Peritoneal disseminated disease, regardless of its origin, should currently be considered a locoregional disease stage, and thus a candidate for an intention to treat therapeutic option with debulking surgery and hyperthermic intraperitoneal chemotherapy.
ObjectiveTo determine whether or not the collagen sponge with fibrinogen 5.5mg and thrombin 2IU, applied as a tissue sealant and gastrointestinal reinforcement sutures, contributes to the reduction of anastomotic leak.
Material and methodsQuasi-experimental, comparative, prospective, case/control study conducted on patients with peritoneal carcinomatosis of colorectal origin, operated on in our Peritoneal Cancer Surgery Unit from 2011 to April 2014. The study included 73 patients, 43 (59%) men and 30 (41%) women with peritoneal carcinomatosis of colorectal origin, candidates for debulking surgery and hyperthermic intraperitoneal chemotherapy. Gastrointestinal anastomoses were performed on 49 (67%) patients. These patients were randomised into 2 groups: A control (27) and B hypothesis (22) reinforced with sponge suture.
ResultsThe total number of anastomoses performed was 49 (mean: 1.9), with 27 in the control group A (mean: 1.88) and 22 in B (mean: 2.16). The debulkings achieved were: complete debulking 0.38 (77.5%), complete debulking 1: 7 (14.8%), and 4 (8.1%) did not undergo hyperthermic intraperitoneal chemotherapy due to complete debulking>2. Intestinal fistula: 3 cases (6.1%) in A group vs 0 in B.
ConclusionsIn our series, the use of a fibrinogen and thrombin sponge has contributed to a significant reduction in the risk of gastrointestinal fistulas in high risk oncology patients.
La enfermedad peritoneal diseminada debe considerarse como un estadio locorregional de la enfermedad, y por tanto, candidata a una opción terapéutica con intención curativa mediante cirugía citorreductora y quimioterapia intraperitoneal hipertérmica perioperatoria.
ObjetivoComprobar si la esponja de colágeno con fibrinógeno 5.5mg y trombina 2UI, aplicada como sellante tisular, contribuye a la reducción del número de dehiscencia anastomóticas.
Material y métodosEstudio cuasi-experimental, comparativo, prospectivo, caso y controles en pacientes con carcinomatosis peritoneal de origen colorrectal, intervenidos en nuestra Unidad de Cirugía Oncológica Peritoneal desde 2011 hasta abril de 2014. Nuestra serie consta de 73 pacientes, 43 (59%) hombres y 30 (41%) mujeres, diagnosticados de cáncer de origen colorrectal y candidatos a cirugía citorreductora y quimioterapia intraperitoneal hipertérmica perioperatoria. En 49 pacientes (67%) se realizaron anastomosis digestivas; dichos pacientes se aleatorizaron en 2 grupos: A control (27), y B hipótesis (22) con sutura reforzada con esponja.
ResultadosEl número total de anastomosis fueron 49 (x: 1.9), 27 en el grupo A (x: 1.88) y 22 en B (x: 2.16). Las citorreducciones alcanzadas fueron: citorreducción completa 0: 38 (77.5%), citorreducción completa 1: 7 (14.8%), y en 4 (8.1%) no se realizó quimioterapia intraperitoneal hipertérmica perioperatoria por citorreducción completa>2. Fístula intestinal: 3 casos (6.1%) en el grupo A (sin esponja medicamentosa) frente a 0 en el grupo B.
ConclusiónEl empleo en nuestra serie de la esponja de fibrinógeno y trombina ha contribuido a una reducción significativa del riesgo de fístulas digestivas, en pacientes oncológicos de alto riesgo.
At present, peritoneal disseminated disease or peritoneal carcinomatosis should be considered a locoregional disease stage of the disease regardless of its origin and thus a candidate for an intention-to-treat therapeutic option with debulking surgery and hyperthermic intraperitoneal chemotherapy.
