Even though cytology remains the gold standard to assess the nature of thyroid nodules, up to 30% of the results are indeterminate (Bethesda III and IV). In these cases, current guidelines recommend performing diagnostic surgery, which proves malignancy in only 15%–30% of cases. A more precise method is needed to avoid unnecessary surgeries, surgical complications and costs in the process of diagnosing indeterminate nodules. Complementary use of molecular profiling tests seems to help in this complex scenario. We present a review of the current literature on the usefulness of molecular profiling of thyroid nodules so as to define its indications, costs and usability for clinical practice.
El análisis citológico tiene un papel fundamental en el estudio de los nódulos tiroideos. Sin embargo, hasta un 30% de estos muestran citologías indeterminadas (BethesdaIII o IV). En estos casos, se realizan cirugías diagnósticas que únicamente demuestran malignidad en un 15–35% de los pacientes. Se precisa una herramienta de mayor precisión para determinar la benignidad o malignidad del nódulo tiroideo con citología indeterminada sin precisar cirugías diagnósticas, evitando así posibles complicaciones y/o costes innecesarios. El uso complementario de paneles moleculares junto con la citología ha sido, de momento, la única herramienta que parece ayudar en este difícil escenario. Se realiza una revisión de la bibliografía sobre el estudio molecular complementario de los nódulos tiroideos para tratar de resumir las características intrínsecas de cada uno de los test disponibles, su coste-efectividad, y determinar sus indicaciones y su aplicabilidad en la práctica clínica habitual.
Thyroid nodules are a common problem, with a prevalence in the general population between 3 and 8%, although in autopsy series the percentage is close to 50%, the majority being benign nodules. Only about 5% of thyroid nodules are malignant and generally have a good prognosis, but their incidence has been increasing in recent years.1 The fundamental importance of this common pathology lies in differentiating benign nodules from thyroid cancer. In general, ultrasonography and diagnosis by fine needle aspiration (FNA) cytology correctly classify most cases. However, when the cytological result is indeterminate (fundamentally Bethesda classification of Bethesda III and IV), the patient frequently undergoes surgery for diagnostic purposes. In this situation, in up to three-quarters of the patients the final diagnosis is usually a benign lesion (follicular or Hürthle cell adenoma), but approximately 15%–35% will be cancer (follicular, follicular variant of papillary cancer or of Hürthle cells).2
Various cytological studies and imaging tests have tried to increase diagnostic accuracy in these cases, but until now they have not reached conclusive results that avoid surgery in cases where the nodule is finally shown to be benign. Both radiological advances and research in molecular biology, including gene expression studies, pillars in the diagnosis of thyroid cancer, have provided a multitude of data. However, today there is still no single marker with sufficient discriminative capacity between benign and malignant nodules when cytology is inconclusive. Recent studies seem to indicate that including molecular studies in the preoperative study of thyroid nodules with indeterminate cytology can increase diagnostic accuracy in the classification of thyroid nodules, thereby reducing the rate of unnecessary diagnostic hemithyroidectomies in our setting.
This review analyzes the literature on the molecular study of thyroid nodules, with the aim of trying to clarify its possible indications, advantages and potential for use in clinical practice.
Molecular Markers and Thyroid CancerThyroid molecular markers are genetic mutations presented by malignant thyroid cells that can be studied in fragments of FNA thyroid tissue biopsies using molecular biology techniques. In papillary thyroid carcinoma, the best known is the BRAF mutation, present in up to 40%–45% of cases, followed by RAS and RET/PTC in about 20% of cases. In follicular thyroid cancer, RAS mutation is found in 40%–50% of cases, followed by PAX8/PPARγ in 30%–35%.3 There are currently several molecular panels that include the diagnosis of most of the mutations involved in thyroid cancer. Among these are Thyroseq®, ThyGenX™, ThyraMIR™, Afirma® and RosettaGx Reveal. They include the main mutations (BRAF, KRAS, NRAS, HRAS) and genomic rearrangements (RET/PTC1, RET/PTC3 and PAX8/PPARγ), studying somatic mutations from DNA and rearrangements from mRNA extracted from cytological material. The analysis is performed by new generation sequencing after amplification of DNA and mRNA by real-time PCR and the use of microarrays.
