Enhanced recovery after surgery (ERAS) is a multimodal perioperative care program which replaces traditional practices concerning analgesia, intravenous fluids, nutrition, mobilization as well as a number of other perioperative items, whose implementation is supported by evidence-based best practices. According to the RICA guidelines published in 2015, a review of the literature and the consensus established at a multidisciplinary meeting in 2015, we present a protocol that contains the basic procedures of an ERAS pathway for resective esophageal surgery. The measures involved in this ERAS pathway are structured into three areas: preoperative, perioperative and postoperative. The consensus document integrates all the analyzed items in a unique time chart. ERAS programs in esophageal resection surgery can reduce postoperative morbidity, mortality, hospitalization and hospital costs.
La rehabilitación multimodal constituye un conjunto de medidas perioperatorias que sustituye prácticas tradicionales respecto a la analgesia, la fluidoterapia, la nutrición y la movilización, entre otros. Su implementación está basada en criterios de medicina basada en la evidencia. Con base en la vía recuperación intensificada en cirugía abdominal publicada en el año 2015, una amplia revisión de la bibliografía y el consenso establecido en una reunión multidisciplinar del Grupo de Trabajo de Cirugía Esofagogástrica del Grupo Español de Rehabilitación Multimodal celebrada en 2015, se presenta un protocolo de rehabilitación multimodal en cirugía resectiva esofágica. Las medidas a aplicar se dividen en 3bloques: preoperatorio, perioperatorio y postoperatorio. Su conjunto da lugar al documento de consenso que integra todas las medidas perioperatorias en una matriz temporal. La aplicación de protocolos de rehabilitación multimodal en cirugía resectiva esofágica reduce la morbimortalidad postoperatoria, la estancia y los costes hospitalarios.
Clinical guideline for enhanced recovery (ER) or multimodal rehabilitation (MR) in surgery or “enhanced recovery after surgery” (ERAS) can be defined as an agreed-upon, consensual, multimodal, evidence-based set of perioperative measures that restructure perioperative care.1
Traditionally, surgeons, anesthetists and nurses worked in individual “compartments” instead of integrating the multiple individual elements of perioperative care.1
The creation of these clinical pathways has meant a substantial change in the philosophy of perioperative care, when compared with traditional care. They have made it possible to “standardize” the processes, avoiding variability, creating predetermined trajectories of routine processes, better informing patients and family members and reviewing each of the items according to evidence-based medicine guidelines.1
In many areas of general surgery, this has meant making postoperative care more efficient, resulting in a reduction of hospital costs by optimizing resources and reducing hospital stay, as well as reducing morbidity and mortality. In this manner, perioperative care is restructured and adjusted to the minimum possible timeframe, while still providing patients improved comfort/well-being and shortened recovery, without compromising safety.
Kehlet and Wilmore2 were the first to implement this type of measures in colorectal surgery. Over the last 5–10 years, there has been noticeable development of ERAS clinical guidelines in many areas of general surgery.
In 2015, the ER guidelines for abdominal surgery (RICA) were created in Spain, resulting from the close collaboration between the Spanish Group of Multimodal Rehabilitation (GERM) and the Ministry of Health, Social Affairs and Equality. In it, the perioperative management of abdominal surgery patients is compiled in a protocol.3
From the GERM, a multidisciplinary workgroup was created at the beginning of 2016 with the aim to develop ER recommendations for esophagogastric resection surgery.
In this paper, we present the resulting protocol, which was developed and agreed upon by GERM members based on a thorough review of the literature currently available and the clinical experience of a group of experts.
MethodsA total of 42 physicians from different specialties and work centers (32 surgeons, 5 anesthetists, 3 nurses and 2 nutritionists), with proven experience in the management of patients with esophageal disease, have developed this protocol, creating a time matrix that was agreed upon at the Second National Multimodal Rehabilitation Congress in 2016.
In addition to the RICA3 recommendations for any type of abdominal surgery, an extensive search of the literature was carried out in the following databases: Cochrane Library, Medline, EMBASE, Scopus, Tryp database and DARE. The results obtained were evaluated using the National Institute for Health and Care Excellence (NICE) methodology, establishing the levels of evidence and degree of recommendations according to the GRADE4 methodology (Tables 1 and 2).