The high rate of morbidity with this technique (30–65%) can be significantly reduced by maximising the selection of candidates for surgery by referrals to specialist units with multidisciplinary cohesive teams, and by the use of a refined surgical technique to reduce the amount of complications which include intestinal fistulae, amongst others.1 Perioperative anastomotic dehiscence after debulking surgery and hyperthermic intraperitoneal chemotherapy varies between 5% and 12%. Many procedures have been developed to reduce the risk of anastomotic leakage, including the use of materials to reinforce suture lines.2
The medicated sponge contains (per cm2) human fibrinogen 5.5mg and human thrombin 2IU, and is indicated in adults as supportive treatment in several surgical procedures to improve haemostasis, promote tissue sealing and as reinforcement for sutures in vascular surgery.
The objective of the study was to check whether the collagen sponge with 5.5mg of fibrinogen and 2IU of thrombin applied as a tissue sealant and as gastrointestinal reinforcement sutures contributes or not to a reduced number of anastomotic leakages. This compound has been demonstrated as effective in reducing the rate of gastrointestinal anastomotic leakage in animal models. The presence of fibrinogen and thrombin around the suture promotes sealing of the suture and forms biological bridges, which promote healing. The material disappears through the endogenous metabolic pathways of fibrin and this avoids the complications associated with other non-degradable prosthesis materials.
