Enhanced recovery after surgery programs in abdominal surgery are being established progressively. The aim of this study is to evaluate the application of different perioperative care measures in gastric surgery by Spanish surgeons.
MethodsA descriptive study of 162 surveys answered from September to December 2017 about the management and perioperative care in non-bariatric gastric resection surgery.
ResultsAntibiotic and antithrombotic prophylaxis are always used by 96.9 and 99.4%, respectively; 62.7% recommend a fasting time for liquids >6h and only 3% use preoperative carbohydrate drinks. Only 32.4 and 13.3% of subtotal and total gastrectomies are performed laparoscopically, respectively; 56.8% use epidural analgesia, and drains are always placed by 53.8% in total gastrectomy. Nasogastric tubes are used selectively by 34.6% and always by 11.3%. Bladder catheters are removed during the first 48h by 77.2%. In the first 24 postoperative hours, less than 20% indicate oral intake and 15.4% mobilize their patients; 49.3% indicate walking after the first 24h; 30.4% apply a clinical pathway for the care of these patients and only 15.2% used an enhanced recovery after surgery protocol.
ConclusionsThe implementation of enhanced recovery after surgery measures in non-bariatric gastric resection surgery is not widespread in our country.
Las medidas de rehabilitación multimodal en cirugía abdominal se están instaurando progresivamente. El objetivo del estudio es evaluar la aplicación de diferentes cuidados perioperatorios en la cirugía gástrica por parte de los cirujanos españoles.
MétodosEstudio descriptivo de 162 encuestas contestadas desde septiembre a diciembre de 2017 acerca del manejo y cuidados perioperatorios en cirugía de resección gástrica no bariátrica.
ResultadosLas profilaxis antibiótica y antitrombótica son empleadas siempre por el 96,9 y 99,4%, respectivamente. El tiempo de ayuno para líquidos es mayor de 6horas para el 62,7%, empleando solo bebidas con sobrecarga de hidratos de carbono prequirúrgicamente el 3%. Tan solo el 32,4 y el 13,3% de las gastrectomías subtotales y totales son realizadas laparoscópicamente. El 56,8% emplea analgesia epidural y los drenajes son colocados siempre por un 53,8% en la gastrectomía total. La sonda nasogástrica es empleada selectivamente por el 34,6% y siempre por el 11,3%. La retirada del catéter vesical es realizada durante las primeras 48horas por el 77,2%. En las primeras 24horas postoperatorias, menos del 20% indica la ingesta oral y un 15,4% moviliza a sus pacientes, comenzando la deambulación a partir de las 24horas el 49,3%. El 30,4% emplea una vía clínica para el cuidado de estos pacientes y solo un 15,2% utiliza un protocolo de recuperación intensificada.
ConclusionesLa aplicación de medidas de rehabilitación multimodal en la cirugía de resección gástrica no bariátrica se encuentra poco extendida en nuestro país.
Since Henrik Kehlet began to transmit his knowledge and experience about what is known as multimodal rehabilitation1 in colon surgery at the end of the last century, there have been numerous advances and studies demonstrating that this form of perioperative management is safe, feasible and, in addition, improves patient recovery after surgical trauma.
In 2007, the Spanish Group for Multimodal Rehabilitation (GERM) was created, which, in close collaboration with the Spanish Ministry of Health, Social Affairs and Equality, published the RICA guidelines (Intensified Recovery in Surgery Abdominal) in 2015.2 This protocol identifies the necessary stages and key points for enhanced recovery in the perioperative management of patients undergoing abdominal surgery.
Currently, there are numerous major abdominal surgical procedures whose perioperative care could include the application of this type of measures, including surgery for non-bariatric gastric resection. Thus, at the beginning of 2017, an enhanced recovery protocol in gastric surgery was published in the Cirugía Española journal,3 together with a time matrix summarizing the measures to be applied in this setting, based on the consensus of experts. Even so, there are few high-quality studies that demonstrate proven evidence about enhanced recovery recommendations in esophagogastric surgery, although there is growing evidence about its safety and clinical benefits, in addition to its better cost-effectiveness compared to traditional management.4–8
In this study, using an online survey, we intend to evaluate the trends and measures applied by surgeons in Spain in the perioperative care of elective non-bariatric gastric resection surgery.