Quality of Evidence According to the GRADE Methodology.
| Quality of the evidence | Definition |
|---|---|
| High | High confidence that the estimated effect is very close to the actual effect |
| Moderate | Moderate confidence in the estimated effect: it is likely that the estimated effect is close to the actual effect, but substantial differences are possible |
| Low | Confidence in the estimated effect is low: the estimated effect may be substantially different from the actual effect |
| Very low | There is very little confidence in the estimated effect: it is very likely that the estimated effect is substantially different from the actual effect |
Recommendation Grades According to the GRADE Methodology.
| Recommendation grades | Definition |
|---|---|
| Strong | High quality of evidence |
| Favorable balance between benefits/disadvantages | |
| Weak | Moderate or higher quality of evidence |
| The benefit/disadvantage balance leads to a weak recommendation (if based on consensus) | |
| Low, very low or no quality of evidence but with strong criteria of benefit>disadvantage |
This document presents recommendations and perioperative measures for esophageal resection surgery. These have been grouped into three stages: preoperative, perioperative and postoperative (Appendix 2).
ResultsIndications and ContraindicationsCandidates for the application of the recommended measures included patients who were undergoing esophagectomy (codes CIE-9: 42.40, 42.41, 42.42, 42.43, 42.99) and met the following criteria3:
- –
Ages 18–85
- –
Adequate cognitive ability (able to understand and cooperate)
- –
ASA I, II and III
The patients excluded from the application of this protocol were pediatric patients and those treated with urgent surgery.
Protocol and Time Matrix (Appendix 2)The time matrix was divided into three sections: pre-, peri- and postoperative measures.
Preoperative PeriodThe following points should be emphasized:
- –
Complete information about the healthcare process for patients and family members. The only randomized trial in esophageal surgery showed that the transmission of audio-visual information reduced patient anxiety levels and increased information retention.5 Similar results were obtained in a prospective observational trial with information from the informed consent information.6 (Recommendation: weak; Level of evidence: moderate.)
- –
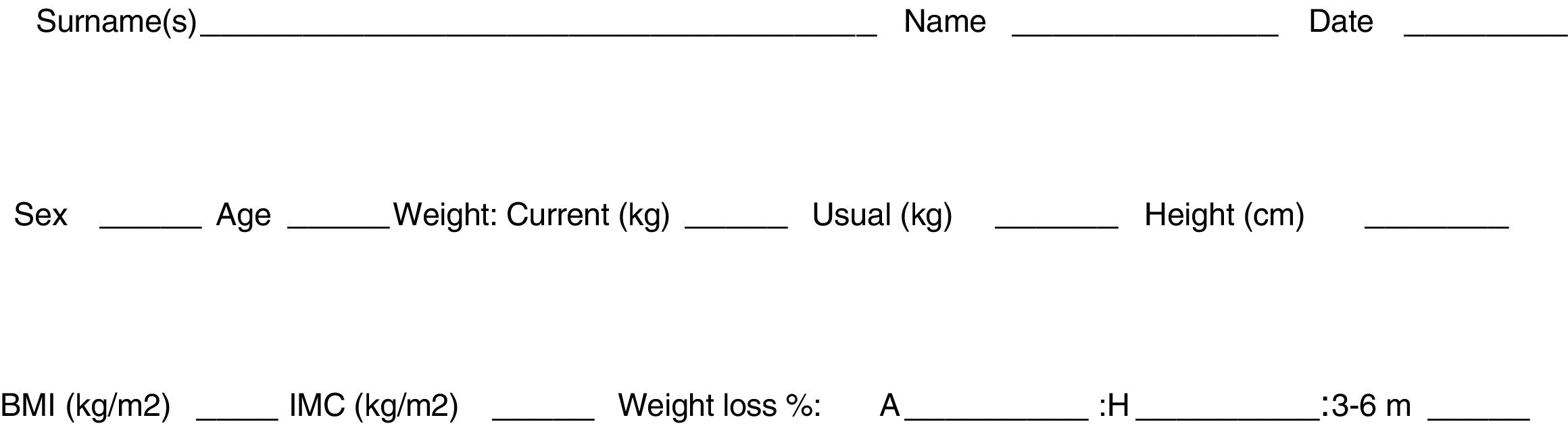
Optimization of nutritional status: although malnutrition favors the appearance of postoperative complications,7 preoperative nutritional interventions for esophageal surgery have not been evaluated. There are two randomized controlled trials that were not able to demonstrate any advantage in the postoperative course after preoperative administration of formulas with immunonutrients.8,9 (Recommendation: strong; Level of evidence: high.) However, nutritional status should be optimized before surgery,10,11 and assessment with the MUST scale3,10,11 is useful (Fig. 1). (Recommendation: weak, level of evidence: moderate.)