The main indications of use proposed for these panels (although still not validated) are as follows4:
- -
In nodules with indeterminate cytology (Bethesda III and IV), to try to improve the diagnosis and reclassify them as probably benign or malignant.
- -
In benign nodules by cytology (Bethesda II), in which the ultrasound characteristics are highly suspicious (disagreement between complementary tests).
- -
In malignant nodules (Bethesda V and VI), in which molecular characteristics are intended to guide surgical extension.
On the one hand, the molecular panels (Thyroseq® and ThyGenX™) analyze specific genes whose mutations are related to thyroid cancer. On the other hand are the gene expression classifiers (Afirma® and ThyraMIR™, RosettaGx) that analyze the expression levels of multiple genes simultaneously in microarrays to create a gene expression profile of the nodule under study. As a summary, the characteristics of these panels are:
- -
ThyroSeq® includes 60 genes. Its version Tyroseq V2 improved its characteristics, especially the positive predictive value (PPV),5 including more than 1000 mutations of 14 genes for DNA study and 42 genetic fusions in 16 genes to evaluate RNA expression. It has been evaluated mainly for follicular-type neoplasms. In addition, it includes genes to determine whether the tissue being studied is parathyroid in origin.
- -
ThyGenX™ includes sequencing of the eight most common genes related to thyroid cancer, including PIK3CA (involved in the progression of follicular and anaplastic cancer), for use in indeterminate cytologies, regardless of type.
- -
Afirma® analyzes the mRNA of the expression of 167 genes, searching for expression patterns associated with benignity through algorithms. Unlike the previous panels, Afirma is a test designed to rule out malignancy, not to increase suspicion. It helps to select low-risk patients. There are two specific variants for assessing BRAF mutations or those associated with medullary carcinoma (Afirma® BRAF and Afirma® MTC, respectively).6
- -
ThyraMIR™ is another gene expression panel that analyzes 10 microRNAs involved in the proliferation and progression of thyroid tumor cells and uses algorithms to classify them as probably benign or suspicious of malignancy. This panel has been designed to increase the power of the ThyGenX panel.
- -
RosettaGx Reveal is the most recent of gene expression panels. After validation studies, it includes 24 mRNA, including mRNA hsa-miR-375, related with medullary thyroid carcinoma.
At the moment, these tests give dichotomous results (probably benign/probably malignant) or positivity/negativity of specific mutations, although in the case of gene expression classifiers it would be interesting to be able to obtain a probabilistic result.
What differences are found between the main molecular panels?Depending on the risk of malignancy of the nodule itself and whether we seek to confirm a malignancy or discard it, we must choose the most appropriate test. We will therefore choose a “rule-out” test (high negative predictive value [NPV] and specificity [Sp]) when we want to identify nodules in which surgery can be avoided, and a “rule-in” test (high PPV and sensitivity [S]) when looking for nodules to indicate surgery.
Afirma® offers an S of 90%, Sp of 52%, with an NPV of 94%–95% and a PPV of 37%–38%. Its main use would be not so much to corroborate cytological suspicion, but to rule out malignancy (rule-out test), thereby being able to identify benign nodules that can avoid surgery. Afirma® is designed to obtain the best results in populations with a prevalence of malignancy in indeterminate nodules of between 15 and 21% (range 12%–25%).4 It has been observed that Afirma® can give false-positives with the presence of Hürthle cells, so its result is less reliable. The study by Hang et al.7 does not recommend the molecular study of nodules with indeterminate cytology that have Hürthle cells, since the low Sp makes their result unreliable. In addition, they refer to the possible worsening of Afirma® properties after reclassification of follicular thyroid nodules with nuclear alterations similar to papillary carcinoma as benign nodules (previously considered follicular subtypes of papillary carcinomas).