Material and methodsWe designed this quasi-experimental, comparative, prospective case and control study in patients with gastrointestinal peritoneal carcinomatosis of colorectal origin (colorectal carcinoma), operated in our Surgical Peritoneal Oncology Unit; between 1 January of 2011 and 1 January 2014 where we used the medicated sponge in patients with gastrointestinal anastomoses. Our series comprised 73 patients, 43 (59%) men and 30 women (41%), all of whom had been diagnosed with peritoneal carcinomatosis and were candidates for potentially curative surgical treatment using debulking techniques and perioperative hyperthermic intraperitoneal chemotherapy. Gastrointestinal anastomoses were performed on 49 (67%) of these patients, 28 men and 21 women, with peritoneal carcinomatosis of colorectal origin. These patients were divided randomly into 2 groups: Group A/control (n=27): mean age 61.5 (range: 39–72). The medicated sponge was not used in this group.
Group B/cases (n=22): mean age 56.2 (range: 35–69), the anastomosis line was reinforced with the medicated sponge.
The procedures used in the patients (cases) and in the controls were carried out after informed consent had been given and our centre's established protocols were followed.
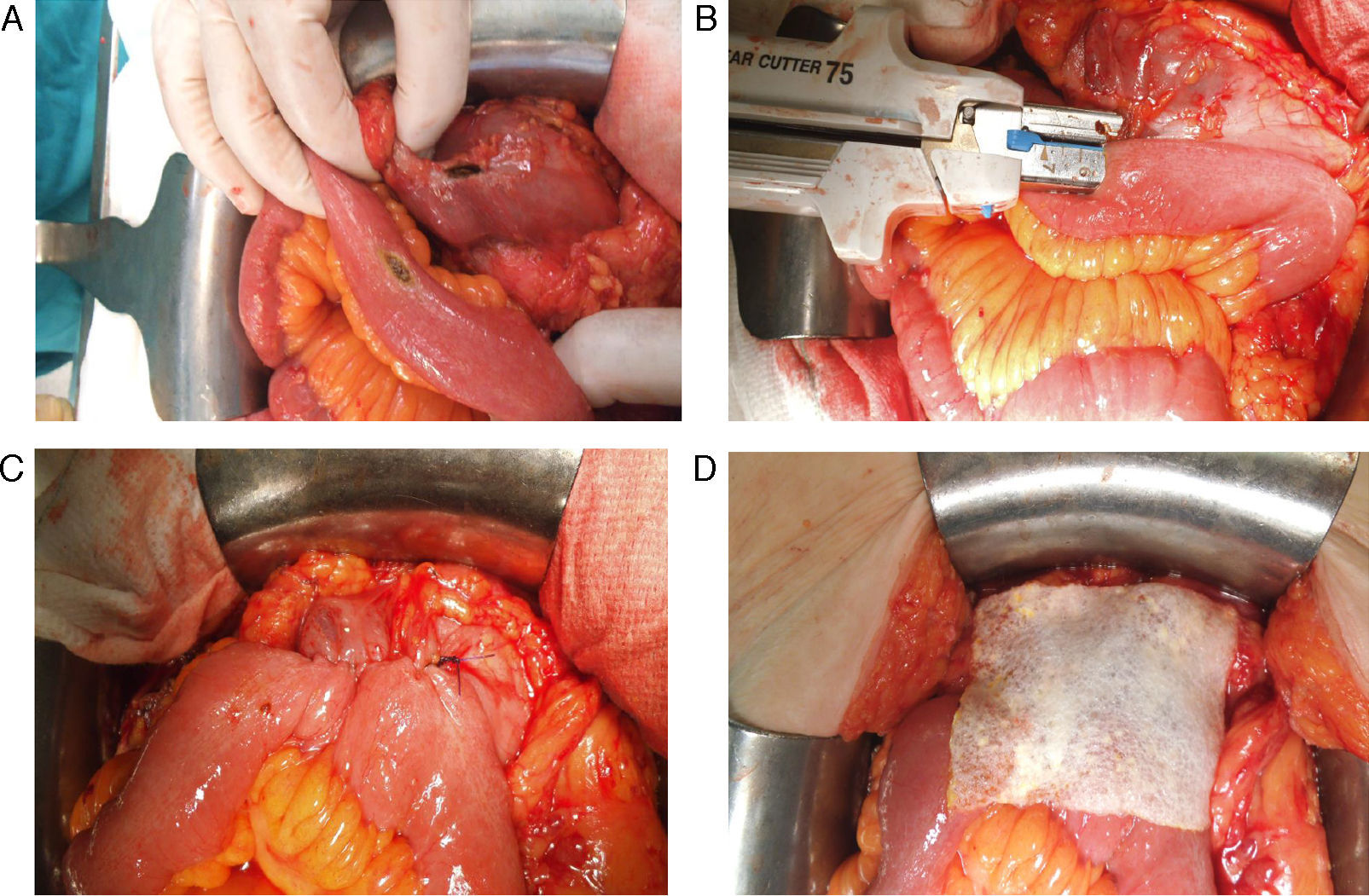
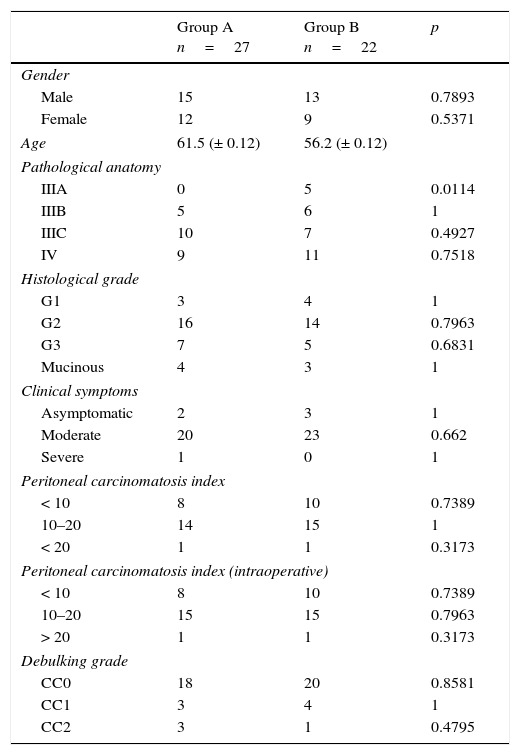
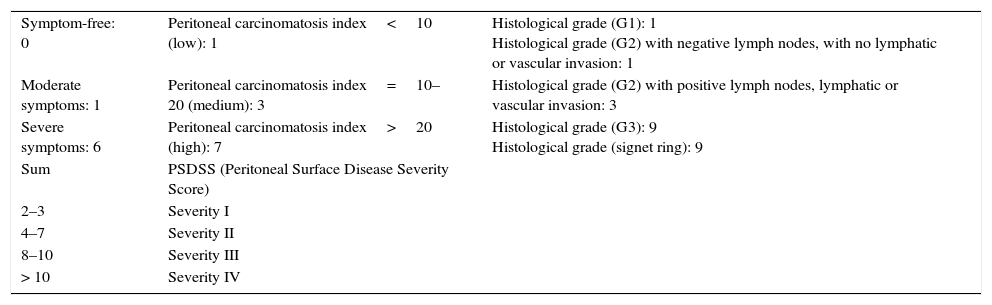
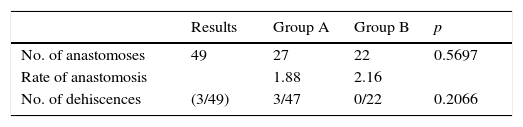
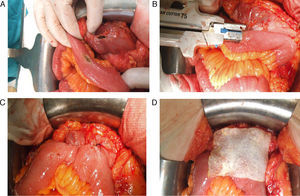
All the sutures were mechanical and always placed by the 2 same surgeons. The sponge was placed covering the entire anastomosis, overlapping at least 0.5–1cm at each side of the suture line and exercising slight pressure on it (Fig. 1). The size of the sponge used was 9.5cm×4.8cm.