- –
Evaluation and treatment of preoperative anemia: although there are no studies done exclusively in esophageal surgery, it can be concluded that preoperative anemia makes the transfusion of blood products more likely, thereby increasing postoperative morbidity and mortality. (Recommendation: weak; Level of evidence: moderate.) In patients with iron deficiency anemia, preoperative treatment with oral iron (ferrous sulfate) is recommended. (Recommendation: weak; Level of evidence: high.) Blood hemoglobin levels take approximately 2 weeks to rise or even 2 months to normalize after the start of oral iron therapy. This delay is not usually relevant since most of these patients receive neoadjuvant chemotherapy. In cases where there is no time for oral administration of iron, administration is intravenous. The benefit of erythropoietin is unclear.3,7,11–13
- –
Respiratory exercises: the use of respiratory incentive-stimulating devices improves lung function. Only in cardiac surgery has this improvement in lung function resulted in better postoperative results, with extrapolated results for esophageal surgery.14–20 (Recommendation: strong; Level of evidence: high.)
The following points should be highlighted:
- –
Diet and preoperative fasting: The guidelines of the European Society of Anaesthesiology21 consider preoperative fasting of 6h for solids and 2h for liquids to be safe (Recommendation: strong, Level of evidence: high), as well as the administration of a carbohydrate drink (250mL, 12.5% maltodextrin) 2h before the intervention, which will result in an improvement of subjective well-being, less thirst and hunger and a lower resistance to insulin. (Recommendation: strong; Level of evidence: high.) There are no trials after esophagectomy, and accepted results have been extrapolated from other surgeries. In patients with dysphagia, caution should be exercised and these measures should be applied individually. It is not possible to interpret benefits from these measures in postoperative morbidity and mortality or length of hospital stay.22,23
- –
Anesthesia: the following anesthesia-related measures are applied; these coincide with recent publications for gastric surgery by Bruna Esteban et al.24
- •
Fluid therapy: goal-directed fluid therapy (GDFT) is a strategy designed to define the appropriate volume to be administered perioperatively, avoiding excessive (liberal approach in volume replacement) as well as deficient (restrictive approach) volumes.25 The benefit of GDFT is low in patients with little surgical risk but high in high-risk patients26,27 or those treated by surgical procedures with high intravascular losses. In this group of patients, the benefit of advanced hemodynamic monitoring is high.28–30 Conceptually, GDFT provides a rational and individualized basis for obtaining and maintaining adequate perioperative hemodynamic optimization to deliver an adequate oxygen supply. To achieve adequate tissue oxygenation, essential factors include adequate cardiac output and dynamic preload parameters at the outset to define the best perioperative volume management strategy. Available methods for the evaluation of cardiac output are transesophageal Doppler ultrasound (obviously, not usable in these cases), pulmonary artery catheterization and pulse wave analysis.11 In abdominothoracic esophagectomy, it is difficult to determine which patients require greater volume replacement, since commonly proposed mechanisms to determine cardiac output and pulse variation are not predictors of the response to volume and have not been validated in open thoracic surgery. To this, if we add the fact that there are no exclusive esophagectomy studies assessing GDFT, the weak recommendation is comprehensible.11,31,32 (Recommendation: weak; Level of evidence: high.) The association between the excessive administration of perioperative fluids and the appearance of post-esophagectomy pulmonary complications has been clearly demonstrated.33 An excessively positive perioperative fluid balance increases pulmonary complications,34 and the vulnerability is especially great in the early postoperative phases.31,35,36 What we commonly call “fluid restriction” is really aimed at maintaining a situation of normovolemia,35,36 while avoiding a situation of preoperative hypovolemia. (Recommendation: moderate; Level of evidence: high.) When using crystalloid solutions, it is preferable that these be balanced. There are two controlled, randomized trials that show a lower rate of complications using balanced crystalloids compared to 0.9% saline.37–40 (Recommendation: strong; Level of evidence: high.)
