Transarterial chemoembolization (TACE) is considered a therapeutic option. It is mostly used in hepatocellular carcinoma or liver colorectal, neuroendocrine or melanoma metastases. Although it is considered a safe procedure, TACE presents complications, such as acute cholecystitis, which is the most common. Other procedure-related complications include pulmonary embolism, hepatic abscess, bile duct injury, gastric mucosa injury and, less frequently, acute pancreatitis. The aim of this study is to review the complications following TACE for liver tumors.
MethodsWe performed a retrospective study including all the TACE procedures performed in a single center during the period between January 2013 and December 2016.
ResultsOut of the 196 patients with liver tumors who had undergone 322 TACE, 258 (80%) were male and 64 (20%) were female. Mean patient age was 66.5 years. Major complications after chemoembolization included: decompensation with edema/ascites (6 patients), acute cholecystitis (4), acute pancreatitis (3), liver rupture (1), liver abscess (1) and renal failure (1). Postembolization syndrome appeared in 71 (20%) patients. On multivariate analysis, it was observed that concomitant cardiovascular disease (OR: 4.5; 95% CI: 1.2–17; P=.025) is a risk factor for the development of complications.
ConclusionsTACE is a safe and effective procedure for liver tumor treatment. The majority of the complications are rare and present a low incidence of mortality.
La quimioembolización transarterial (QETA) es considerada una opción terapéutica utilizada en el tratamiento del carcinoma hepatocelular y de las metástasis hepáticas secundarias del carcinoma colorrectal, tumores neuroendocrinos y melanoma ocular. Aunque es un procedimiento seguro, no está exento de complicaciones, siendo la más frecuente la colecistitis aguda. Otras complicaciones descritas son el tromboembolismo pulmonar, el absceso hepático, lesiones de la mucosa gastrointestinal, lesiones de la vía biliar, etc. El objetivo principal del estudio es revisar y describir las complicaciones derivadas de la QETA en el tratamiento de los tumores hepáticos.
MétodosSe ha realizado un análisis retrospectivo de todas las QETA practicadas en nuestro centro entre enero de 2013 y diciembre de 2016. En dicho periodo se realizaron 322 QETA en 196 pacientes.
ResultadosDel total de procedimientos, 258 (80%) fueron realizados en hombres y 64 (20%) en mujeres. Además, la edad media de los pacientes fue de 66,5años. Las complicaciones mayores derivadas de la QETA fueron descompensación edemo-ascítica (6casos), colecistitis aguda (4), pancreatitis aguda (3), rotura hepática (1), absceso hepático (1) y deterioro de la función renal (1). Además, el síndrome postembolización se objetivó en 71 (22%) casos. En el análisis multivariante se observó que el antecedente cardiovascular (OR: 4,5; IC95%: 1,2-17; p=0,025) es un factor de riesgo para el desarrollo de complicaciones post-QETA.
ConclusionesLas complicaciones derivadas de la QETA son poco frecuentes y con una baja incidencia de mortalidad.
Transarterial chemoembolization (TACE) is a therapeutic option commonly used in the treatment of primary hepatocellular carcinoma (HCC) and secondary hepatic metastases of colorectal cancer, neuroendocrine tumors and ocular melanoma.1–3 Likewise, it can be used as adjuvant treatment before or after surgical resection or as a bridge therapy before liver transplantation.
The various TACE techniques include selective or supraselective catheterization and the use of different chemotherapeutic and embolization agents, which can influence outcome.4 In recent years, calibrated synthetic microspheres have been developed that are loaded with the chemotherapeutic agent. These provide a more uniform, prolonged release of the drug and achieve high concentrations of the chemotherapeutic agent in the tumor cells, reducing its passage though the systemic circulation and thereby minimizing side effects.4,5 This technique is known as DEB-TACE (Drug Eluting Beads) to differentiate it from conventional TACE, in which the chemotherapeutic agent is administered together with lipiodol and subsequently the occlusion material. Furthermore, the safety of both treatments has been evaluated, and no differences have been found in their safety profiles. In addition, this method does not increase survival or decrease local recurrence,6 and the incidence of adverse effects within the first 30 days is similar,7 although DEB seems to be better tolerated.
Even though TACE is considered a safe procedure, it is not free of complications, the most frequent of which are acute cholecystitis and leukopenia.8 Other complications described are pulmonary thromboembolism, hepatic ischemia, liver abscess, bile duct lesions and, less frequently, acute pancreatitis.9–11 The main objective of this study was to review and describe the complications derived from performing TACE in the treatment of hepatic tumors (primary or metastatic). Likewise, the secondary objectives were to describe the epidemiological, clinical and analytical characteristics of the patients who underwent said procedure.
MethodsWe present a retrospective analysis of all TACE performed at our hospital between January 2013 and December 2016. During that period, 322 TACE were performed in 196 patients.
