Auxiliary heterotopic liver transplantation with portal vein arterialization (AHLT-PVA) is a model that has been hardly studied, despite its therapeutic potential.
MethodsHemodynamic and biochemical characterisation was carried out during graft implantation, in a pig-to-pig model (n=15 AHLT-PVA). Furthermore a histopathological study was performed to establish microscopic alterations due to PVA.
ResultsReperfusion of the arterialized graft produced an increase in heart rate (HR) vs baseline (P=.004) and vs inferior vena cava clamping phase (P=.004); and a decrease in systemic vascular resistance vs cava clamping phase (P=.021). At the end of implantation, cardiac output remained elevated (P=.001), likewise HR remained increased vs baseline phase (P=.002). Mean arterial pressure decreased with cava clamping, but was not affected by the reperfusion of the graft, nor the skin closure. The histopathological study at 3, 10, and 21 days post-PVA revealed that functional liver structure was maintained although it is common to find foci of perilobular necrosis on day 3 (P=.049), and perilobular connective tissue proliferation at day 10 (P=.007), vs native liver.
ConclusionsThe described arterialized liver graft model minimises the number of vascular anastomoses vs previously described models. It is hemodynamically and metabolically well tolerated and the double arterial vascularisation of the graft does not cause significant changes in liver histology.
El trasplante auxiliar heterotópico hepático con arterialización de la vena porta (TAHH-AVP) es un modelo poco estudiado a pesar de su potencial terapéutico. El objetivo del estudio es valorar la respuesta hemodinámica y bioquímica durante el implante y analizar la repercusión de la arterialización portal en la funcionalidad y morfología hepática.
MétodosSe realizó un estudio hemodinámico y bioquímico durante el implante auxiliar en un modelo porcino (n=15 TAHH-AVP). Además, se analizaron las consecuencias de la arterialización portal sobre la arquitectura hepática mediante un estudio ultraestructural.
ResultadosLa reperfusión del injerto arterializado aumentó la frecuencia cardiaca (FC) respecto a los valores basales (p=0,004) y a la fase del pinzamiento de la vena cava (p=0,004) y disminuyó las resistencias vasculares sistémicas respecto a la fase del pinzamiento de la vena cava (p=0,021). Al final del implante, el gasto cardiaco permaneció elevado (p=0,001), al igual que la FC respecto a la fase basal (p=0,002). La presión arterial media disminuyó con el pinzamiento venoso, pero no se vio afectada ni por la reperfusión del injerto ni por el cierre de la piel. Todas las muestras histológicas obtenidas a los 3, 10 y 21 días conservaron su morfología y arquitectura hepáticas. Si bien se observaron algunos focos de necrosis perilobular el día 3 (p=0,049) y proliferación conectiva perilobular el día 10 (p=0,007), respecto al hígado nativo.
ConclusionesEl trasplante del injerto hepático arterializado descrito minimiza el número de anastomosis vasculares respecto a los modelos previamente publicados, presenta una buena tolerancia hemodinámica y metabólica, y la arterialización portal del injerto no produce cambios significativos en la histología hepática.
Orthotopic hepatic transplant (OHT) is the treatment of choice for chronic terminal hepatic diseases.1–3 Although auxiliary hepatic transplant (AHT) has more restricted indications, it may be an alternative to OHT in certain situations, such as: (a) non-cirrhotic metabolic hepatopathologies, or sudden potentially reversible liver failure in which recovery of the native liver is possible4–9; (b) extremely small liver grafts10,11 and (c) even in xenotransplant, as a bridging solution until a compatible human graft becomes available for OHT.12
Portal vein arterialisation (PVA) is a technical variant that when associated with auxiliary heterotopic hepatic transplant (AHHT) makes it possible to conserve the native hepatic hilus intact, preventing the phenomenon of competition for the portal flow between the two livers, while supplying the native liver with optimum conditions for its potential regeneration.13–18 However, portal vein arterialisation will subject the hepatic portal territory to high pressure arterial flow.
The aim is to analyse the hemodynamic, electrolytic and acid base balance alterations which occur in a porcine model of AHHT-PVA during implant in the recipient, and to analyse the histopathological changes that arise in the graft.