- •
Ventilation strategies: in open and thoracoscopic surgery in lateral decubitus, single-lung ventilation is used (bronchial blockers and double-lumen endotracheal tubes are comparable in clinical efficacy), while in thoracoscopic surgery in the prone position, bilateral pulmonary ventilation can be performed when pneumothorax with CO2 is used, maintaining an insufflation pressure of 6–8mmHg. In patients who are treated with single-lung ventilation, pulmonary protection maneuvers should be performed, including: maintaining low tidal volumes (6mL/kg), PEEP between 5 and 10cmH2O and peak and plateau inspiratory pressures less than 25 and 30cmH2O; also, alveolar recruitment maneuvers at least before and after single-lung ventilation.1,30 Protective ventilation during single-lung ventilation has been shown to decrease the release of post-esophagectomy inflammatory mediators.35 In a recent randomized controlled trial, protective ventilation measures were associated with fewer pulmonary complications after minimally invasive esophagectomy (MIE).41 In open surgery, PEEP is applied in the dependent or ventilated lung, and CPAP in the non-dependent or collapsed lung. The use of CPAP in the collapsed lung reduces local inflammation and should help reduce lung injury.42
- •
Thoracic epidural catheter: thoracic epidural anesthesia is considered the basic pillar of analgesia after esophagectomy. In 3-field esophagectomy, pulmonary complications and anastomotic dehiscence have decreased, with a higher incidence of hypotensive episodes and bladder catheterizations.43 With Ivor-Lewis esophagectomy, the systemic inflammatory response is lower and analgesic control is improved44 compared to intravenous analgesia with opioids. In a recent retrospective analysis, Intensive Care Unit stays have decreased.45 Long hypotensive periods should be avoided, since they have been found to correlate directly with anastomotic dehiscence.46 The role of thoracic epidural anesthesia has yet to be determined in MIE.11
- •
Surgical approach: to date, there is no randomized controlled trial comparing MIE to the open approach, and therefore the focus is on large hospital series and multicenter databases.31 MIE is increasingly carried out more generally, and related publications have increased notably since 2007. MIE is similar to open surgery in postoperative morbidity and mortality, readmissions and 5-year survival.47–51 While the hospital stay (one day) is reduced, the incidence of postoperative ileus, incidence of wound infections and the need for transfused blood products also decreases.47 MIE entails a longer surgical time and a higher rate of reoperations.47 (Recommendation: strong; Level of evidence: high.)
The systematic use of nasogastric (NG) tubes is recommended to decompress the esophageal repair. (Recommendation: moderate; Level of evidence: high.) NG intubation avoids distension and associated vomiting, pain or bronchial aspirations, while also avoiding dilatation, thereby reducing anastomotic tension, compression, or ischemia.11 A recent randomized trial has demonstrated that early withdrawal of the NG tube is safe,52 although there are a few specific trials in favor of not using NG intubation, with questionable results.53 In practically all trials evaluating ERAS in esophagectomy, decompressive NG intubation is used.54–58,61–69
The use of chest drain tubes is mandatory because this prevents lung compression and monitors hemorrhage as well as air, chymal, or anastomotic leaks.13 Their use can be minimized, and it is sufficient to use at least one multiperforated tube. (Recommendation: weak; Level of evidence: high.) Withdrawal will be possible when the 24-h discharge is <200ml, there is no air leakage and the drainage characteristics are serous. (Recommendation: weak; Level of evidence: high.)