Demographic, clinical, analytical, radiological and treatment data were extracted from the hospital's electronic medical records. We also recorded the associated comorbidity of each patient: cardiovascular (hypertension, heart disease, valvulopathy or peripheral arterial disease), pulmonary and renal. Laboratory tests included complete blood count, coagulation, liver and kidney function tests, serum alpha-fetoprotein (AFP) and viral markers for hepatitis B and C. In addition, the computed tomography (CT) and magnetic resonance (MRI) findings were analyzed.
All the cases were presented before a multidisciplinary committee (oncologist, diagnostic and interventional radiologist, surgeon and hepatologist), where the indication of TACE in each patient was discussed following the Barcelona Clinic Liver Cancer (BCLC) guidelines for cases of HCC.12,13 In addition, the diagnosis of primary HCC was based on the criteria of the European Association for the Study of the Liver (EASL).14 In patients with intrahepatic cholangiocarcinoma (ICC) and liver metastasis, palliative TACE was considered. The absolute contraindications for the procedure included: decompensated cirrhosis (Child–Pugh B>8), jaundice, encephalopathy and refractory ascites or hepatorenal syndrome; severe portal flow obstruction; tumor invading both lobes; portal vein tumor thrombosis; evident arteriovenous shunt; and renal function deterioration (creatinine≥2mg/dL or clearance <30mL/min).
All procedures were performed by interventional radiologists with similar experience, using Dyna-CT for imaging guidance. By means of the Seldinger technique, the femoral artery was punctured, and an angiography of the superior mesenteric artery and right and left hepatic arteries was performed to determine the main artery that supplied and nourished the tumor. After selective or supraselective catheterization, chemoembolization was performed with a mixture of doxorubicin hydrochloride (Accord Healthcare Laboratory) and lipiodol (Guerbet Laboratory) for cases with HCC and ICC, and irinotecan for cases of metastasis. In addition, in certain cases, drug-eluting beads (DEB) (HepaSphere®) were used, preferably in multiple-lobe tumors, although the decision to use or not use the microspheres was made by the interventional radiologists.
In all patients, a follow-up abdominal CT scan was performed 4–6 weeks after the procedure to assess the response, based on the modified criteria from the Response Evaluation Criteria In Solid Tumors (RECIST).15,16 Likewise, the decision to repeat the procedure or perform another imaging test 3 months later was made at that moment, depending on the CT results, with a minimal interval of 2–3 months between the TACE. In addition, all adverse effects occurring within the first 6 weeks after the procedure were recorded. Adverse effects were grouped according to the classification of The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE): grade 1 or mild (asymptomatic or mild symptoms), grade 2 or moderate, grade 3 or severe (do not endanger life, but prolong hospitalization), grade 4 (endanger life) and grade 5 (death).17
On the other hand, in order to facilitate the analysis of complications, we have divided them into minor and major. The minor complications reduced the quality of recovery and prolonged the hospital stay, while the major complications also endangered the patient's life.
Statistical AnalysisThe categorical variables have been expressed according to frequencies, while the quantitative variables have been expressed by mean and standard deviation. A multivariate analysis was conducted using binary logistic regression to evaluate the risk of developing complications. The final model was described through the odds ratio, providing its 95% confidence interval together with the P value. A P≤.05 was considered statistically significant. The data analysis was carried out with the SPSS version 20.0.
ResultsOut of the total number of procedures, 258 (80%) were performed in men and 64 (20%) in women. The average patient age was 66.5 years, and the average size of the liver tumors was 31.6mm.