Reinforcement of an anastomosis using a medicated sponge. Mechanical jejunocolic latero-lateral anastomosis after right hemicolectomy in a patient with peritoneal carcinomatosis of colorectal origin. A. Preparation of the anastomosis: incision with an electrosurgical unit of the jejunum and transverse colon. B. Insertion of GIA 75mm through both incisions for anastomosis. C. Final result of mechanical jejunocolic latero-lateral anastomosis. D. Placing the medicated sponge on the intestinal anastomosis.
Most of the anastomoses were covered with a single sheet, if 2 or more were required, this was recorded in the patient's clinical history. Neither group presented significant populational differences (Table 1).
Patient characteristics.
| Group A n=27 | Group B n=22 | p | |
|---|---|---|---|
| Gender | |||
| Male | 15 | 13 | 0.7893 |
| Female | 12 | 9 | 0.5371 |
| Age | 61.5 (± 0.12) | 56.2 (± 0.12) | |
| Pathological anatomy | |||
| IIIA | 0 | 5 | 0.0114 |
| IIIB | 5 | 6 | 1 |
| IIIC | 10 | 7 | 0.4927 |
| IV | 9 | 11 | 0.7518 |
| Histological grade | |||
| G1 | 3 | 4 | 1 |
| G2 | 16 | 14 | 0.7963 |
| G3 | 7 | 5 | 0.6831 |
| Mucinous | 4 | 3 | 1 |
| Clinical symptoms | |||
| Asymptomatic | 2 | 3 | 1 |
| Moderate | 20 | 23 | 0.662 |
| Severe | 1 | 0 | 1 |
| Peritoneal carcinomatosis index | |||
| < 10 | 8 | 10 | 0.7389 |
| 10–20 | 14 | 15 | 1 |
| < 20 | 1 | 1 | 0.3173 |
| Peritoneal carcinomatosis index (intraoperative) | |||
| < 10 | 8 | 10 | 0.7389 |
| 10–20 | 15 | 15 | 0.7963 |
| > 20 | 1 | 1 | 0.3173 |
| Debulking grade | |||
| CC0 | 18 | 20 | 0.8581 |
| CC1 | 3 | 4 | 1 |
| CC2 | 3 | 1 | 0.4795 |
Inclusion criteria: patients diagnosed with peritoneal carcinomatosis of colorectal origin and candidates for potentially curative surgical treatment using debulking techniques and perioperative intraperitoneal hyperthermic chemotherapy.
Exclusion criteria: patients diagnosed with peritoneal carcinomatosis which was not colorectal in origin. Patients who had undergone a different operation (peritoneal normothermic chemotherapy). No patients were lost to follow-up.
The study variables were gender, age, pathological anatomy, histological grade, clinical signs, peritoneal carcinomatosis index, peritoneal carcinomatosis index (intraoperative) and degree of debulking.
All the patients included in this study fulfilled strict inclusion criteria for surgical treatment using debulking surgery and intraperitoneal perioperative hyperthermic chemotherapy and were informed, orally and in writing, of their participation in the study. The patient's signature was required for their inclusion and that of a close relative. In all cases, we complied with current legislation including the current Data Protection Act.
According to the pathological anatomy of the surgical piece, the tumours were staged as: IIIA (one), IIIB (11), IIIC (17) and IVB (20). G1: 7 (14%), G2: 30 (61%) and G3:12 (24%). Mucinous 7 (15%). According to the symptoms presented: 5 (10%) asymptomatic, 43 (88%) with moderate symptoms and one (2%) with major symptoms. The radiological peritoneal carcinomatosis index was ≤ 10: 18 cases (36%), between 10 and 20: 29 (59%) and 2 with a peritoneal carcinomatosis index ≥ 20. Thirty-seven (75.5%) patients received adjuvant chemotherapy stages IIIC and IVB.