Urinary catheterization is used to monitor diuresis and for comfort and well-being. In return, patients have less mobility, a greater risk of infections and longer hospital stays.13 The catheters should be withdrawn as soon as possible, once they have fulfilled their function. (Recommendation: weak; Level of evidence: very low.) Withdrawal on the first postoperative day reduces the rate of urinary tract infections, with an incidence of resuscitations of <10% if the patients did not have a history of urological pathologies, even with a functioning thoracic epidural catheter.70 (Recommendation: weak; Level of evidence: high.)
Although a pyloroplasty favors the drainage of the esophageal repair, its role in the “final evolution” of these patients is not clear, so no recommendation can be made. Opponents argue that it increases bile reflux, shortens the esophageal repair and prolongs surgical time; supporters claim that it reduces episodes of bronchial aspiration, obstruction and esophageal suture dehiscence.
There are no prospective studies in esophageal surgery comparing the use of drainage versus no drainage.71
Immediate postoperative period: during the first 48h, the patient will remain in the Post-anesthesia Recovery Unit or Postoperative Recovery Unit.
Early enteral nutrition through a jejunostomy tube (J-tube) seems logical if the evidence from other non-esophageal surgeries in favor of early and preferably enteral nutrition is extrapolated. Current evidence does not allow us to recommend the best way to administer enteral nutrition. Although J-tubes are used more frequently than nasojejunal (NJ) tubes, the risk of serious complications with J-tubes is low but not zero, while the main problem of NJ tubes is their frequent dislodgement.11,72
Late postoperative period: after the initial 48h post-op, the transfer of the patient to the hospitalization floor will be assessed.
There are no specific recommendations regarding the ideal time to initiate oral intake. In most of the reviewed ERAS studies in esophagectomy56–69 (except for the study by Jianjun et al.73 and partially by Cao74), oral intake was not initiated before the third or fourth postoperative day. The Mayo Clinic group, which delays oral intake for up to 28 days and uses J-tubes during this period, has been able to reduce esophageal dehiscence from 12 to 2.7%, while also reducing hospital stay.75
Although most groups of experts in esophageal surgery advocate early mobilization, the level of evidence after esophagectomy is very low.
Radiological follow-up of the intrathoracic esophagogastric anastomosis in Ivor-Lewis esophagectomy using computed tomography or esophageal transit seems logical before the chest drain tube is withdrawn, but this is based on poor-quality studies, so its routine use cannot be recommended.11
In the absence of warning signs, patients may be assessed for hospital discharge on the seventh postoperative day if they meet the following criteria76:
- 1.
Adequate pain control with oral analgesia
- 2.
Correct walking and independence for basic daily activities
- 3.
Good oral tolerance
- 4.
Correct comprehension on the discharge instructions and actions in situations of concern
- 5.
No signs for concern or of any complications
- 6.
Acceptance by the patient
MR guidelines, involving the implementation of a group of pre-, peri- and postoperative measures, are aimed at reducing surgical stress and favoring postoperative recovery.1,76 Alone, many of these measures do not have a positive impact on the final evolution of these patients, yet they do when applied together, as many are interrelated.1,77
The pathogenesis of paralytic ileus and the mechanisms involved in insulin resistance exemplify this interrelationship.