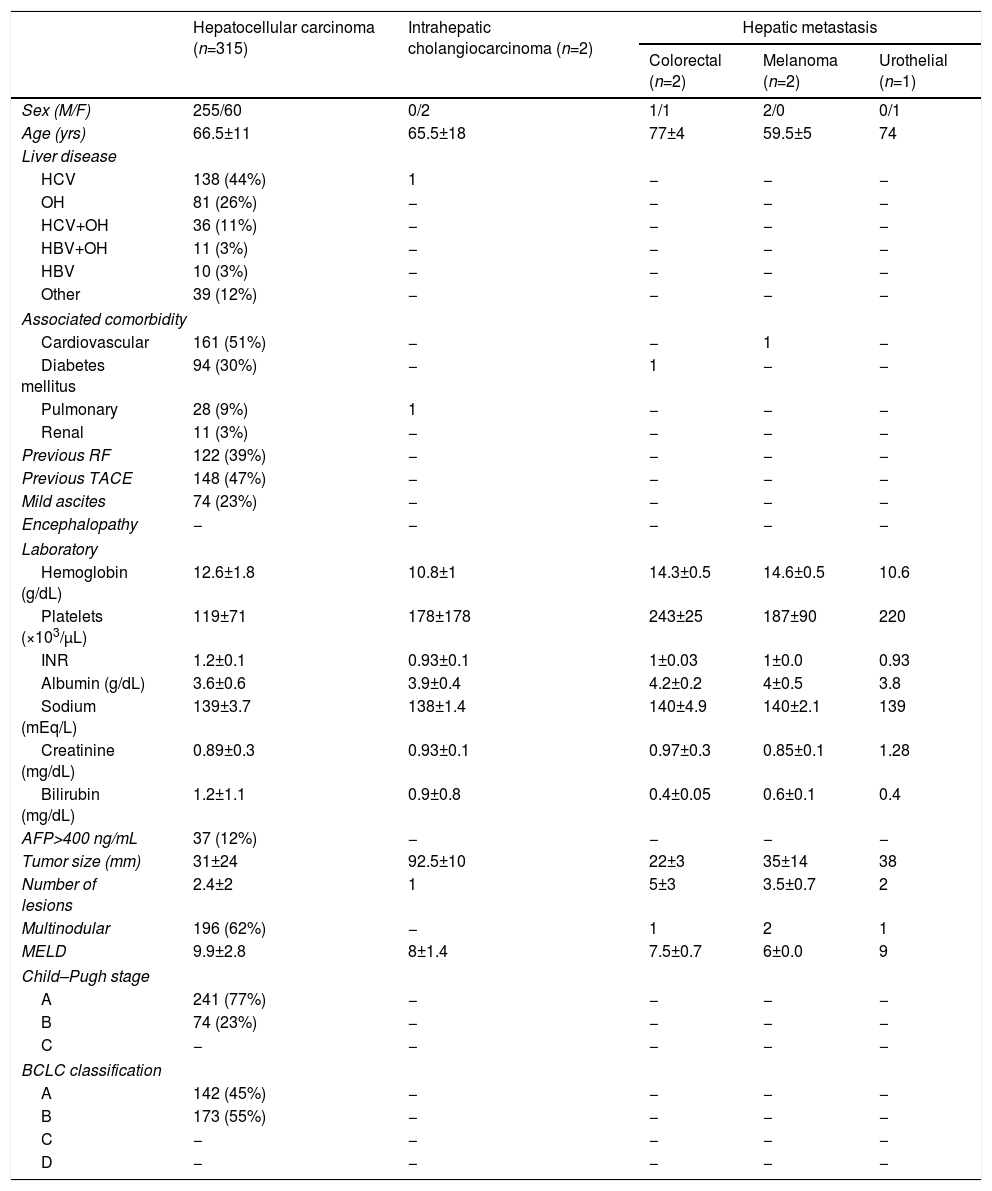
The primary diagnosis with indication for TACE was primary HCC, representing 97.8% (315 cases); other causes were liver metastases and ICC. All patients with HCC had chronic liver disease, the main etiologies being hepatitis C virus (44%) and alcohol (26%). The main comorbidities listed in the patient history were cardiovascular disease (50%) and diabetes (30%) (Table 1).
Epidemiological, Clinics, Analytics and Radiological Characteristics of the Patients Treated With Transarterial Chemoembolization.
| Hepatocellular carcinoma (n=315) | Intrahepatic cholangiocarcinoma (n=2) | Hepatic metastasis | |||
|---|---|---|---|---|---|
| Colorectal (n=2) | Melanoma (n=2) | Urothelial (n=1) | |||
| Sex (M/F) | 255/60 | 0/2 | 1/1 | 2/0 | 0/1 |
| Age (yrs) | 66.5±11 | 65.5±18 | 77±4 | 59.5±5 | 74 |
| Liver disease | |||||
| HCV | 138 (44%) | 1 | − | − | − |
| OH | 81 (26%) | − | − | − | − |
| HCV+OH | 36 (11%) | − | − | − | − |
| HBV+OH | 11 (3%) | − | − | − | − |
| HBV | 10 (3%) | − | − | − | − |
| Other | 39 (12%) | − | − | − | − |
| Associated comorbidity | |||||
| Cardiovascular | 161 (51%) | − | − | 1 | − |
| Diabetes mellitus | 94 (30%) | − | 1 | − | − |
| Pulmonary | 28 (9%) | 1 | − | − | − |
| Renal | 11 (3%) | − | − | − | − |
| Previous RF | 122 (39%) | − | − | − | − |
| Previous TACE | 148 (47%) | − | − | − | − |
