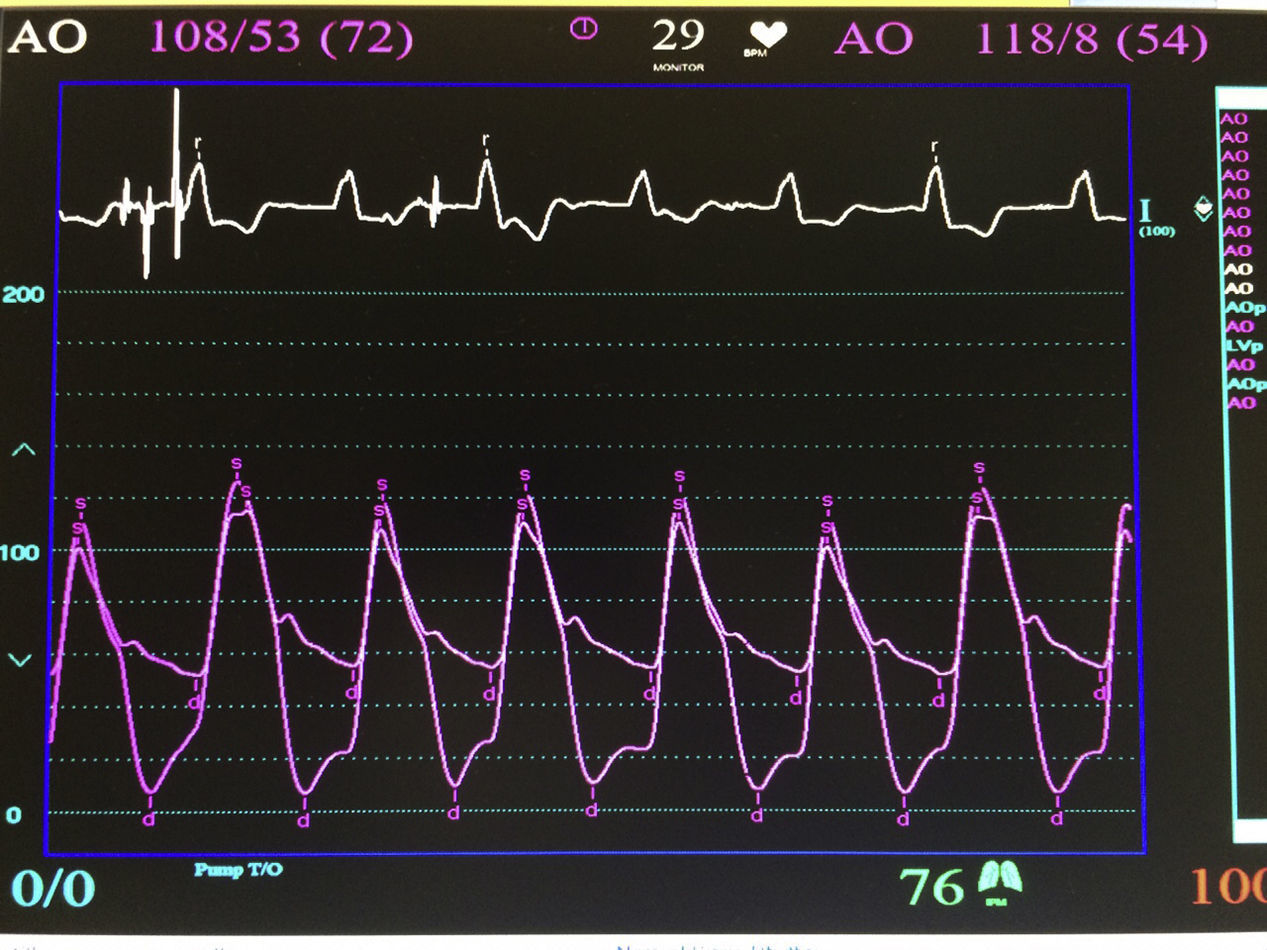
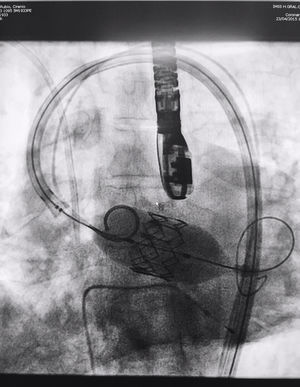
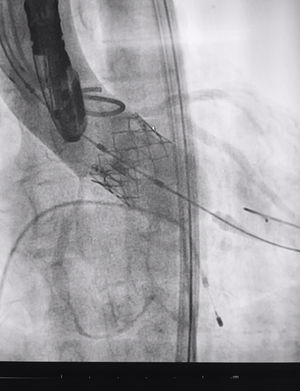
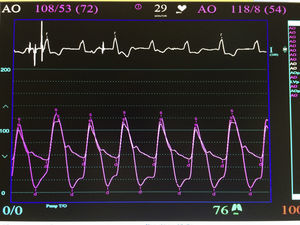
The procedure was performed under general anaesthesia, intubation of the airway and with prior monitoring through radial arterial catheter, central venous catheter, vesical catheter and electrocardiogram. Transfemoral access was used with surgical exposure of the femoral artery in all cases and transesophageal echocardiographic and fluoroscopic control of images. A temporary electrode for an endocardial pacemaker in the right ventricle was implanted. After this balloon aortic valvuloplasty was performed and angiographic control with cardiac stimulation to 180lpm. Later, once the patient had been haemodynamically stabilised, the valve was implanted, through the femoral artery and the aorta and with angiographic control, at aortic ring level, where again with pacemaker stimulation at 180lpm, it was attached to the wall by dilating the valve (Fig. 1). Pacemaker stimulation was suspended, and once cardiac frequency and mean blood pressure had been stabilised, valve function was assessed by transophageal echocardiogram. Once correct valve function had been confirmed and the presence or non presence of periprosthetic leakage had been confirmed, angiographic images and pressure strokes were taken in the left ventricle and the aortic root (Figs. 2 and 3) and the endovascular catheters were subsequently removed so as to proceed with closure of vascular accesses and surgical site.