ThyGenX and ThyroSeq combine good NPV and PPV, ThyroSeq v2 being the best except when complementing the use of ThyGenX with ThyraMIR™, at which time the NPV rises to 96%. These two panels could therefore be used to rule out malignancy as well as to confirm it (rule-out and rule-in tests), but they are usually used to confirm malignancy.
At the moment, RosettaGx, the last to come on the market, has only been included in the latest comparative study with a single meta-analysis article.8 However, unlike the rest, it is the only test applicable in samples that have been previously fixed and stained, frozen or in paraffin blocks, without the need for fresh tissue for study.9 It does not therefore require a second biopsy (after indeterminate cytological result) and can be used in the same sample.
The most recent study is a meta-analysis conducted by Vargas-Salas et al.8 comparing the different panels available, including 26 studies from US medical centers (19 Afirma®, 5 ThyroSeq®, 1 ThyGenX™/ThyraMIR™ and RosettaGx Reveal), 8 of which were multicentric. Comparing the different tests, no significant differences were found regarding the S (around 90%) and NPV (92%–96%), although it is striking that the S of ThyGenX™/ThyraMIR™ was higher in the Bethesda IV cases. In terms of Sp, ThyGenX™/ThyraMIR™ (92%) and ThyroSeq® (85%) were notably better than Afirma® (52%) and RosettaGx Reveal (74%). The same goes for ThyGenX™/ThyraMIR™ and ThyroSeq®, presenting a PPV around 78% (which increased up to 82% in the Bethesda IV cases) compared to Afirma® (37%) and RosettaGx Reveal (43%), which also showed no differences in the subgroups. Not all studies had control of the outcome after surgery, and also the studies included using ThyGenX™/ThyraMIR™ and RosettaGx Reveal, in addition to being less numerous, had overall worse scientific quality, so the results must be taken with caution.
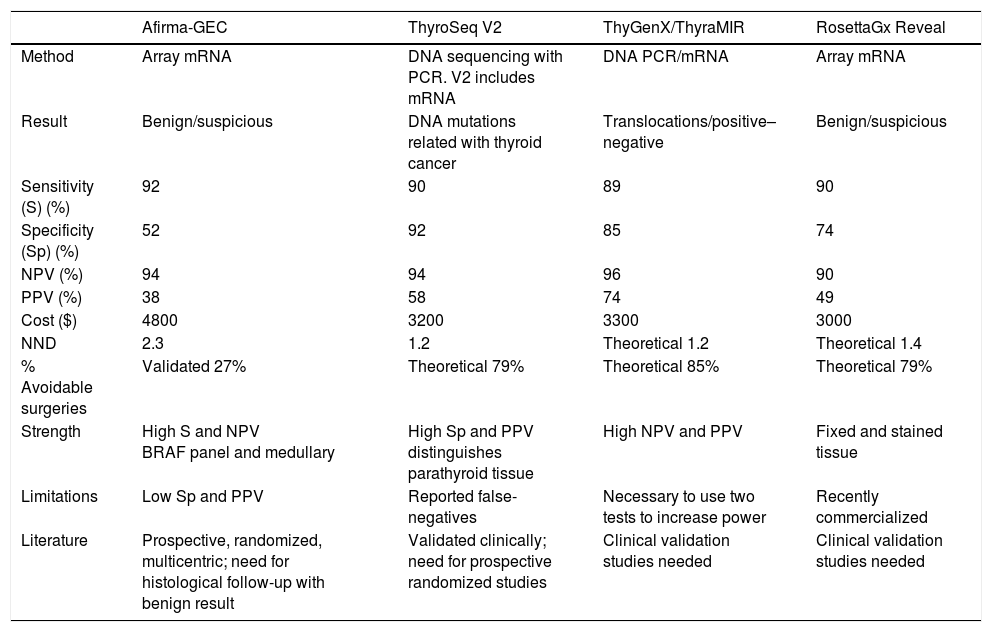
Table 1 summarizes the characteristics of each test derived from the meta-analysis, including the theoretical number of surgeries avoided (NND) and their market prices in the United States.