MethodAnimals15 AHHT-PVA were performed on female crossed Large White and Landrace pigs. The donors weighed 12kg and the recipients weighed 25kg, as did those used for hemotherapeutic resources. Crossed agglutination tests were used to determine donor-recipient compatibility.19
All of experimental protocols were approved by the Ethics Committee of our University, which is responsible for the application of Directive 2010/63/EU. The animals were anaesthetised with isofluorane (1.5%–2%) and fentanyl (0.03–0.05mg/kg/h).
Surgical ProcedureStandard hepatectomy was performed on the donor, apart from retrogastric dissection of the hepatic artery.18
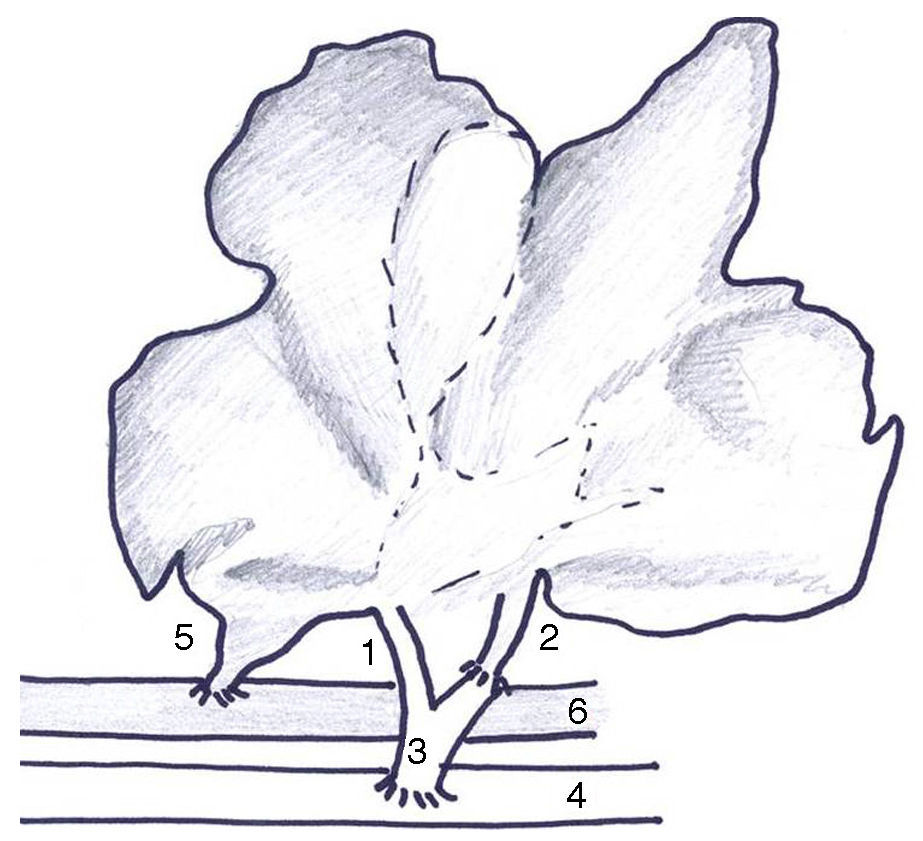
The type of AHHT-PVA previously described by our group18 was modified in 3 ways: (1) arterial anastomosis to the infrarenal aorta of the recipient due to the high rate of thrombosis (Fig. 1); (2) section of the infrahepatic cava vein at the level of the caudate lobe to prevent folding; (3) complete auxiliary transplant due to the frequency of bleeding from the resection surface, and (4) external drainage of the common bile duct to evaluate bile production.
Hemodynamic AnalysisFor the hemodynamic study a central venous catheter was used in the internal jugular vein, together with a catheter in the femoral artery connected to a Picco® monitor (Pusion Medical Systems, Feldkirchen, Germany).20 This measures cardiac output (CO) using transpulmonary thermodilution and the arterial pulse wave.
The hemodynamic variables analysed were average arterial pressure, central venous pressure, CO, systemic vascular resistances (SVR), total telediastolic volume, intrathoracic blood volume, extravascular lung volume and cardiac frequency (CF). Measurements were taken at 4 moments during the implant: (A) after making the incision in the skin (basal), (B) after clamping the infrahepatic caudal cava vein, (C) after reperfusion and (D) when the skin is closed.21 The basal values were used as the control.