Echocardiogram with trans-esophageal transducer and fluoroscopy were used for procedure control.
The valve used was Sapien XT® (Edwards Lifesciences Inc., Irvine, CA, U.S.A.), with diameters of 23mm and 26mm in accordance with anatomical measurements of the patient's valve ring and the distance between the ring and the coronary ostia.
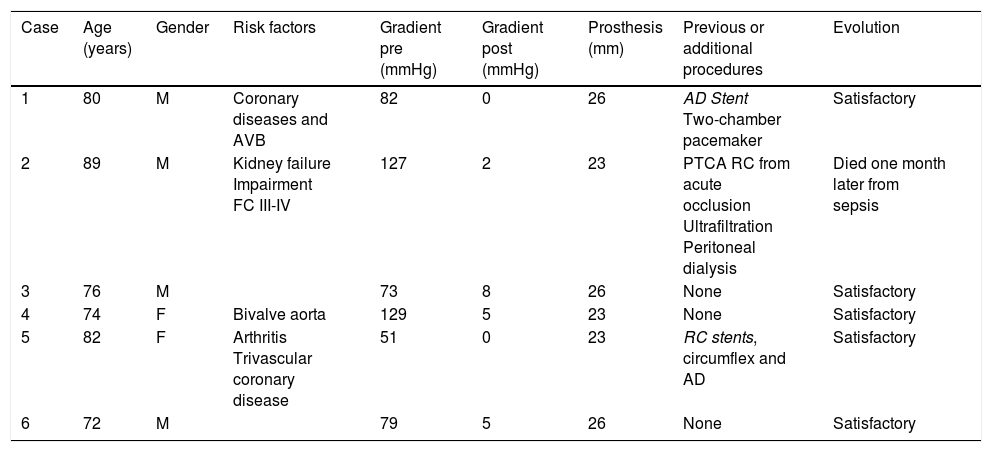
ResultsBetween 1st November 2014 and 31st May 2015 6 aortic valves were implanted using this technique. Four were implanted in men and 2 in women (Table 1), with an average age of 78.83±5.66 years (range between 72 and 89). The average transvalve gradient prior to the procedure was 90.16±28.53mmHg (range between 51 and 129), and after the implant, was 3.33±2.92mmHg (range between 0 and 8) (p<0.05).
Patient characteristics.
| Case | Age (years) | Gender | Risk factors | Gradient pre (mmHg) | Gradient post (mmHg) | Prosthesis (mm) | Previous or additional procedures | Evolution |
|---|---|---|---|---|---|---|---|---|
| 1 | 80 | M | Coronary diseases and AVB | 82 | 0 | 26 | AD Stent Two-chamber pacemaker | Satisfactory |
| 2 | 89 | M | Kidney failure Impairment FC III-IV | 127 | 2 | 23 | PTCA RC from acute occlusion Ultrafiltration Peritoneal dialysis | Died one month later from sepsis |
| 3 | 76 | M | 73 | 8 | 26 | None | Satisfactory | |
| 4 | 74 | F | Bivalve aorta | 129 | 5 | 23 | None | Satisfactory |
| 5 | 82 | F | Arthritis Trivascular coronary disease | 51 | 0 | 23 | RC stents, circumflex and AD | Satisfactory |
| 6 | 72 | M | 79 | 5 | 26 | None | Satisfactory |
PTCA: percutaneous transluminal coronary angioplasty; AVB: AV block; RC: right coronary; FC: functional class; AD: anterior descending; F: female; M: male; post: post procedure; pre: prior to procedure.