MethodsA descriptive study was conducted of the data collected from the surveys answered between September 15 and December 15, 2017, by Spanish surgeons regarding perioperative measures applied to patients undergoing non-bariatric gastric resection. We invited surgeons who were members of the Spanish Association of Surgeons (AEC) by e-mail to participate and anonymously complete an online survey. Currently, out of the 4612 active members of the AEC, only 308 surgeons are involved in the Esophagogastric Surgery Division. At the end of the 3 months of the study period, a total of 162 surgeons had correctly completed the questionnaire.
The survey consisted of 61 questions and was designed to evaluate the following aspects:
- 1.
Membership data:
- -
Age and sex
- -
Experience and professional position
- -
Characteristics and location of their place of work
- -
Volume and type of procedures done
- 2.
Preoperative care and preparation:
- -
Antibiotic prophylaxis
- -
Antithrombotic prophylaxis
- -
Preoperative nutrition
- -
Preoperative fasting
- 3.
Perioperative care:
- -
Prevention of hypothermia
- -
Epidural analgesia
- -
Catheters and drain tubes
- 4.
Postoperative care:
- -
Postoperative oxygen therapy
- -
Respiratory exercises
- -
Fluid therapy and parenteral nutrition
- -
Test for leaks
- -
Initiation of mobilization
- -
Initiation of oral intake
- 5.
Hospital discharge:
- -
Hospital discharge criteria
- -
Days of postoperative hospital stay
- 6.
Follow-up of the perioperative care of these patients
The descriptive study was carried out using IBM® SPSS® Statistics version 20. The descriptive results are presented as number of cases and percentage, and as mean and standard deviation (SD).
ResultsA total of 162 surveys were answered during the 3 months of the study period. 63.1% of the participants were male, their mean age was 47.3 years (SD: 6.6) and the average professional career was 17.6 years (SD: 7.2).