Biochemical Analysis and Blood GasesPhysiological saline serum was used as the maintenance liquid as well as following reperfusion. Anticoagulated total blood was transfused, with sodium citrate if hemoglobin was <10g/dl.
Gasometries were taken using the Irma® gas analyser (Diametrics Medical, Minnesota, U.S.A.), measuring arterial pH, partial oxygen pressure (pO2), partial carbon dioxide pressure (pCO2), standard bicarbonate (HCO3), excess bases, potassium (K+), sodium (Na+), ionised calcium (Ca2+) and hemoglobin (Hb).
Biochemical and gas parameters were analysed in the same phases hemodynamic variables were determined, using basal values as the control.
Immunosuppressor ProtocolThe immunosuppressor protocol followed has been described previously.22
Monitoring ProtocolBile production was measured every day, and daily blood analysis (hemogram, coagulation and hepatic profile) took place until day 4 post-AHHT. After this they were measured weekly and whenever a complication arose.
The following diagnostic techniques were used:
- 1.
Ultrasound checks with an ECCO-CEE® two dimensional colour ecoDoppler (Toshiba Corporation Medical Systems Division, Tokyo, Japan) at 3 and 10 days, and also in case of suspicion of graft dysfunction.
- 2.
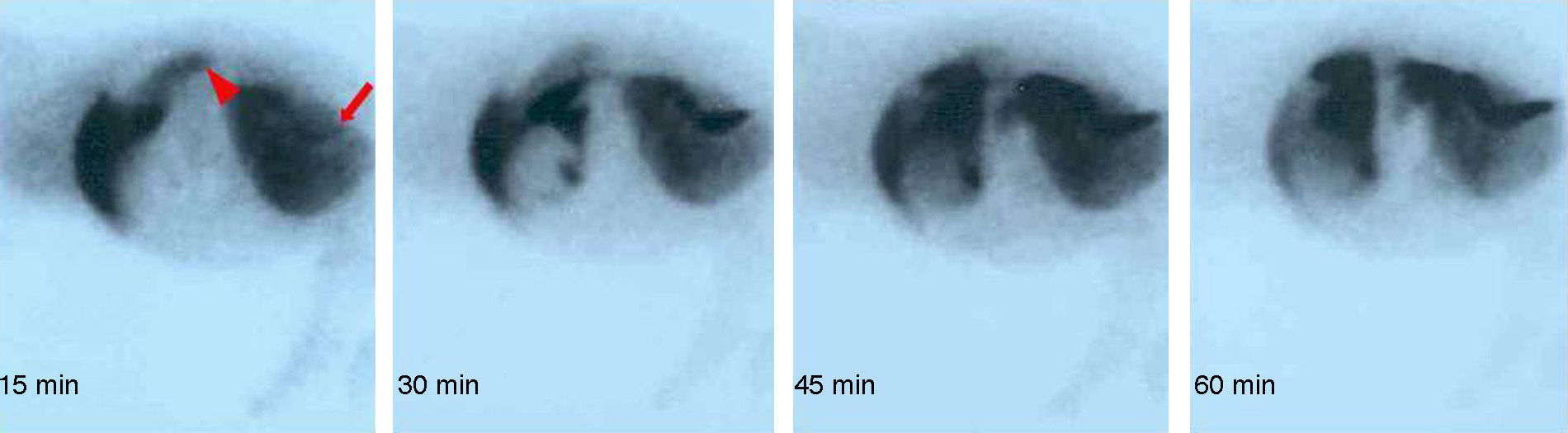
To evaluate graft hepatic integrity and function, gammagraphic studies were used, with an E.CAM Single® gammacamara (Siemens, Erlangen, Germany). Studies were of 2 types: (a) gammagraphy with human colloidal albumin aggregate marked with 99mTc, and (b) dynamic gammagraphy with diisopropyl-iminodiacetic acid (DI-sida) marked with 99mTc. Tests were performed at 7 and 14 days or if an animal deteriorated.