| Mild ascites | 74 (23%) | − | − | − | − |
| Encephalopathy | − | − | − | − | − |
| Laboratory | |||||
| Hemoglobin (g/dL) | 12.6±1.8 | 10.8±1 | 14.3±0.5 | 14.6±0.5 | 10.6 |
| Platelets (×103/μL) | 119±71 | 178±178 | 243±25 | 187±90 | 220 |
| INR | 1.2±0.1 | 0.93±0.1 | 1±0.03 | 1±0.0 | 0.93 |
| Albumin (g/dL) | 3.6±0.6 | 3.9±0.4 | 4.2±0.2 | 4±0.5 | 3.8 |
| Sodium (mEq/L) | 139±3.7 | 138±1.4 | 140±4.9 | 140±2.1 | 139 |
| Creatinine (mg/dL) | 0.89±0.3 | 0.93±0.1 | 0.97±0.3 | 0.85±0.1 | 1.28 |
| Bilirubin (mg/dL) | 1.2±1.1 | 0.9±0.8 | 0.4±0.05 | 0.6±0.1 | 0.4 |
| AFP>400 ng/mL | 37 (12%) | − | − | − | − |
| Tumor size (mm) | 31±24 | 92.5±10 | 22±3 | 35±14 | 38 |
| Number of lesions | 2.4±2 | 1 | 5±3 | 3.5±0.7 | 2 |
| Multinodular | 196 (62%) | − | 1 | 2 | 1 |
| MELD | 9.9±2.8 | 8±1.4 | 7.5±0.7 | 6±0.0 | 9 |
| Child–Pugh stage | |||||
| A | 241 (77%) | − | − | − | − |
| B | 74 (23%) | − | − | − | − |
| C | − | − | − | − | − |
| BCLC classification | |||||
| A | 142 (45%) | − | − | − | − |
| B | 173 (55%) | − | − | − | − |
| C | − | − | − | − | − |
| D | − | − | − | − | − |
AFP: alpha-fetoprotein; BCLC: Barcelona Clinic Liver Cancer; M: male; F: female; MELD: Model for End-stage Liver Disease; OH: alcohol; RF: radiofrequency; HBV: hepatitis B virus; HCV: hepatitis C virus.