After the various surgical techniques in peritonectomy, electrofulguration and visceral resection had been performed, only the patients who achieved CC0–CC1 underwent perioperative intraperitoneal hyperthermic chemotherapy using the Sugabaker protocol with mytomicin+doxorubicin at 42°C for 90min. The peritoneal carcinomatosis index was ≤ 10 in 15 cases (30%), between 10 and 20 in 32 (65%) and ≥ 20 in 2 (5%). They were all staged preoperatively according to the Peritoneal Surface Disease Severity Score (Table 2).
Peritoneal Surface Disease Severity Score (PSDSS).
| Symptom-free: 0 | Peritoneal carcinomatosis index<10 (low): 1 | Histological grade (G1): 1 Histological grade (G2) with negative lymph nodes, with no lymphatic or vascular invasion: 1 |
| Moderate symptoms: 1 | Peritoneal carcinomatosis index=10–20 (medium): 3 | Histological grade (G2) with positive lymph nodes, lymphatic or vascular invasion: 3 |
| Severe symptoms: 6 | Peritoneal carcinomatosis index>20 (high): 7 | Histological grade (G3): 9 Histological grade (signet ring): 9 |
| Sum | PSDSS (Peritoneal Surface Disease Severity Score) | |
| 2–3 | Severity I | |
| 4–7 | Severity II | |
| 8–10 | Severity III | |
| > 10 | Severity IV |
After a mean follow-up of 24 months, the patients were classified according to their clinical situation as: disease-free, alive with disease, deceased due to and without disease. In both groups, the intestinal tract was reconstructed after administration of intraoperative intraperitoneal hyperthermic chemotherapy. All the sutures were mechanical and always placed by the same 2 surgeons. In group A (control) the anastomoses were not reinforced with medicated sponge, and in group B they were, following the manufacturer's standards.
Statistical studyPearson's χ2 test was used for the baseline comparisons of the patients’ characteristics or the Fisher test as appropriate. The differences in the incidence of anastomotic fistulae were measured using Fisher's bilateral exact test. For all the analyses the level of statistical significance was set at 5%, value α=0.05.
ResultsThere were a total of 19 anastomoses (x˙: 19), 27 in Group A (49.4%, x˙: 1.88) and 22 in group B (54.7%, x˙: 2.16). The debulkings achieved were: CC0 in 38 patients (77.5%), CC1 in 7 (14.8%), and in 4 (8.1%) perioperative intraperitoneal hyperthermic chemotherapy was not undertaken due to CC2–CC3. There was anastomotic dehiscence and intestinal fistula in 3 cases (6.1%); 2 of them in the CC0–CC1 group and one in the CC2–CC3 group. The 3 in group A/control (without medicated sponge) compared to none in group B/hypothesis. Two of them required reoperation (stomas) with significantly increased postoperative time. The mean admission time in our series for uncomplicated patients was 14 days compare to a mean of 26 days in the cases complicated with intestinal fistula. Two patients (4.1%) presented haemoperitoneum, one per group, and both were reoperated. The overall morbidity rate was: 31.7%, grade I–II (21.5%), grade III–IV (10.2%) (Table 3).
DiscussionPer cm2 the medicated sponge contains 5.5mg human fibrogen and 2IU human thrombin in the form of a dry layer on the surface of a collagen matrix. When in contact with physiological fluids (blood, lymph or saline solution, for example) the components of this layer dissolve and partially spread over the surface of the wound. Then a reaction of the fibrinogen and the thrombin is produced which starts the last stage of physiological blood coagulation. Fibrinogen converts into fibrin monomers that polymerise spontaneously to form a fibrin coagulate, which keeps the collagen matrix firmly adhered to the wound surface. The fibrin cleaves in cross-links due to endogen factor XIII, creating a firm and mechanically stable net with good adhesive properties, and therefore at the same time serves as a sealant.