Samples were taken from the graft at day 3 (early study), day 10 (intermediate study) and day 21 (late study) and a sample of native liver was used as the control tissue. Hepatic samples were obtained using an ultrasound-guided Tru-cut or an open biopsy in those cases when the sample taking coincided with a laparotomy. Due to the risk of hemorrhage, portal endothelial samples were only taken on day 21 before the animals were sacrificed.15 All samples were stained with hematoxylin–eosin Masson trichrome, and they were evaluated by 2 pathologists who did not know the sample codes.
To determine the percentage of tissue that was damaged, the area of parenchyma affected was calculated on a known surface (10 4× fields, 800000μm2/field). To analyse other parameters we use the following scoring procedure:
- 1.
Sinusoidal dilation and congestion: (−) negative; (+) 0%–10% of parenchyma affected; (++) 10%–20%; (+++) more than 20%.
- 2.
Non-specific mononuclear inflammatory infiltrate: (−) negative; (+) perivascular periportal cuffs.
- 3.
Hyperplasia of the bile ducts: (−) negative; (+) periportal bile ducts neoformation.
To ensure reproducibility, in hemodynamic and biochemical measurements the average of 3 consecutive measurements was calculated.23 To determine whether they changed throughout the surgery, each phase was compared with the previous phase and with the basal value by using Student's t test.
For the histopathological study, to evaluate the changes over time each phase was compared with the others using Wilcoxon's test. Additionally, each phase was compared with native liver samples using the Mann–Whitney test.
ResultsHemodynamic Analysis (n=15)Once surgery was finished the transplant was considered to be successful if arterial flow to the graft was observed, together with appropriate venous drainage and bile production.
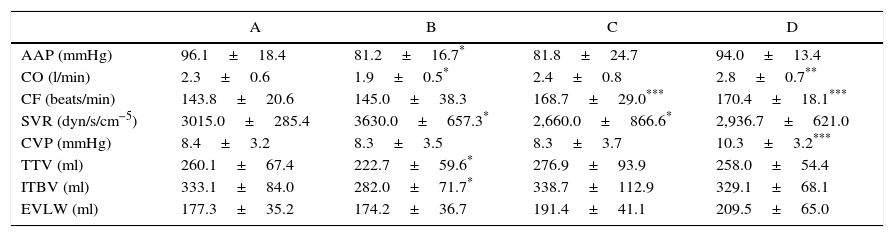
Reperfusion of the arterialised graft caused an increase in the CF over the basal figure (P=.004) and at the venous clamping phase (P=.004); and a reduction in vascular resistances compared with the clamping phase (P=.021). At the end of the implant, CO remained high (P=.001), as did CF compared to the basal phase (P=.002) (Table 1).
Hemodynamic Measurements in the Basal Phase (A), After Clamping the Inferior Cava Vein (B), After Reperfusion (C) and After Closing the Skin (D).
| A | B | C | D | |
|---|---|---|---|---|
| AAP (mmHg) | 96.1±18.4 | 81.2±16.7* | 81.8±24.7 | 94.0±13.4 |
| CO (l/min) | 2.3±0.6 | 1.9±0.5* | 2.4±0.8 | 2.8±0.7** |
| CF (beats/min) | 143.8±20.6 | 145.0±38.3 | 168.7±29.0*** | 170.4±18.1*** |
| SVR (dyn/s/cm−5) | 3015.0±285.4 | 3630.0±657.3* | 2,660.0±866.6* | 2,936.7±621.0 |
| CVP (mmHg) | 8.4±3.2 | 8.3±3.5 | 8.3±3.7 | 10.3±3.2*** |
| TTV (ml) | 260.1±67.4 | 222.7±59.6* | 276.9±93.9 | 258.0±54.4 |
| ITBV (ml) | 333.1±84.0 | 282.0±71.7* | 338.7±112.9 | 329.1±68.1 |
| EVLW (ml) | 177.3±35.2 | 174.2±36.7 | 191.4±41.1 | 209.5±65.0 |
Student's t. Values expressed as an average±SD.
EVLW: extravascular lung volume (EVLW); CF: cardiac frequency; CO: cardiac output; TTV: total telediastolic volume; ITBV: intrathoracic blood volume; AAP: average arterial pressure; CVP: central venous pressure; SVR: systemic vascular resistances (SVR).