Two patients also had coronary artery disease which was treated prior to surgery for valve implantation with drug-eluting coronary stents. One patient had also been implanted with a two-chamber endocardial pacemaker due to a conduction system disease. One patent with a diagnosis of bivalve aorta disease mild aortic impairment with no haemodynamic or clinical repercussions.
One patient (the second case) presented with acute occlusion of the right coronary artery during valve implantation, which was satisfactorily and immediately resolved with a drug-eluting stent. There were no signs of myocardial infarction during the patient's evolution. However, due to the background of kidney failure and postoperative aortic failure, the patient presented with exacerbation of kidney failure, heart failure and stroke with neurological recovery. The patient died one month after the procedure from sepsis and respiratory failure.
The other cases progressed satisfactorily with an average hospital discharge of 7 days, with no complications in follow-up, the mean of which was 4.5 months, and with a range of between 3 and 8 months.
DiscussionThe treatment of aortic valve disease, as has already been commented upon, basically involves replacing the valve.1,3 However, a person over 80 or with a EuroSCORE>20% was considered to have contraindications for surgery.1,8,9 until the appearance of the valve implant option through peripheral venous access.5
This type of procedure has been under analysis because it was a new alternative which involved a series of considerations such as risk-benefit, cost of resource, appropriate patient selection and associated complications, among other variables.10,11 It has been demonstrated that with the use of a peripheral venous approach hospital stay time is lower than with conventional surgery.9 However, paravalvular leakage, adverse vascular events and the need for a definitive postoperative pacemaker is higher with the vascular approach.9,10 These situations should be taken into consideration due to clinical repercussions. In the majority of cases the paravalvular leakage is mild and has no significant repercussions, especially in the cases where there is a previous double aortic lesion (stenosis and valve failure) confirmed by medical assessment and additional studies such as echocardiogram or cardiac catheterisation.12
In one review Cao et al.9 reported that approximately 40,000 transcatheter aortic valve implants were not able to be medically justified or based on cost-benefit, because in the medium term mortality in cases treated with surgery and with transcatheter approaches were similar. In contrast, patients who had not been referred to surgery and who benefited from a peripheral venous access valve implant had better survival than with standard medical treatment, although a greater complication rate of cerebral vascular events was observed.9
Based on these findings, it is important to consider that patients referred to transcatheter aortic valve surgery only when there is very solid clinical evidence and not due to personal preference of the patient who rejects open heart surgery or the attending physician who is keen to market it, nor in cases where the patient has a short life expectancy due to another disease.1 Furthermore, the iliac vessels should be between 6 and 9mm in diameter, the patient should have no history
aorto femoral surgery and there should be no torsion in the aorta, abdominal aneurism or severe atheroma in the aortic arch.1
In the majority of cases surgery is performed under general anaesthesia although in patient groups with greater experience it may be sufficient to apply sedation and analgesia for a transfemoral approach.1 Intensive therapy postoperative care is required for at least 24h after surgery for the follow-up of kidney function and monitoring of blood and venous accesses.1
Transcatheter aortic valve implantation is considered to be successful when an aortic valve area of between 1.5 and 1.8cm2 is obtained; observed survival at 2 years is between 70% and 80%, and mortality at 30 days is between 5% and 18%.1 In contrast, a frequency of vascular complications of between 10% and 15% has been identified, vascular cerebral events of between 3% and peri procedural coronary occlusion <1%.1 Due to this type of data it is important to insist on a very careful selection of patients to be treated in this way.
In approximately 50% of cases, coronary lesions coexist with the aortic valve lesion. In our experience conservative criteria have been chosen in the treatment of previous lesions, although reports are now in existence of simultaneous treatment of coronary obstructions and valve implantations with the use of greater contrast agents, vascular accesses and other variables that should be taken into consideration.13 Here it is important to remember that coronary occlusion may present in acute form during the procedure of calcium displacement of the valve to the coronary orifices or inappropriate implantation. This is a serious and possibly even lethal complication and requires immediate treatment with mechanical circulatory support at times.14 In our experience a complication of acute right coronary closure was resolved by the implantation of a drug-eluting stent in a patient who was classified as high risk due to pre-existing kidney failure and who died the following month due to kidney failure complications and sepsis, following a prolonged hospital stay.
The findings in our patient group were those expected based on the comparison with those published in the literature we were able to consult and are promising in that they confirm that the well indicated procedure offers a different therapeutic option in our centre for aortic valve stenosis with observed clinical improvement in treated patients.
ConclusionThis initial experience shows several satisfactory outcomes in the implantation of a percutaneous aortic valve, in keeping with results reported in the international medical literature, with the required safety and it shows the viability of the procedure for our centre in well selected cases.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Careaga-Reyna G, Lázaro-Castillo JL, Lezama-Urtecho CA, Macías-Miranda E, Dosta-Herrera JJ, Díaz JG. Tratamiento de la estenosis valvular aórtica con implante de prótesis valvular transcatéter. Experiencia inicial. Cir Cir. 2017;85:375–380.