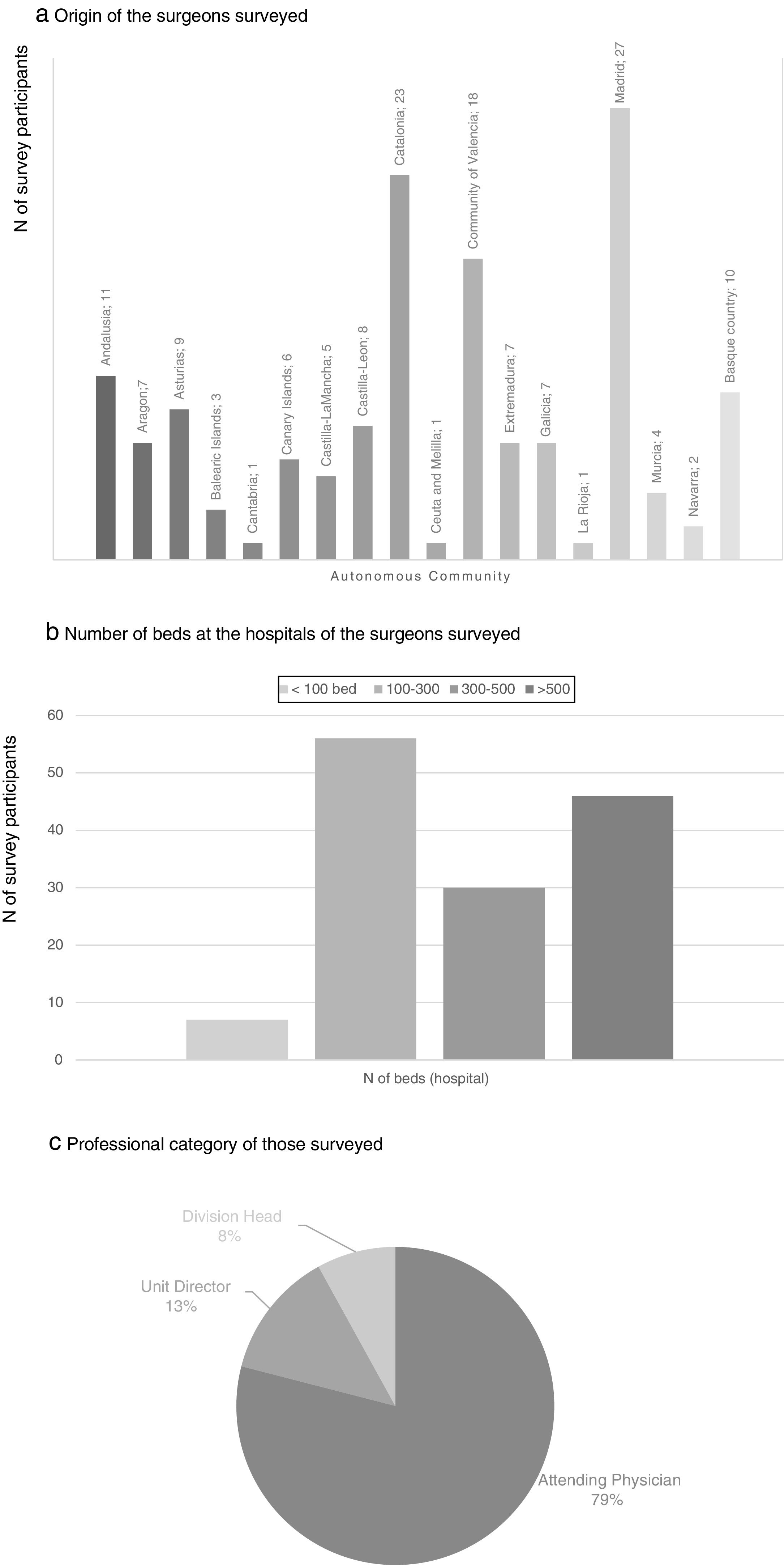
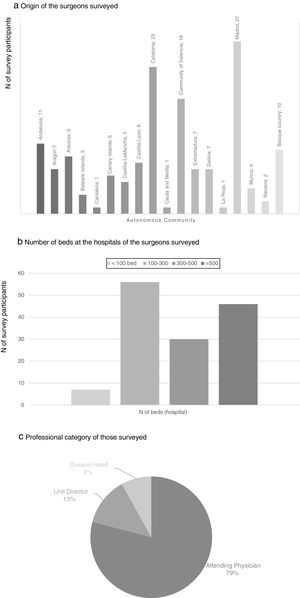
There were participants from all the autonomous communities; the regions with the greatest representation were the Community of Madrid (27 participants), Catalonia (23), Community of Valencia (18), Andalusia (11) and the Basque Country (10). 36.4% of the participants worked in a hospital with between 100 and 300 beds and 29.9% in hospitals with more than 500 beds. None of the participating surgeons worked exclusively in private practice, and most of them were attending physicians (79%) (Fig. 1).
The average number of subtotal gastrectomies performed annually by the participants was 8.8 (SD: 2.3), with an average percentage of 32.4% laparoscopic procedures. Regarding total gastrectomy, the average annual number of procedures performed by each of the participants was 4.9 (SD: 1.7), 13.3% of which were laparoscopic.
Antibiotic prophylaxis was used by the majority (96.9%) and was administered during anesthetic induction by 80.3% of the participants. Although 27.7% maintained antibiotic therapy during the first 24h post-op, 62.3% used a single dose. The most commonly used antibiotics were amoxicillin-clavulanic acid and cephalosporins, such as cefazolin or cefuroxime. Antithrombotic prophylaxis with low-molecular-weight heparin was routinely used (99.4% of participants); enoxaparin and bemiparin were the most commonly used formulas. This type of therapy was maintained during the postoperative period for 15–30 days by 62.3% of the participants, and more than 1 month by 22%. The fasting time for solids before surgery required by almost all participants (97.5%) was 6 or more hours. In the case of liquids, 62.7% required a minimum period of 6h, 19.6% a minimum of 4–5h and only 17.7% a period of 2–3h. The use of sugary drinks or those with a carbohydrate overload a few hours before surgery was not widespread; only 3% did so systematically and 73% had never indicated it.