During graft reperfusion sodium increased (P=.014) over the previous value; bicarbonate (P=.009) and excess bases (P=.03) reduced in comparison with basal values. At the end of surgery, pO2 remained high (P=.01) and bicarbonate fell (P=.037) in comparison with basal values (Table 2).
Metabolic Changes in the Basal Phase (A), After Clamping the Inferior Cava Vein (B), After Reperfusion (C) and After Closing the Skin (D).
| A | B | C | D | |
|---|---|---|---|---|
| pH | 7.4±0.1 | 7.4±0.1** | 7.3±0.1 | 7.4±0.1 |
| pCO2 (mmHg) | 44.9±19.3 | 39.8±16.7** | 44.3±14.4 | 37.1±6.0 |
| pO2 (mmHg) | 497.3±92.2 | 532.1±101.0 | 559.3±103.6 | 555.9±82.8** |
| Htc % | 29.1±4.6 | 28.5±2.9 | 30.4±2.7 | 30.6±7.2 |
| Na+(mmol/l) | 135.7±5.0 | 132.5±4.1** | 136.6±3.2* | 135.7±3.9 |
| K+(mmol/l) | 4.1±3.2 | 6.2±5.0 | 3.7±2.3 | 5.6±3.8 |
| iCa2+(mmol/l) | 1.1±0.1 | 1.0±0.2 | 1.1±0.2 | 1.1±0.3 |
| HCO3− (mmol/l) | 27.3±2.7 | 26.0±2.7** | 22.8±4.6** | 23.8±3.2** |
| TCO2 (mmol/l) | 28.7±3.2 | 27.1±3.0** | 24.1±4.9** | 25.0±3.3** |
| BEb (mmol/l) | 2.6±1.1 | 1.9±2.2 | −3.0±4.0** | −0.5±3.6 |
| BEecf (mmol/l) | 2.8±1.4 | 2.1±2.2 | −5.0±5.4 | −1.7±4.4 |
| O2 Sat % | 99.8±0.1 | 99.9±0.1 | 99.8±0.0 | 99.9±0.7 |
| tHb (g/dl) | 9.9±1.5 | 10.0±1.4 | 10.3±0.9 | 10.4±2.4 |
Student's t. Values expressed as average±SD.