The medicated sponge is indicated for epilesional use only. Intravascular administration is contraincidated. As a consequence, pharmacokinetic studies have not been performed on humans. Fibrin/haemostatic sealants that metabolise in the same way as endogenous fibrin by fibrinolysis and phagocytosis. In studies on animals, the medicated sponge biodegrades after administration on the wound surface, and little remains after 13 weeks. Complete degradation of the medicated sponge was observed in some animals 12 months after administration on a wound in the liver, while in others small remains were seen. Degradation was associated with the infiltration of granulocytes and the formation of resorbable granulation tissue that encapsulated the remains of the gradual degradation of the medicated sponge. No evidence of local intolerance was observed in the studies on animals. From experience gained in humans, a few isolated cases have been found where remains found incidentally did not cause functional damage.
The morbidity of multidisciplinary treatment in peritoneal carcinomatosis is significant.1–3 Specific surgical morbidity is 30% and essentially corresponds to gastrointestinal suture dehiscences, perforations and intestinal fistulae, collections or intrabdominal abscesses and postoperative bleeding, requiring 10% of patients to be reoperated once or several times.4
The incidence of dehiscence or anastomotic leakage as a postoperative complication after colonic surgery is estimated at around 2–5%, associated with increased morbimortality. Determining the exact cause of a fistula is difficult, various factors are involved including, malnutrition, hypoproteinaemia, inappropriate surgical technique (poor vascularisation of the intestinal ends, anastomosis under tension), amongst others. Whether or not the colon should be prepared prior to surgery is still a matter of debate. The major complications of anastomotic leakage are sepsis from intraperitoneal abscesses (50%), fecaloid peritonitis (25%), wall abscesses and wound infections (25%), with a major impact on hospital stay.1,3–8
Many surgical techniques and procedures have been developed to reduce the risk of anastomotic leakage, including the use of materials to reinforce the suture line. These include fibrin glue, expanded polytetrafluoroethylene, bovine pericardium patches polyglycol and oxidised cellulose patches, amongst many others. However, randomised, controlled prospective studies are necessary to obtain evidence of their efficacy.2
The sponge is indicated in adults as support treatment in various surgical interventions to improve haemostasis, promote tissue sealing and to reinforce sutures in vascular surgery when standard techniques have proved insufficient. Its efficacy and safety have been demonstrated in various types of surgery, for example after liver resections,9,10 reducing biliary drainage, blood loss from the liver bed, and consequently a reduction in hospital stay. Chirletti et al.11 demonstrated its efficacy in the prevention of pancreaticojejunal anastomotic leakage after cephalic duodenopancreatectomy. Rickenbacher et al.2 and Toro et al.12 published reviews on the use of the tissue sealant in abdominal surgery, they reported no complications associated with immunogenicity or safety. Its efficacy was demonstrated in reducing the number of oesophagogastric and intestinal anastomotic leakages in various experimental models.13 However, the number of publications which refer to its use in preventing anastomotic dehiscence in colorectal surgery is very limited. Parker et al.14 observed fewer leaks in 26 patients who underwent anterior colorectal resections in the group of patients with anastomoses reinforced using the medicated sponge. These results are very similar to those obtained by De Stefano et al.15 in 63 patients, with a significant reduction in the number of colorectal anastomotic leakages in the group treated using this compound. Sabino et al.16 recently reported, in an experimental rat model, the effect of immediate postoperative intraperitoneal chemotherapy on the healing processes which take place in manual colocolic anastomoses in left hemicolectomies. It can be observed how 5-fluorouracil impedes these mechanisms, promoting anastomotic dehiscence. On the other hand, fewer anastomotic leakages are seen in the group of animals whose suture line had been protected with the medicated sponge.