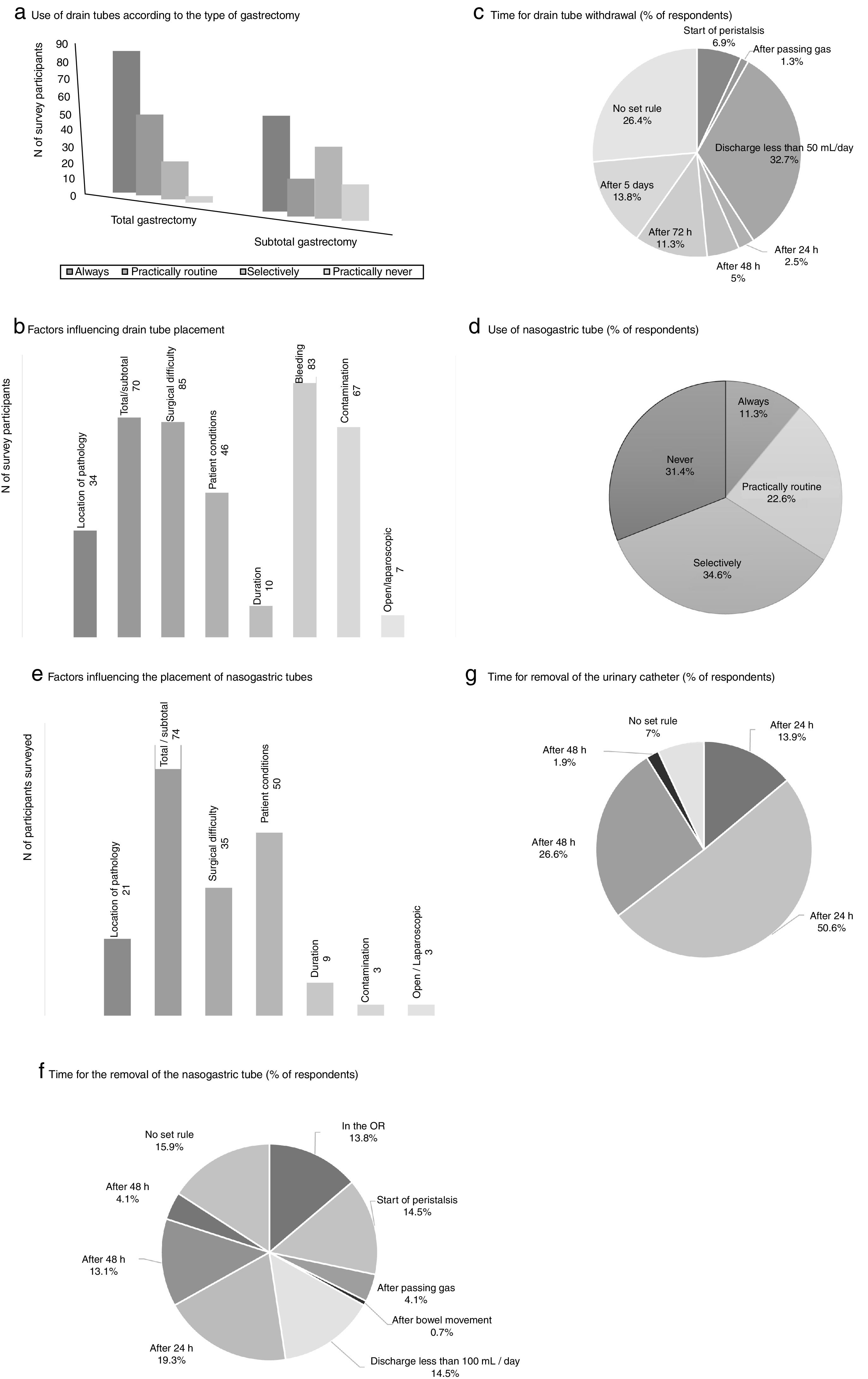
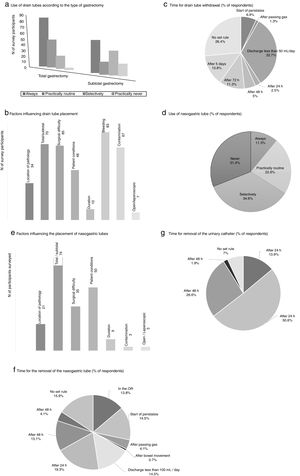
The measures to achieve the maintenance of normothermia intraoperatively were used by most of participants (89.3% always and/or practically routinely use warm air and 63.5% warm fluids). 56.8% of the participants used a catheter for epidural analgesia in this type of surgery, and more than half (62%) maintained it up to 48h postoperatively. Drain tubes were always placed by a greater percentage of surgeons in total gastrectomy (53.8%%) than in the subtotal procedure (34.8%) (Fig. 2). The main factors that participants considered for drain placement were operative difficulty, bleeding, type of surgery (total/subtotal) and contamination (Fig. 2). The majority of the respondents stated that drain tube withdrawal was done without a set standard (26.4%) or when the daily discharge was <50mL (32.7%) (Fig. 2).