Beb: excess bases; BEecf: excess bases extracellular fluid; HCO3: standard bicarbonate; Htc: hematocrit; iCa2+: ionised calcium; O2 Sat: oxygen saturation; pCO2 partial carbon dioxide pressure; pH: arterial pH; pO2: partial oxygen pressure; TCO2: total concentration of CO2 in plasma; tHb: total hemoglobin.
Except for one animal which had thrombosis in the arterial anastomosis, in the others gammagraphy clearly showed that the arterialised graft was functioning (Fig. 2).
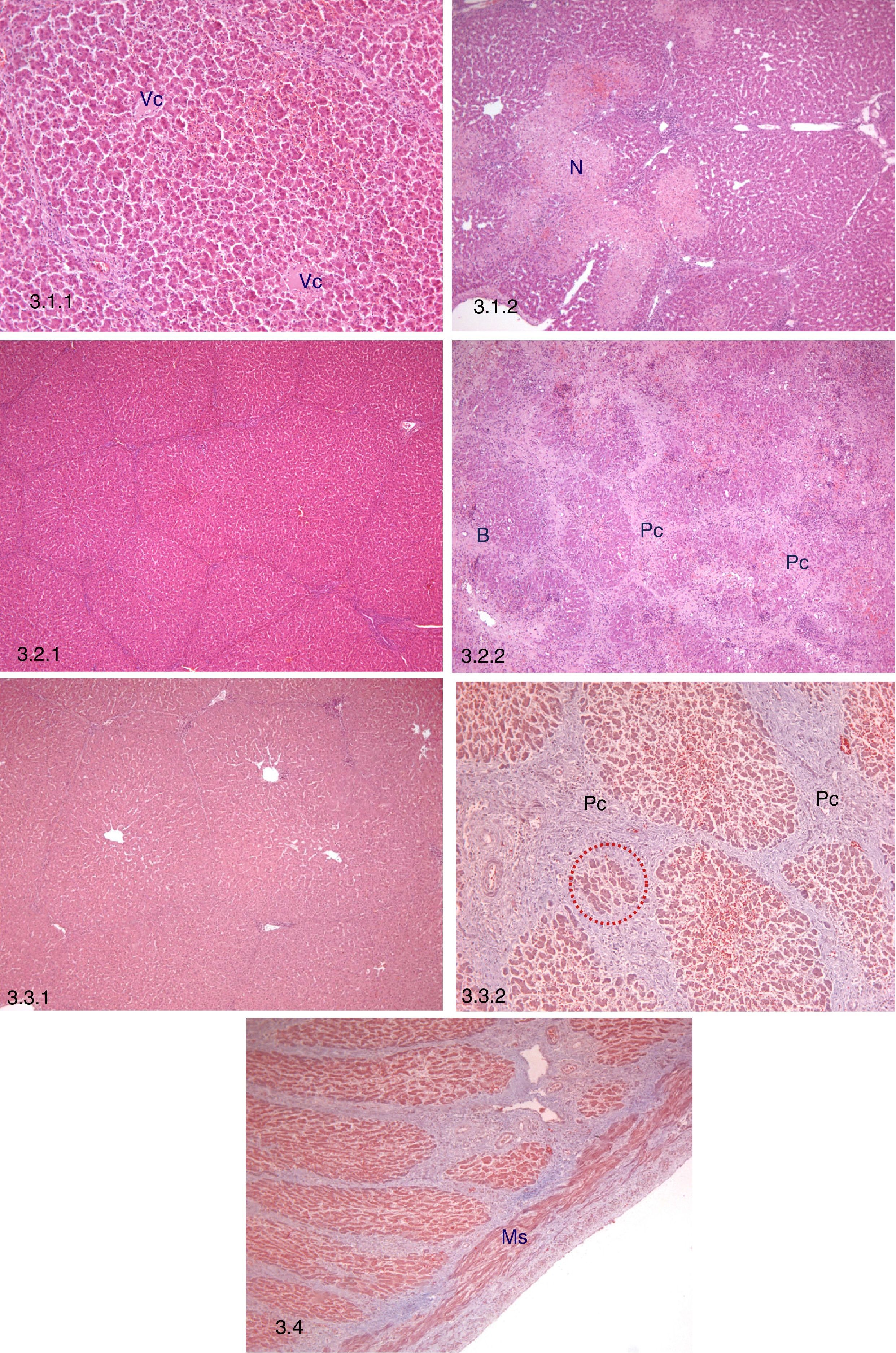
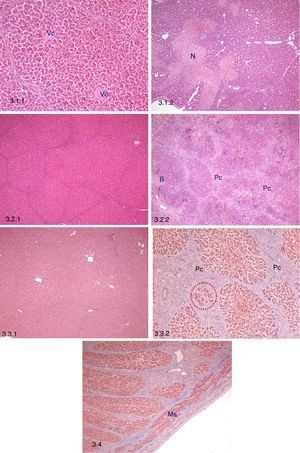
Early Histopathological Study (n=12)All of the samples conserved hepatic morphology and architecture. The main finding was an increase in the percentage of tissue with perilobular foci of necrosis together with hemorrhages and thrombosis in comparison with the native liver (P=.048) (Table 3; Fig. 3.1). Non-specific mononuclear inflammatory infiltrate, congestion and sinusoidal dilation were also observed.
Histopathological Findings in the AHHT-PVA Model.
| Control | 3 days | 10 days | 21 days | |
|---|---|---|---|---|
| Perilobular connective proliferation (%) | 10.6±1.0 | 15.5±7.5 | 15.6±3.7* | 16.3±8.1 |
| Necrosis (%) | 0.0±0.0 | 7.9±9.7* | 0.0±0.0 | 0.0±0.0 |
| Post-necrotic areas (%) | 0.0±0.0 | 0.0±0.0 | 7.6±8.5* | 6,0±7,5 |
| Sinusoidal dilation | − | ++ | +++ | ++ |
| Congestion | − | + | ++ | + |
| Non-specific mononuclear infiltrate | − | + | + | + |
| Hyperplasia of bile ducts | − | − | + | + |
Values expressed as average±SD.
Mann–Whitney test.