In 148 (46%) cases, anterior chemoembolization was performed. In addition, 201 (62%) cases presented more than one liver injury. The mean AFP was 795ng/mL and only in 37 (11%) cases was the AFP≥400ng/mL. Likewise, in patients with stage A (BCLC), the indication for TACE was as a bridge therapy to liver transplantation.
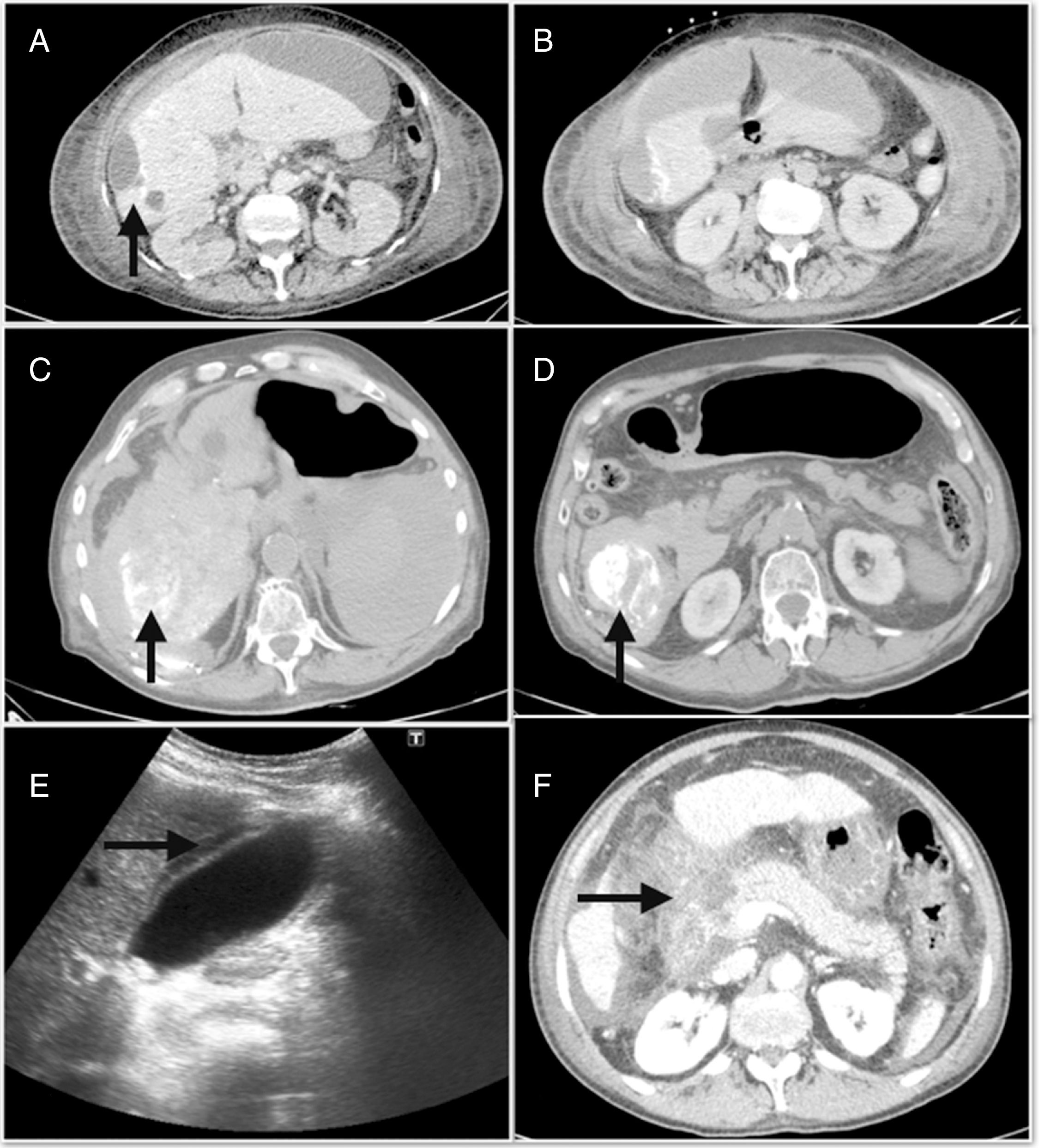
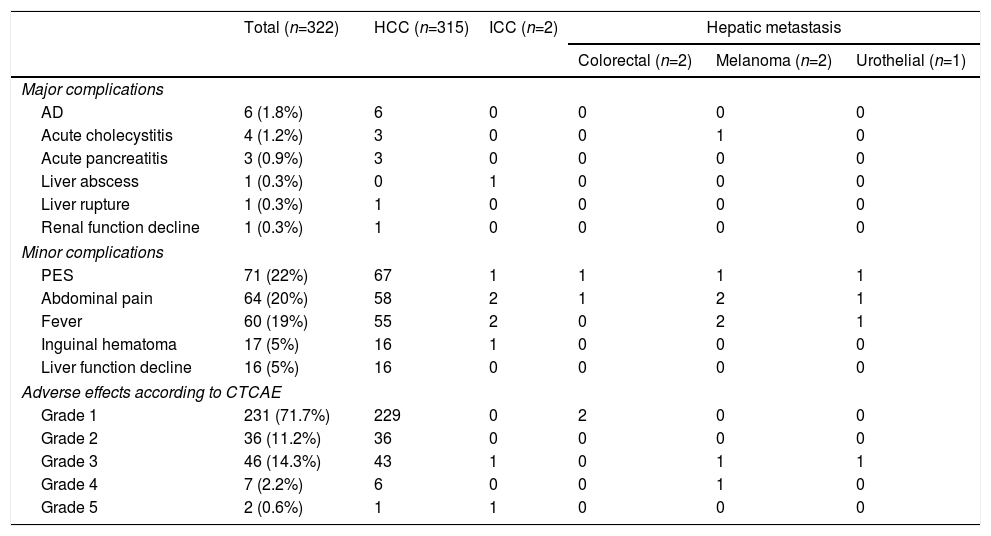
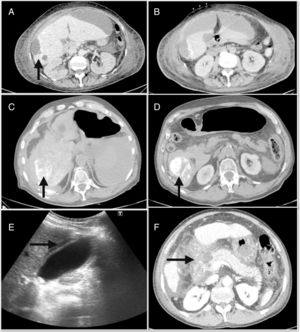
The major complications derived from the TACE had an incidence of 4.9% and were mainly edemo-ascitic decompensation,6 acute cholecystitis4 and acute pancreatitis.3Fig. 1 shows some of the major complications, such as hepatic abscess, hemoperitoneum and pancreatitis. In addition, among the minor complications, post-embolization syndrome (PES) was detected in 71 (22%) cases. However, when we analyzed the adverse effects, 71.7% of the patients were asymptomatic or had mild symptoms (Table 2).
(A) Post-chemoembolization hepatic abscess; (B) subcapsular peri-hepatic collection that continues with the peripheral lesion of segment 6; (C) hemoperitoneum occupying predominantly the right flank; (D) foci of lipiodol extravasation toward the abdominal cavity; (E) abdominal ultrasound with signs of acute cholecystitis; (F) extensive necrosis of the head of the pancreas associated with necrosis of the peripancreatic fat and the root of the mesentery.