Results of the survey about the use of drain tubes (a–c), nasogastric tubes (d–f) and urinary catheters (g). (a) Use of drain tubes according to the type of gastrectomy; (b) factors influencing drain tube placement; (c) time for drain tube withdrawal (% of respondents); (d) use of nasogastric tube (% of respondents); (e) factors influencing the placement of nasogastric tubes; (f) time for the removal of the nasogastric tube (% of respondents); (g) time for removal of the urinary catheter (% of respondents).
The use of nasogastric tube (NG) intubation was selective according to more than one-third of the respondents (34.6%), while 11.3% used them always (Fig. 2). The factors that most influenced the decision for placement were the type of surgery (total/subtotal), patient conditions and surgical difficulty. There was no clear trend regarding when to remove the NG tube; 19.3% removed it 24h after surgery, while 15.9% did not follow a set standard (Fig. 2).
The bladder catheter was removed between the first 24 and 48 postoperative hours by most participants (77.2%) (Fig. 2), and only 13.9% were withdrawn on the same day of surgery.
The use of oxygen therapy in the postoperative period (63.9%) with nasal cannula (90.1%) was quite common, and most maintained this therapy for the first 24–48h (86.6%). Similarly, 89.8% of the participants prescribed the use of an incentive spirometer device either always or almost always.
The daily fluid therapy volumes most frequently administered in the postoperative period were 2500mL (47.5%), 2000mL (28.4%) and 3000mL (13%). Parenteral nutrition was always or almost routinely used by 28.4% in the case of a subtotal gastrectomy and by 60.5% in the case of total gastrectomy. 73.2% of the respondents did not systematically insert a jejunostomy feeding tube in this type of patients, although 19.1% place it routinely in cases of total gastrectomy.