To the best of our knowledge there is no study which refers to the potential role in the prevention of anastomotic leakage in patients with peritoneal carcinomatosis, who have undergone total debulking techniques and intraoperative intraperitoneal hyperthermic chemotherapy (multimodal treatment). In relation to the rates of anastomotic dehiscence, most authors1,3,5–8 report values of 6.5% associated with the duration of the operation and the extent of the implant. Sugarbaker1,17 stresses that the duration of surgery, the number of peritonectomy procedures and the number of sutures are independent risk factors of anastomotic dehiscence. Glockzin et al.4 report a strong relationship between the extent of debulking and the development of postoperative complications with an 8.9% rate of anastomotic leakage and an intraabdominal infection rate of 11.3%. Baratti et al.6 describe a 6.9% rate of anastomotic dehiscence. In their study, multivariable logistic regression indicates that the duration of surgery (> 540min) and the peritoneal carcinomatosis index (> 19) are independent predictive factors. For their part, Bartlett et al.18 on 795 patients operated due to gastrointestinal and gynaecological peritoneal carcinomatosis using total debulking techniques and intraoperative intraperitoneal hyperthermic chemotherapy observe, in their multivariable analysis that aged over 60, albumin levels of below 3g/dl, operation time (> 500min) and requirements for transfusion are associated with an increase in morbimortality. As is logical, the number of anastomosis performed plays an important role in the percentage of postoperative complications. There is no evidence that the various perfusion techniques or the intraperitoneal hyperthermic chemotherapy itself influence postoperative complications.1,5–8 It is evident that the development of operative complications has a negative impact on the patient's cancer outcome.
In our series, the rate of anastomotic leakages was 3 cases, all the group of patients in whom the fibrin-thrombin collagen sponge was not used. All the patients were included because they presented peritoneal (carcinomatosis) of colorectal origin: one anastomotic fistula after right hemicolectomy, one after sigmoidectomy and pelvic peritonectomy after systemic neoadjuvant chemotherapy for synchronous colorectal peritoneal carcinomatosis, and a third leakage from a mechanical latero-lateral jejuno-jejunal fistula in a patient with implants for a left colon tumour (synchronous peritoneal carcinomatosis) and with various implants in the mid jejunum which were infiltrating the muscle layer and which required resections of segments of small intestine. The first 2 occurred after intraoperative intraperitoneal hyperthermic chemotherapy, whereas another occurred in a patient with CC2–CC3 (incomplete debulking), and therefore, not candidates for intraoperative chemotherapy. The mortality described varies between 0% and 14%, although figures of 2%-6% appear as the most frequent in most of the studies. It is 3% in our overall series. Mortality is associated with the origin of the peritoneal carcinomatosis (greater in peritoneal pseudomyxoma in relation to a high peritoneal carcinomatosis index). Furthermore, it has also been associated with the intensity of surgical aggression; the number of peritonectomy procedures performed reflects this, and the peritoneal carcinomatosis index (more than 5 anatomical regions affected), the number of gastrointestinal anastomosis and the volume of blood transfused preoperatively. Although the mean mortality described with this complex treatment does not exceed that referenced in major digestive surgery, the percentage and importance of morbidity and surgical complications that imply reoperating very frail patients, mean that a very careful selection of patients should be made. The morbimortality relates directly to the experience of the surgical team.1–3
Although we are aware that there are too few cases (n=49) in our study for us to extract concrete and significant conclusions, the study can serve to start and incentivise the design of further randomised, prospective studies recruiting a larger number of patients in order to clarify the role of the medicated sponge in the prevention of intestinal anastomotic leakage.
In conclusion, in a selected group of patients, we consider that peritoneal carcinomatosis of gastrointestinal and gynaecological origin is potentially curable. The best therapeutic option is total debulking surgery and intraoperative intraperitoneal hyperthermic chemotherapy. We must ensure that we assess these patients in specialised units with multidisciplinary teams. In our series, the use of the medicated sponge to reinforce intestinal anastomoses proved safe and effective and contributed to a reduction in the risk of digestive fistula in patients who, as oncology patients, are at high risk of dehiscence having undergone extensive and prolonged surgery; almost all of them treated previously with systemic chemotherapy and generally malnourished. After a thorough search of the major literature databases, this study is the first to demonstrate the preventive role in the development of anastomotic dehiscence of the application of the medicated sponge in patients with peritoneal implants treated surgically with curative intent by total debulking surgery and intraoperative intraperitoneal hyperthermic chemotherapy.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Torres-Melero J, Motos-Micó JJ, Lorenzo-Liñán M, Morales-González Á, Rosado-Cobián R. Aplicación de sellante tisular como refuerzo de las anastomosis digestivas realizadas en pacientes con carcinomatosis peritoneal tratados con intención curativa mediante procedimiento quirúrgico de citorreducción y quimioterapia intraperitoneal intraoperatoria hipertérmica. Cir Cir. 2016;84:100–106.