Incidence of Complications and Adverse Effects Associated With Transarterial Chemoembolization.
| Total (n=322) | HCC (n=315) | ICC (n=2) | Hepatic metastasis | |||
|---|---|---|---|---|---|---|
| Colorectal (n=2) | Melanoma (n=2) | Urothelial (n=1) | ||||
| Major complications | ||||||
| AD | 6 (1.8%) | 6 | 0 | 0 | 0 | 0 |
| Acute cholecystitis | 4 (1.2%) | 3 | 0 | 0 | 1 | 0 |
| Acute pancreatitis | 3 (0.9%) | 3 | 0 | 0 | 0 | 0 |
| Liver abscess | 1 (0.3%) | 0 | 1 | 0 | 0 | 0 |
| Liver rupture | 1 (0.3%) | 1 | 0 | 0 | 0 | 0 |
| Renal function decline | 1 (0.3%) | 1 | 0 | 0 | 0 | 0 |
| Minor complications | ||||||
| PES | 71 (22%) | 67 | 1 | 1 | 1 | 1 |
| Abdominal pain | 64 (20%) | 58 | 2 | 1 | 2 | 1 |
| Fever | 60 (19%) | 55 | 2 | 0 | 2 | 1 |
| Inguinal hematoma | 17 (5%) | 16 | 1 | 0 | 0 | 0 |
| Liver function decline | 16 (5%) | 16 | 0 | 0 | 0 | 0 |
| Adverse effects according to CTCAE | ||||||
| Grade 1 | 231 (71.7%) | 229 | 0 | 2 | 0 | 0 |
| Grade 2 | 36 (11.2%) | 36 | 0 | 0 | 0 | 0 |
| Grade 3 | 46 (14.3%) | 43 | 1 | 0 | 1 | 1 |
| Grade 4 | 7 (2.2%) | 6 | 0 | 0 | 1 | 0 |
| Grade 5 | 2 (0.6%) | 1 | 1 | 0 | 0 | 0 |
ICC: intrahepatic cholangiocarcinoma; HCC: hepatocellular carcinoma; CTCAE: The National Cancer Institute Common Terminology Criteria for Adverse Events; AD: ascitic decompensation; PES: post-embolization syndrome.
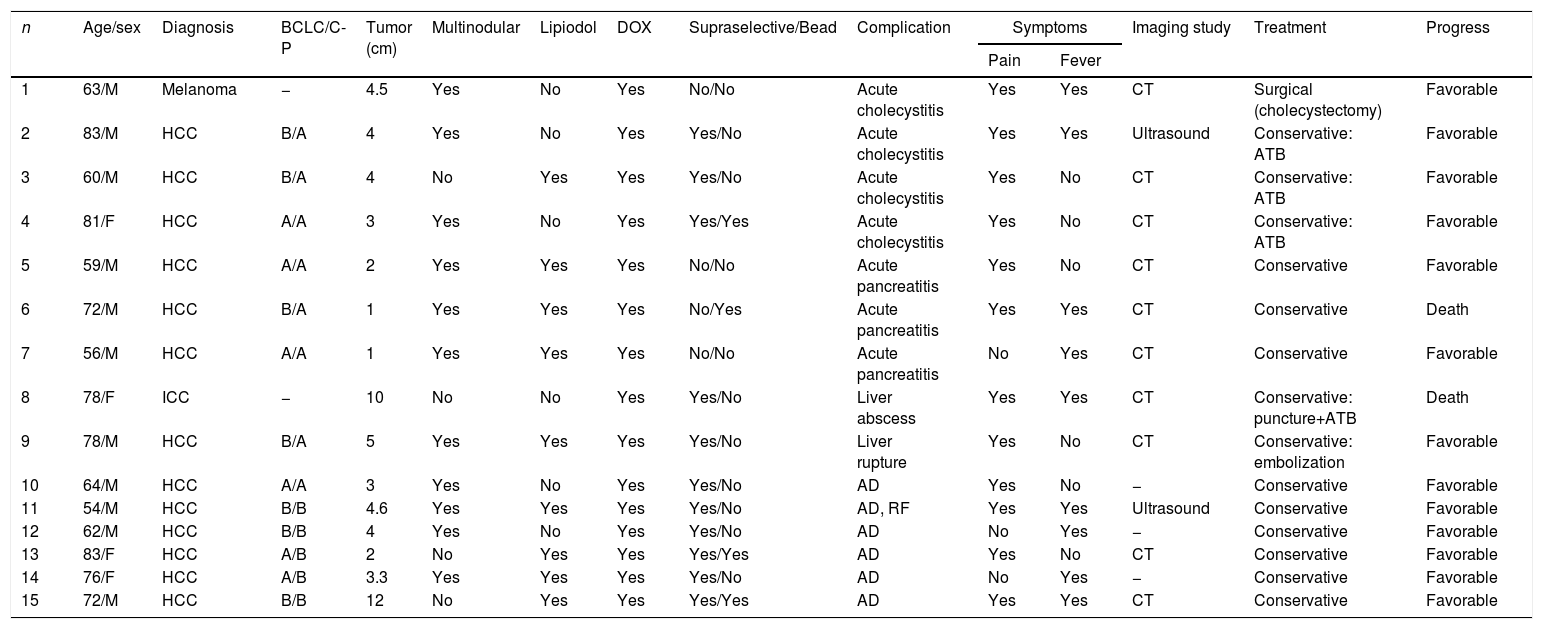
Table 3 demonstrates the 15 patients who presented a major complication derived from the procedure, which was treated conservatively in 14 (93%) patients. Only one patient with a diagnosis of cholecystitis underwent surgical treatment (cholecystectomy). In contrast, mortality related to the procedure occurred in 2 (0.6%) cases. When the multivariate analysis was carried out, we observed that the cardiovascular history (OR: 4.5, 95% CI: 1.2–17, P=.025) was a risk factor for developing post-TACE complications.