Leak tests before initiating oral intake was always indicated by 19.9%, practically routine in 32.1%, selectively by 29.5% and practically never by 18.6% (3.7% did not respond).
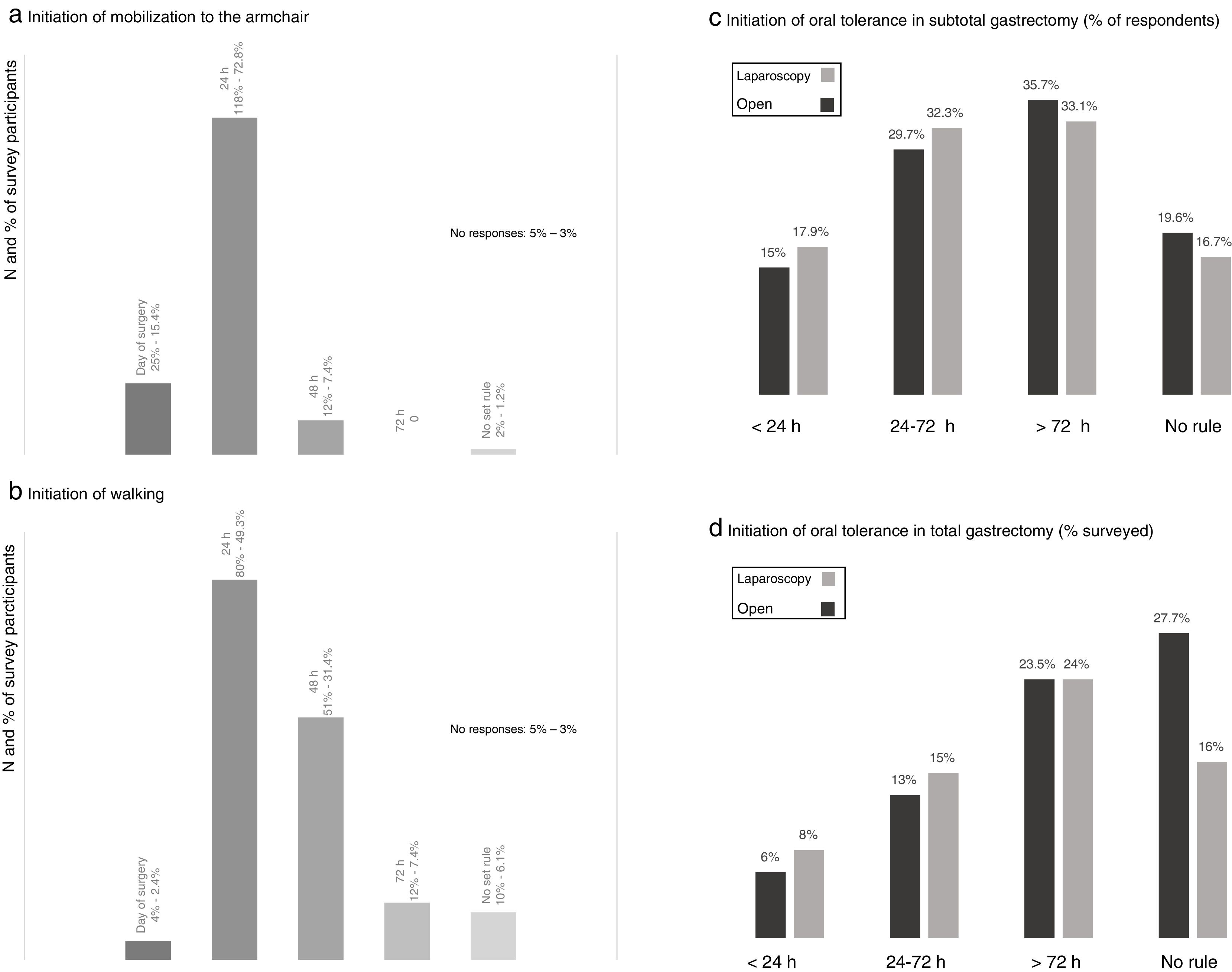
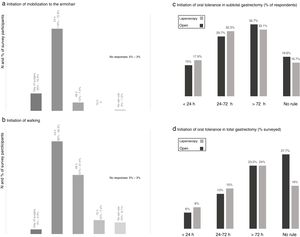
When evaluating the degree of early postoperative mobilization, the majority of the respondents (72.8%) indicated that these patients were transferred to an armchair 24h after surgery and only 15.4% within the first postoperative day (Fig. 3). Walking was usually prescribed after the first 24h (49.3%) and 48h (31.4%) post-op (Fig. 3).
Results of the survey about mobilization from the bed to an armchair (a) and ambulation (b), and postoperative oral intake in subtotal (c) and total (d) gastrectomy. (a) Initiation of mobilization to the armchair; (b) initiation of walking; (c) initiation of oral tolerance in subtotal gastrectomy (% of respondents); (d) initiation of oral tolerance in total gastrectomy (% surveyed).
Most of the participants indicated the beginning of oral intake after the second postoperative day in cases of subtotal gastrectomy and after the third day post-op in total gastrectomy, while around 15–18 and 6–8% initiated oral intake in the first 24h after surgery, respectively (Fig. 3). There was a slight tendency toward initiating oral intake earlier in cases of laparoscopic surgery.
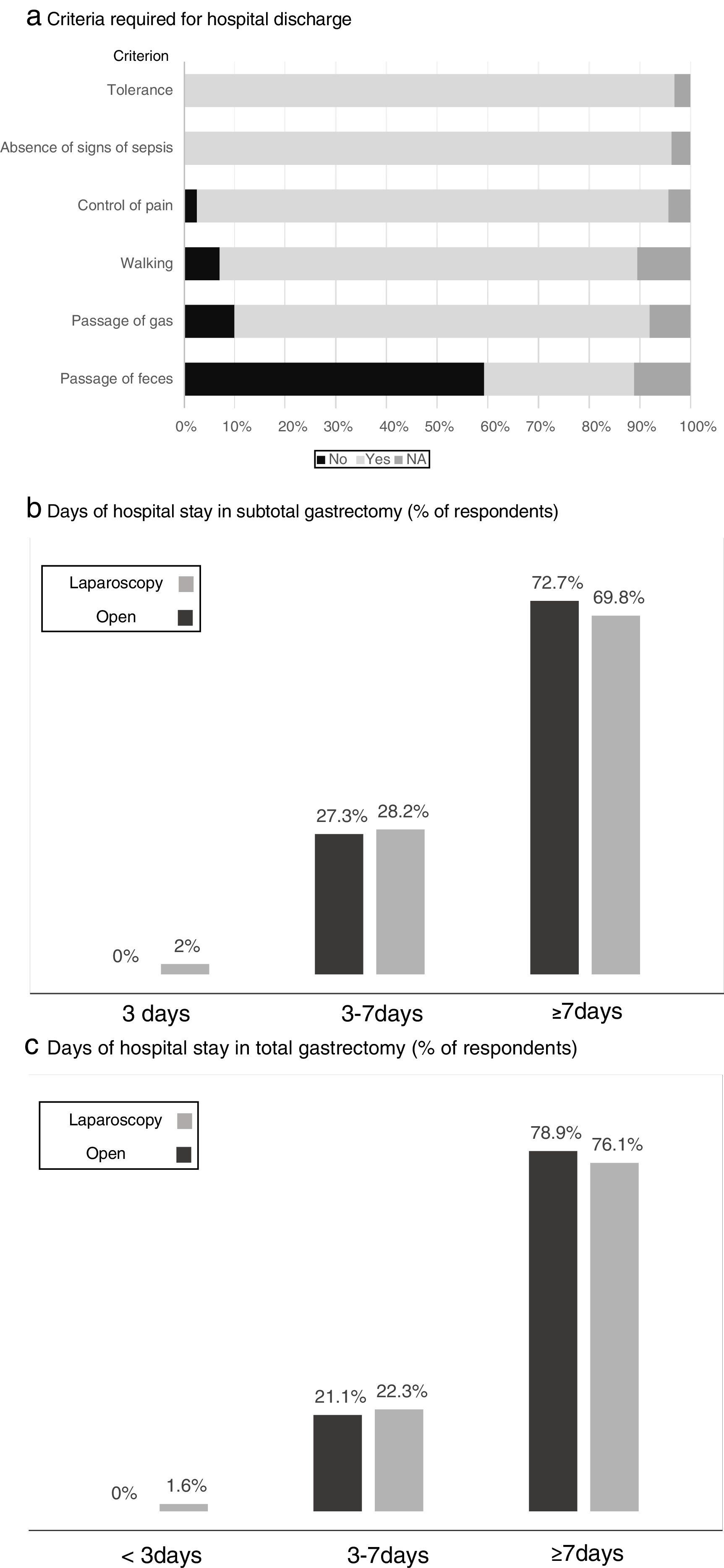
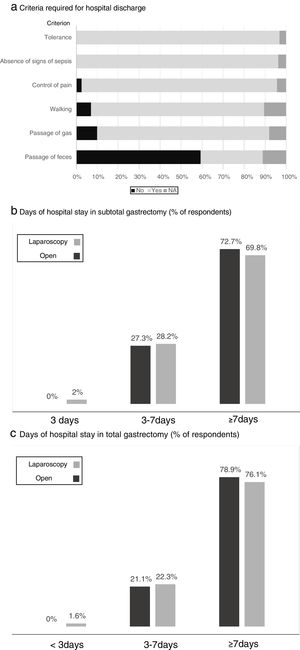
Fig. 4 shows the criteria required by the participants before discharge, where tolerance to diet and the absence of signs of local or general sepsis are required by all. The estimated days of hospital stay for total and subtotal gastrectomy with the open or laparoscopic approach are shown in Fig. 4, while “more than 7 days of stay” was the response of most surgeons, somewhat lower in the case of laparoscopic subtotal gastrectomy.