The main pathogenic factors of postoperative ileus are counteracted by measures included in the ERAS guidelines, such as the reduction of surgical trauma and the poor intestinal manipulation achieved with minimally invasive surgical techniques, restrictive fluid therapy and the use of non-opioid analgesia.7,76
Another critical factor is insulin resistance, which is directly related to the magnitude of surgical aggression (being lower in minimally invasive surgery) as well as postoperative morbidity, mortality and length of hospital stay.7,78 Short preoperative fasting periods, the intake of carbonated beverages 2h before anesthetic induction, the use of thoracic epidural catheters and the early initiation of both oral intake and mobilization will decrease insulin resistance; meanwhile, insulin resistance will increase with non-compliance with these measures.7,78
The first MR guidelines were published in 2012 for colorectal surgery and pancreaticoduodenectomy, followed by guidelines for gastric resection in 2014 and bariatric and hepatic surgery in 2016. The ERAS Society is currently developing MR recommendations for esophagectomy.79
According to how the main MR steps are defined, they range between 18 and 24, not all of which are mandatory at the same time.1,10,14,25,56–69,73,74,76,77
The recent publication by Bruna Esteban et al.24 reviewed MR protocols for gastric resection surgery. It is noteworthy that some of the measures that could be considered “aggressive” a priori, such as not using NG intubation or drains and tending to initiate oral intake very early on, have been shown to be safe.24,80 This is reflected in the high percentage of implementation of these measures in the studies published. The application of MR guidelines in gastric resection surgery has reduced the average hospital stay as well as hospital costs, without increasing postoperative morbidity or mortality rates.24
MR guidelines in esophageal resection surgery have represented a radical change over traditional perioperative measures. Until a few years ago, NG intubation and parenteral nutrition were maintained for long periods, the urinary catheter until the epidural catheter was withdrawn and the chest drain tube until the start of oral intake.77
Most likely, the MR recommendations in esophageal resection surgery, within the set of MR recommendations for surgery, are the most “conservative” if the compliance of measures that could be considered “risky” is evaluated, such as not decompressing the esophageal repair with a NG tube, not using chest drain tubes or initiating oral diet very early. Even so, partial implementation of the different perioperative measures has resulted in a clear decrease in postoperative morbidity and mortality, as well as in shorter hospital stay and lower hospital costs.56–69,73,74 None of the trials published so far is multicentric, randomized and controlled, and most of them are retrospective observational studies with a small number of patients, so the quality of the evidence is low.56–69,73,74
Esophageal resection surgery occupies a special place in surgical procedures since it has a high rate of associated postoperative morbidity. For minimally invasive transthoracic esophagectomy, a recent multicenter study has established that a complication rate of 50% is acceptable.81 This is even more remarkable if we take into account that only high-volume centers participated and patients included were low risk.81
Considering that the number of patients treated surgically at Spanish hospitals is generally low, as these procedures have not been regionalized, it is logical that the implementation of “aggressive” perioperative measures should be slow and progressive.
Conflicts of InterestThe authors have no conflict of interests to declare.
Enhanced Recovery after Esophagogastric Surgery Workgroup of the Spanish Enhanced Recovery Group
Maria Asunción Acosta Merida, Maria Dolores Alonso Herreros, Rosario Aparicio Sánchez, Laura Armañanzas Ruiz, Carmen Balagué Ponz, Helena Benito Naverac, José A. Casimiro Pérez, Vanessa Concepción Martín, Roberto de la Plaza Llamas, Marta de Vega Irañeta, Carlos J. Díaz Lara, Ismael Diez del Val, Maria del Lluch Escudero Pallardó, Mónica García Aparicio, Francisca García-Moreno Nisa, Lorena Gómez Diago, Maria Luz Herrero Bogajo, Yolanda López, Rafael López Pardo, Ezequiel Martí-Bonmatí, Javier Martín Ramiro, José Martínez Guillén, Luis Enrique Muñoz Alameda, Inmaculada Navarro García, Ana Cristina Navarro Gonzalo, María Posada González, Pablo Priego Jiménez, Maria Quiles Guerola, Elizabeth Redondo Villahoz, Mário Ribeiro Gonçalves, Javier Riera Castellano, Elena Romera Barba, David Ruíz De Angulo, Jesús Salas Martínez, Cristina Sancho Moya, Amparo Valverde Martínez, Ramon Vilallonga Puy, Camilo Zapata Syro, Jorge Zarate Gomez.
Members of the Enhanced Recovery after Esophagogastric Surgery Workgroup of the Spanish Enhanced Recovery Group are listed in Appendix A.
Please cite this article as: Vorwald P, Bruna Esteban M, Ortega Lucea S, Ramírez Rodríguez JM y Grupo de Trabajo de Cirugía Esofagogástrica del Grupo Español de Rehabilitación Multimodal (GERM). Rehabilitación multimodal en la cirugía resectiva del esófago. Cir Esp. 2018;96:401–409.