Histopathological study. 3.1 Early histopathological study (3 days post-AHHT). 3.1.1: Preservation of hepatic architecture, central lobular vein (Vc), H–E, 10×. 3.1.2: Perilobular focal necrosis (N), H–E, 5×. 3.2. Intermediate histopathological study (10 days post-AHHT). 3.2.1: Persistence of hepatic architecture, H–E, 5×. 3.2.2: Connective proliferation (Pc) and hyperplasia of bile ducts (B), H–E, 5×. 3.3. Late histopathological study (21 days post-AHHT). 3.3.1: Persistence of hepatic architecture, H–E, 5×. 3.3.2: Post-necrotic nodular regeneration, connective proliferation (Pc) and hyperplasia of hepatocytes between the connective tissue (red dots), Masson trichrome, 10×. 3.4 Intrahepatic portal vein in a sample taken on day 21, smooth muscle fibres (Ms), Masson trichrome, 5×.
All of the samples conserved hepatic morphology and architecture. The main findings were: (1) a significant increase in perilobular connective proliferation vs native liver (P=.007) and (2) a significant increase in areas with post-necrotic regeneration vs native liver (P=.046) (Table 3; Fig. 3.2). Additionally, non-specific mononuclear inflammatory infiltrate was observed, together with persistent sinusoidal congestion and dilation, as well as hyperplasia of the bile ducts associated with areas of post-necrotic regeneration.
Late Histopatholgical Study (21 Days After Transplant, n=10)All of the samples conserved hepatic architecture, while non-specific mononuclear inflammatory infiltrate, sinusoidal congestion and dilation were still present (Table 3; Fig. 3.3).
Histopathological Study of the Portal VeinHistopathological analysis of the portal vein at 21 days showed no significant changes (Fig. 3.4).
Histopathological Study of the Native LiverAtrophy was not detected in any of the histopathological study phases.
DiscussionAHHT was the technique used during the early days of liver transplant, although it was not widely used because it was associated with competition by the native and graft livers, venous drainage obstruction and lack of room in the abdomen.4 PVA in association with AHHT is a technical variant that prevents the phenomenon of competition between the 2 livers for the portal flow, and it leaves the native hepatic hilus intact.13–16,18 Our current version reduces the number of vascular anastomoses in comparison with other versions,13,15–17 reducing the risk of thrombosis. This is especially important in the context of xenotransplant, as porcine blood is highly liable to coagulate.24
In the hemodynamic study, during the clamping of the infrahepatic cava vein a fall in the preload was observed due to a reduction in venous return. This was reflected in the fall in overall telediastolic volume and intrathoracic blood volume. In turn, the fall in preload caused a reduction in CO and, as a result of this, in average arterial pressure. An attempt is made to compensate for this situation with an increase in SVR.25 During reperfusion, the freeing of the aortic clamp and the inclusion of the graft reduce the SVR, while the preload increases due to the inclusion of the volume that had been retained.25,26 These 2 facts produce a compensating increase of CO, to which the increase in CF contributes.
After transplant CO increases over the basal value, ensuring reperfusion of the graft. The increase in CO basically occurs at the expense of CF, which rises after reperfusion, and this fact may be due to conservative replacement of the volemia. The fact that preload markers, overall telediastolic volume and intrathoracic blood volume only vary after vascular clamping seem to corroborate this. Thus more aggressive replacement of the volemia would have raised CO with a smaller rise in CF. An increase in CO may be said to be harmful in the case of acute liver failure in which CO remains high. Nevertheless, this phenomenon has also been seen in the clinical reaction to liver transplant following graft reperfusion, and it is normally well-tolerated by recipients.25 On the other hand, central venous pressure increases at the end of surgery, and this may be due to the closure of the abdominal cavity more than to changes in the volemia, so that this would not be a reliable indicator of preload.
There are no major biochemical variations, and this is probably due to the functional nature of the native liver and the lower volume of liquid infusion than in a clinical liver transplant.27 Moreover, the variations in sodium, bicarbonate and pCO2 are within standard values. Although the native liver hinders study of the biochemical response, the implant of arterialised graft does not, in itself, cause significant metabolic changes.