Results of the survey regarding the criteria required for hospital discharge (a) and days of hospital stay in subtotal (b) and total (c) gastrectomy. (a) Criteria required for hospital discharge; (b) days of hospital stay in subtotal gastrectomy (% of respondents); (c) days of hospital stay in total gastrectomy (% of respondents).
Based on the data collected from this survey, only 30.4% of participants used a clinical pathway for the care and management of these patients and only 15.2% used a specific enhanced recovery protocol for this type of surgery.
DiscussionThe use of different guidelines and protocols that systematize the care of patients undergoing surgery is one of the most used and recommended tools to improve the safety of the process and optimize the results. Multimodal rehabilitation, enhanced recovery, fast-track or enhanced recovery after surgery (ERAS) are different names that have been given to the set of measures aimed at improving the quality of care, reducing stress, reducing the number of complications and accelerating the recovery of patients undergoing surgical trauma.
At the end of the last century, Dr. Henrik Kehlet demonstrated that this type of perioperative management is safe, feasible and, furthermore, improves the recovery of these patients. In Spain, the GERM published the RICA2 clinical pathway in 2015. In 2016, the “Clinical practice guidelines for perioperative care in major abdominal surgery” were also published, which offered a set of recommendations for patient management before, during and after surgery in order to improve the quality of care and optimize recovery and postoperative rehabilitation.9 Currently, GERM is working to achieve the implementation of these protocols in most of the hospitals in our country through the IMPRICA project.