Comparison of Main Characteristics of the Molecular Panels Available.
| Afirma-GEC | ThyroSeq V2 | ThyGenX/ThyraMIR | RosettaGx Reveal | |
|---|---|---|---|---|
| Method | Array mRNA | DNA sequencing with PCR. V2 includes mRNA | DNA PCR/mRNA | Array mRNA |
| Result | Benign/suspicious | DNA mutations related with thyroid cancer | Translocations/positive–negative | Benign/suspicious |
| Sensitivity (S) (%) | 92 | 90 | 89 | 90 |
| Specificity (Sp) (%) | 52 | 92 | 85 | 74 |
| NPV (%) | 94 | 94 | 96 | 90 |
| PPV (%) | 38 | 58 | 74 | 49 |
| Cost ($) | 4800 | 3200 | 3300 | 3000 |
| NND | 2.3 | 1.2 | Theoretical 1.2 | Theoretical 1.4 |
| % Avoidable surgeries | Validated 27% | Theoretical 79% | Theoretical 85% | Theoretical 79% |
| Strength | High S and NPV BRAF panel and medullary | High Sp and PPV distinguishes parathyroid tissue | High NPV and PPV | Fixed and stained tissue |
| Limitations | Low Sp and PPV | Reported false-negatives | Necessary to use two tests to increase power | Recently commercialized |
| Literature | Prospective, randomized, multicentric; need for histological follow-up with benign result | Validated clinically; need for prospective randomized studies | Clinical validation studies needed | Clinical validation studies needed |
After commercialization, many groups have incorporated these molecular panels into the diagnostic algorithm to try to refine the classification of thyroid nodules with indeterminate cytologies.
Chaudhary et al.10 compared the outcome of Afirma® versus definitive pathology studies in 158 cases of Bethesda III/IV nodules between 2012 and 2015. After the study with Afirma®, 86% of the nodules classified as suspicious by the test were resected, 62% of which were histologically benign. In the case of nodules with Afirma® results for benignity, 13% were resected, and the benign histology of the lesion was confirmed in 100% (NPV 100% and PPV 38%). Afirma® managed to reclassify 40% of thyroid nodules with Bethesda III/IV category as benign, achieving a reduction in the rate of surgical resections (52% vs 76%) only in the Bethesda IV group but not in the III group, compared to the years 2009–2012 (with no complementary use of Afirma®), possibly due to the sample size.
Kloos6 carried out a systematic review of all publications related to the impact of the use of Afirma® on clinical decisions. From this review, the authors concluded that a non-suspicious Afirma® result carries a risk of malignancy of less than 5% (NPV>94%) for Bethesda III and IV nodules in populations with malignancy prevalence of around 20% in these nodules. This result is comparable to the risk of malignancy presented by nodules with benign cytology (6%–8%), which supports the management of the Bethesda III and IV nodes after the non-suspicious Afirma® result as if they were benign nodules. The rate of surgical indication before and after the implementation of Afirma® was also evaluated, demonstrating a significant reduction from the historical 73 to 10% with Afirma® for Bethesda III/IV nodes, with a 3-year follow-up. From this review, it was determined that the number needed to treat (NNT) in order to avoid surgery in a patient is 2, meaning that for every two patients studied with the molecular panel (Bethesda III and IV), one could potentially avoid surgery.5 In the case of cytologies with Hürthle cells, Kloos6 also described the increase in false-positives, so the NNT increases to 3.