Characteristics of the Patients Who Developed Major Complications After Transarterial Chemoembolization.
| n | Age/sex | Diagnosis | BCLC/C-P | Tumor (cm) | Multinodular | Lipiodol | DOX | Supraselective/Bead | Complication | Symptoms | Imaging study | Treatment | Progress | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain | Fever | |||||||||||||
| 1 | 63/M | Melanoma | − | 4.5 | Yes | No | Yes | No/No | Acute cholecystitis | Yes | Yes | CT | Surgical (cholecystectomy) | Favorable |
| 2 | 83/M | HCC | B/A | 4 | Yes | No | Yes | Yes/No | Acute cholecystitis | Yes | Yes | Ultrasound | Conservative: ATB | Favorable |
| 3 | 60/M | HCC | B/A | 4 | No | Yes | Yes | Yes/No | Acute cholecystitis | Yes | No | CT | Conservative: ATB | Favorable |
| 4 | 81/F | HCC | A/A | 3 | Yes | No | Yes | Yes/Yes | Acute cholecystitis | Yes | No | CT | Conservative: ATB | Favorable |
| 5 | 59/M | HCC | A/A | 2 | Yes | Yes | Yes | No/No | Acute pancreatitis | Yes | No | CT | Conservative | Favorable |
| 6 | 72/M | HCC | B/A | 1 | Yes | Yes | Yes | No/Yes | Acute pancreatitis | Yes | Yes | CT | Conservative | Death |
| 7 | 56/M | HCC | A/A | 1 | Yes | Yes | Yes | No/No | Acute pancreatitis | No | Yes | CT | Conservative | Favorable |
| 8 | 78/F | ICC | − | 10 | No | No | Yes | Yes/No | Liver abscess | Yes | Yes | CT | Conservative: puncture+ATB | Death |
| 9 | 78/M | HCC | B/A | 5 | Yes | Yes | Yes | Yes/No | Liver rupture | Yes | No | CT | Conservative: embolization | Favorable |
| 10 | 64/M | HCC | A/A | 3 | Yes | No | Yes | Yes/No | AD | Yes | No | − | Conservative | Favorable |
| 11 | 54/M | HCC | B/B | 4.6 | Yes | Yes | Yes | Yes/No | AD, RF | Yes | Yes | Ultrasound | Conservative | Favorable |
| 12 | 62/M | HCC | B/B | 4 | Yes | No | Yes | Yes/No | AD | No | Yes | − | Conservative | Favorable |
| 13 | 83/F | HCC | A/B | 2 | No | Yes | Yes | Yes/Yes | AD | Yes | No | CT | Conservative | Favorable |
| 14 | 76/F | HCC | A/B | 3.3 | Yes | Yes | Yes | Yes/No | AD | No | Yes | − | Conservative | Favorable |
| 15 | 72/M | HCC | B/B | 12 | No | Yes | Yes | Yes/Yes | AD | Yes | Yes | CT | Conservative | Favorable |
ATB: antibiotic; BCLC: Barcelona Clinic Liver Cancer; ICC: intrahepatic cholangiocarcinoma; HCC: hepatocellular carcinoma; C-P: Child–Pugh; AD: ascitic decompensation; DOX: doxorubicin; RF: renal failure; M: male; F: female; CT: abdominal computed tomography scan.
The first embolization was described by Doyon et al.18 in 1974. Subsequently, in 1983, Yamada et al.19 added the use of a gelatin sponge and a chemotherapeutic agent, thus creating the concept of transarterial chemoembolization. Liver tumors receive 90% of their blood supply through the hepatic artery. Therefore, embolization causes ischemic necrosis of the tumor, resulting in damage to the membrane receptors of the tumor cell, thereby increasing absorption of the chemotherapeutic agent.20
The indications for TACE of the liver involve hypervascular tumors, and it is most frequently applied in HCC, ICC and liver metastases. Likewise, it is relevant in cases of HCC recurrence after surgical resection, as a bridge therapy to liver transplantation, or as neoadjuvant therapy in patients with potentially resectable tumors, although in these cases it has not been shown to increase survival.21,22
With regard to other types of tumors, its use has also been described in colorectal and neuroendocrine tumor metastases.3,5 However, the non-hypervascular nature of colorectal metastases limits the diffusion of chemoembolization agents. Therefore, TACE is used as a second-line treatment after the absence of response to chemotherapy, with a response rate of 25%. In our study, it has been used in 5 liver metastases: 2 colorectal, 2 uveal melanoma and one urothelial.