More specifically, in 2014, Mortensen et al.10 published a series of recommendations for gastric surgery based on an extensive review of the evidence and expert opinion on the enhanced recovery measures to be applied in gastrectomy patients. And, in 2017,3 an expert consensus was published in Spain for the enhanced recovery measures that should be used in this type of procedures.
That same year, Ripollés et al.11 published the results of a survey about surgery with enhanced recovery in our country with the participation of 272 medical professionals, 45.2% of whom were surgeons. 86.1% of them knew about the multimodal rehabilitation programs, although only 37.9% knew of the GERM group. Most of the participants applied these measures in colorectal surgery (procedures where the greatest experience has been achieved), and only 10% applied this type of protocol in esophagogastric surgery. However, every day there are more studies that support the application of these measures in this field of abdominal surgery, although most of them are based on Asian series.4–8
As the results of this study show, despite the extensive compliance with points such as antibiotic and antithrombotic prophylaxis and the maintenance of normothermia, there are many others whose implementation is still very limited, despite current scientific evidence.2,9
Laparoscopic gastric resection surgery has shown better results compared to the open approach in terms of postoperative recovery and surgical wound complications, without increasing short- and long-term morbidity and mortality.12 Despite this, less than one-third of these procedures were performed laparoscopically by the respondents of this study. There was also low use of carbohydrated drinks up to 2h before surgery, even though this measure has shown in some studies a more accelerated recovery of gastrointestinal function in terms of first flatulence and first defecation, shorter hospital stay, lower cost derived from hospitalization and lower percentage of overall complications.6,13
The use of drains is greater in the case of total gastrectomy, perhaps due to the greater risk of anastomotic leakage of the esophagojejunal anastomosis. However, in most published series, no difference has been established in the use of drainages between total or subtotal gastrectomy. A Cochrane Review published in 201514 concluded that the use of the drain tubes does not provide any benefit in terms of post-operative morbidity and mortality or reoperations, and its non-use accelerates the start of oral intake and shortens hospital stay.
One of the basic pillars of general enhanced recovery protocols is the early initiation of oral intake and mobilization, both related to the reduction of postoperative ileus time.15 However, these measures tend to be delayed until 24h after surgery by most surgeons surveyed, with less than 10% initiating oral tolerance on the same day of surgery, and around 15% encouraging patients to transfer to an armchair in this period.
The urinary catheter should be removed as soon as possible, according to what has been established in the fast-track protocols,2,9 which reduces the risk of infection and facilitates the mobilization of these patients. According to the data of this study, the catheter was usually withdrawn 24h after surgery, with a lower percentage of surgeons indicating its removal the same day of surgery. Likewise, 11% always used NG intubation in this type of surgery, although the scientific evidence16 does not recommend its systematic use in elective surgery for gastric cancer.
Regarding the hospital stay estimated for this type of procedure, the data from the survey showed higher figures than those published by gastrectomy series with enhanced recovery.6,17
Obviously, this study reports information from surveys filled out anonymously and individually, so their results should be evaluated and considered based on the limited evidence of this type of study. In addition, due to the extension of the questionnaire, some of the measures included in the multimodal rehabilitation protocols could not be evaluated in this study, including prehabilitation, psychological therapies, nutritional evaluation and support, evaluation and treatment of preoperative anemia, prophylaxis for nausea and vomiting, etc. However, this study provides much data about the application of multimodal rehabilitation measures in patients undergoing gastric surgery. The fact that only 15% of the participating surgeons report following an enhanced recovery clinical protocol for these procedures demonstrates the limited implementation of these guidelines in standard clinical practice in Spain. Furthermore, we must realize that the creation of a protocol is not enough.18 Many barriers must be overcome19 before reaching the full application of these processes, so there is still a long way to go before multimodal rehabilitation in gastric surgery becomes a reality in our setting.
Therefore, despite the available evidence that supports the use of these multimodal rehabilitation protocols in gastric surgery and that the process of introducing these measures has been well described,20 the results of this study show that their diffusion and application is still limited in Spain.
Conflicts of InterestThe authors have no conflict of interests to declare with regards to the publication of this manuscript.
Please cite this article as: Bruna M, Navarro C, Báez C, Ramírez JM, Ortiz MÁ. Resultados de la encuesta nacional sobre cuidados perioperatorios en cirugía resectiva gástrica. Cir Esp. 2018;96:410–418.