Sacks et al.11 published a study in 2016 comparing patients studied with Afirma® between July 2013 and December 2014 (post-Afirma®) with a retrospective cohort from January 2012 to July 2013 (pre-Afirma®), analyzing a total of 4292 samples obtained by FNA. The increase in the diagnosis of Bethesda III and IV in the post-Afirma® group (with a secondary decrease in Bethesda II diagnoses) was striking in this study. The authors attributed this difference to the fact that the very existence and availability of the molecular test would make pathologists unconsciously prefer including nodules in indeterminate categories to complement their study. The rates of surgery and malignancy did not show significant differences between the two groups. In addition, one-third of the patients were studied with Afirma® after an initial result of indeterminate cytology, without repeating FNA. However, 35% of patients with indeterminate cytologies in which FNA was repeated were reclassified (non-indeterminate) through the second aspiration, without specifying the complementary molecular study. Of note in this study is the possible bias when compared to a retrospective cohort.
Does this mean that a negative Afirma® result is reliable, so surgery could be avoided while patients are followed as if it were a benign nodule? Two recent articles have evaluated the long-term follow-up in these cases (between 20 and 36 months) and concluded that their long-term behavior is comparable to that of nodules with benign cytology, so that follow-up without surgery is feasible and safe in these cases. The article by Sipos et al.12 in 2016 carried out a survey of the doctors involved in the clinical decision-making of these patients and found that 86% of them considered molecular nodule study with Afirma® useful and beneficial for patients. On the other hand, Singer et al.13 published a retrospective study in 2016 comparing the follow-up and resection rates of patients with positive cytologies and those with indeterminate cytologies and the Afirma® molecular study, suggestive of benignity. The authors studied 804 patients (201 molecular studies, 603 only cytology) and observed that there were no differences in the rates of surgery during the first 20 months of follow-up (86.6% of patients with a molecular study suggestive of benignity were managed conservatively), or in the need to repeat imaging tests.
There are no publications about the clinical use of ThyGenX™/ThyraMIR™ or RosettaGx, which was the last to enter the market.
Regarding the Thyroseq® gene expression panel, the number of studies is lower. Shapouran et al.14 published a retrospective study with 66 patients (38 Thyroseq® and 28 Afirma®). 57% of the patients had a suspicious Afirma® result; 69% of these underwent surgery, and 36% presented malignancy. In contrast, of the 43% non-suspicious results, benignity was confirmed in 100% during follow-up or with surgery due to nodule growth. Meanwhile, 34% of the patients studied with Thyroseq® showed a suspicious result; 54% were treated surgically and malignancy was confirmed in 71% (better PPV than Afirma®). However, of the 66% of nodules classified as benign, there was only one false-negative. They concluded that the study with a molecular panel managed to avoid surgery in 51% of the patients.
In a review, Nikiforov15 published several studies with results of the use of Thyroseq® in 465 nodules with indeterminate cytology collected between May 2014 and May 2015. S, Sp and predictive values were comparable to those previously mentioned. It is striking that 77% of the suspicious nodules operated on demonstrated malignancy; out of these, 90% were follicular and 10% papillary. There were also two cases of false-negatives (both papillary carcinomas) and five cases of false-positives, all of which were adenomas with RAS mutation, clonal proliferation with nuclear atypia and immunohistochemistry characteristic of papillary carcinoma. In addition, three thyroid nodules expressed parathyroid genes in the molecular panel (they were found to be parathyroid adenomas, showing analytic hyperparathyroidism), so the authors concluded that not only can it be used to distinguish malignancy from benignity, but it can also distinguish non-thyroid tissue.
There is also controversy in the literature about whether the molecular study of nodules can help us define the extent of a thyroidectomy. Nicholson and Yip16 propose that molecular studies should also be used in thyroid nodules measuring between 1 and 4cm with cytology for papillary carcinoma to guide the extension of surgery.
To date, there have been no studies comparing the alteration of the quality of life perceived after diagnostic thyroidectomy compared to the long-term follow-up of nodules with indeterminate cytology reclassified as benign through the use of a molecular panel.