In the histopathological study, all of the samples from all 3 phases of the study conserve hepatic architecture and morphology. The main early histopathological finding is the presence of perilobular foci of necrosis. This finding has been described in rat models28 and in hepatic regeneration studies.29 Schleimer et al. state that the high concentration of oxygen in the arterialised portal flow may be the cause, as oxygen free radicals that have not been neutralised by glutathione may trigger the apoptosis cascade.29 In our model the foci of necrosis seems to have multiple causes, above all ischemia-reperfusion and portal arterial hyperflow.
At 10 days the main finding is perilobular connective proliferation. This is historically associated with PVA and has been described in portal hypertension surgery as well as in OHT-PVA models.30–33 This is probably a result of the adaptation of the graft to the hyperflow. In clinical models of AHHT-PVA, the arterialisation is created by interposing a similar vascular graft to the one described by our group13–15 and, although these authors did not detect connective proliferation in their grafts, we have to take into account the fact that the hepatic stroma in the pig is far more developed than the human hepatic stroma.34
Likewise, in our model a significant increase was observed in areas with post-necrotic regeneration as an evolution of the perilobular necrosis found in the early study. Additionally, and associated with these post-necrotic areas, bile duct hyperplasia was detected. We do not believe these to be associated with partial obstruction of bile drainage, given that bile production was continuous and dynamic scintigraphy showed that the graft was eliminating bile sufficiently. The damage caused by ischemia-reperfusion which led to ischemic cholangiopathy associated with canalicular stenosis may be the origin of the proliferation of new bile ducts.
Late histopathological study revealed areas of post-necrotic nodular regeneration (disorganised lobes with atrophic hepatocytes and groups of hepatocytes inside connective tissue), together with a reduction in cholangiole hyperplasia.
In all phases of the histopathological study sinusoidal dilation was observed, together with congestion and non-specific mononuclear infiltrate. The sinusoidal dilation fits the description in clinical and experimental models of AHHT-PVA,15,28 while congestion is also described in a model of hepatic regeneration following the resection of 70% of the liver associated with a PVA with flow regulation.29 These findings are probably due to the hyperflow. The non-specific mononuclear infiltrate, which is not associated with rejection,35 has also been described in rat30,36 and dog37 arterialised livers, while Erhard et al. described lymphocytic infiltrate in the sinusoids of a clinical model of AHHT-PVA.14
Finally, in our model we observed no dilation or increase in portal vein collagen fibres, as described by Li et al.38 Nor was steatosis found in any of our preparations, although it is described in similar models.15,32
To conclude, AHHT-PVA is tolerated well hemodynamically, and it leads to a slight increase in CO. The variations in metabolism and the acid–base balance are minimal, and they are due more to the surgical technique than they are to reperfusion of the arterialised graft. This model does not lead to major changes in hepatic histology, and it maintains functional hepatic architecture and structure. Nevertheless, foci of perilobular necrosis are often found at 3 days, as is perilobular connective proliferation at 10 days.
Authorship/collaboratorsThe specific contribution of each author:
Olga M. Fernandez-Rodriguez took part in the development of the study, experimental design, writing the paper and data analysis.
Antonio Ríos took part in the development of the study, experimental design and writing the paper.
Carlos G. Palenciano took part in the development of the study, experimental design and data analysis.
Pablo Ramírez took part in the experimental design.
José Luis Navarro took part in the development of the study.
Laura Martínez-Alarcón took part in the development of the study.
Carlos Martínez took part in the development of the study and data analysis.
Teodomiro Fuente took part in the development of the study.
José Antonio Pons took part in the development of the study.
José Antonio Navarro took part in the development of the study and data analysis.
Maruja Majado took part in the development of the study.
Pedro Martínez took part in the development of the study.
Pascual Parrilla took part in the experimental design.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to thank Dr. Pedro Luis Tornel, Dr. Pablo Pelegrin, Dr. Manuel Canteras and Dr. Guadalupe Ruiz Merino for their essential contribution to this work.
Please cite this article as: Fernández-Rodríguez OM, Ríos A, Palenciano C, Ramírez P, Navarro JL, Martínez-Alarcón L, et al. Estudio hemodinámico, metabólico e histopatológico de un modelo porcino de trasplante auxiliar heterotópico hepático con arterialización portal. Cir Esp. 2016;94:77–85.