The complications of TACE are rare and their frequency is lower than 5%, similar to our study. The main risk factors are portal vein obstruction, impaired liver functional reserve, biliary obstruction, previous biliary surgery, excess lipiodol injection and non-selective embolization.23
PES is a type of minor and pathognomonic complication of TACE. It can appear immediately afterwards or in the 10 days following the procedure, prolonging hospitalization and limiting the application of additional treatments.24,25 It occurs in the form of fever, abdominal pain, nausea and/or vomiting and elevated transaminase levels. The underlying mechanisms have not been well established, and some of the proposed hypotheses are hepatic ischemia, Glisson capsule distention, or gallbladder ischemia due to embolization of the cystic artery.26
The most serious major complication is liver failure. Predisposing factors are hyperbilirubinemia, advanced cirrhosis, or the administration of high doses of the chemotherapeutic agent.27 In our series, major complications included ascitic decompensation (2.8%), acute cholecystitis (1.5%), acute pancreatitis (0.9%) and renal function decline (0.6%).
Dhamija et al.28 reported an incidence of biliary complications of 1.9% due to the exclusive vascularization of the bile duct by branches of the hepatic artery. This can cause necrosis of the bile duct, ectasia, the formation of biliomas or stenosis. Reported predisposing factors include tumor size, existing dilation of the bile duct prior to the procedure, proximal embolization, the interval between two procedures less than 3 months and the injection of lipiodol with the chemotherapeutic agent. Monier et al,29 however, observed an increase in bile duct damage with the use of TACE-DEB compared with TACE. This finding is rather controversial, considering the existence of several randomized controlled studies demonstrating the safety of the use of TACE-DEB compared to TACE in patients with more compromised liver function. However, these studies did not objectively evaluate locoregional toxicity. In addition, patients with advanced cirrhosis have a lower risk of developing locoregional toxicity with the TACE-DEB due to gradual hypertrophy of the peribiliary vascular plexus caused by portal hypertension and collateral vascularization.
Another uncommon and serious complication is acute pancreatitis, and it has been suggested that its pathogenesis resides in the regurgitation of chemoembolization material to the gastroduodenal artery, causing pancreatic ischemia.30 The incidence described in the literature is around 1.5%–2%31 and 0.9% in our study.
With regards to renal function decline (defined in our study as a sudden increase in creatinine greater than 50% over the baseline level or more than 1.5mg/dL within the first 7 days after of the procedure), the underlying mechanism is contrast-induced nephrotoxicity, although it is true that the rate of renal decline is higher in patients with HCC who undergo a TACE than other subjects who undergo another angiographic procedure.32 The risk of renal failure is related to the dose and number of sessions of TACE, and may have a cumulative effect on this risk.
Other complications described in our series are abscess (0.6%) and hepatic rupture (0.6%). The incidence of liver abscess is similar to the cases described in the literature. The risk factors associated with this complication are biliointestinal bypass, advanced age, diabetes mellitus, tumor size and portal vein occlusion.33 In most patients, abscesses present as solitary lesions (66.7%), and the imaging test of choice is CT. Regarding treatment, a meta-analysis found that abscesses measuring less than 5cm may be treatable with antibiotics, and percutaneous or surgical drainage is the preferred option in cases greater than 5cm.34
In our series, we only had one case of hepatic rupture (0.6%), similar to the Tu et al.25 series. The risk factors for hepatic rupture are giant tumors (>10cm), or tumors located on the liver surface. Conservative management can be performed, which is sometimes supplemented with another embolization.
Another serious complication described, although not present in our series, is acute lung injury or respiratory distress syndrome caused by the emboligenic material that reaches the pulmonary vascularization due to an arteriovenous shunt. This complication is rare (0.05% of cases).35
Also, in the multivariate analysis, we observed that cardiovascular history is a risk factor for the development of complications. This data is not reflected in other publications, so we believe it could be novel and a starting point for future studies. However, as the cardiovascular patient history refers to a group of pathologies, it would be interesting for future studies to analyze these separately. Another limitation of this study is its retrospective nature, which may affect the results, because perhaps not all complications were recorded or identified by physicians.
In conclusion, the complications derived from the TACE are uncommon, with a low incidence of mortality, and most are resolved with conservative treatment. In addition, the presence of cardiovascular comorbidity is associated with an increased risk of developing complications after the procedure.
Conflict of InterestsThe authors have no economic, personal or professional conflicts of interests.
Please cite this article as: Marcacuzco Quinto A, Nutu O-A, San Román Manso R, Justo Alonso I, Calvo Pulido J, Manrique Municio A, et al. Complicaciones de la quimioembolización transarterial (QETA) en el tratamiento de los tumores hepáticos. Cir Esp. 2018;96:560–567.