Cost-effectivenessRegarding costs, previous studies that compared genetic testing alone with the costs of thyroidectomy tipped the balance in favor of the use of molecular panels. However, when these studies include the long-term follow-up (5 years) of the nodules, the derived costs were found to be higher.
The latest study published in this regard is the study by Balentine et al.,17 based on a probabilistic economic model that concluded that, although the initial costs of surgery are higher compared to the molecular study of the nodule, the initial surgical approach is more cost-effective, since it avoids the costs derived from monitoring the patient. Wu et al.18 concluded that the follow-up strategy after molecular study is only superior in cost effectiveness in those areas in which the prevalence of malignancy is low (given that the NPV depends on disease prevalence).
At the moment, there are no prospective randomized studies that evaluate the cost-effectiveness of both interventions, nor studies that evaluate the secondary costs of subjective variables derived from the anxiety perceived by patients when avoiding surgery, the perceived safety after performing a genetic test or the alteration in the quality of life in the two treatment branches.
When Should Molecular Profiling Be Used?Both the American Association of Clinical Endocrinologists (AACE) as well as the American College of Endocrinology (ACE) and the Associazione Medici Endocrinologi (AME) decided to recommend its use in the 2016 Guidelines for thyroid nodules:
- -
With indeterminate cytology (not to reassess nodules with clearly benign or malignant cytologies).
- -
As long as the molecular diagnosis does not substitute cytological reevaluation, if necessary.
- -
Always when it is expected that the result will change the clinical management.
Furthermore, for now there is not sufficient evidence for molecular study results to determine the extension of surgery in the case that it is indicated or sufficient result in the long-term in order to be able to distance or suspend follow-up in patients stratified as low risk after molecular study.19
The same recommendations were included in the 2017 Thyroid Carcinoma Guidelines of the National Comprehensive Cancer Network (NCCN), recommending its use for nodules with indeterminate cytology (Bethesda III and IV) in which the diagnosis needs to be refined to help decide on conservative management or diagnostic surgery. It allows for follow-up (evidence 2B) in nodules that the molecular study has stratified as low risk of malignancy.20
The recommendations of the ATA in 2015 add that the patient must be informed at all times of the possible benefits and limitations of the molecular study and that this study must be carried out in specific molecular laboratories that have conducted a prior analytical validation.21 For the moment, they recommend the use of molecular panels as rule-out tests, without being able to recommend one molecular panel over the other until long-term clinical validation studies are available.
In their meta-analysis, Vargas-Salas et al.8 recommend that the need for molecular testing, and which type to use, is determined by clinical and ultrasound characteristics, in addition to the indeterminate characteristics of cytology. Thus, a nodule that is defined as high-risk either clinically or on ultrasound should not be studied molecularly, since, regardless of the result, it is highly likely that it will be treated surgically.
ConclusionsIn up to 30% of the cases of FNA performed during the thyroid nodule study, the cytology is indeterminate. The problem lies in differentiating between which nodules are benign and can be followed over time, and which are malignant and require surgery. The complementary use of molecular panels together with cytology has been, for the moment, the only tool that seems to help in this difficult scenario. To date, only the Afirma® molecular panel has been able to prove (in multicenter, prospective and randomized studies) its efficacy to reclassify nodules with indeterminate cytology as benign, thereby avoiding surgery. Prospective, randomized, multicenter studies are needed to study the true utility in clinical practice, costs and the quality of life of patients, as well as recommendations about long-term follow-up or the need for cytological/molecular re-evaluation of these patients.
FundingThis article has received no funding.
Conflicts of InterestThe authors have no conflict of interests to declare.
Please cite this article as: López Rojo I, Gómez Valdazo A, Gómez Ramirez J. Utilidad del estudio molecular de nódulos tiroideos con citología indeterminada. Cir Esp. 2018;96:395–400.