The outcomes of patients treated with colonic stents as a bridge to surgery (BTS) have recently been questioned in terms of safety and long-term oncologic outcomes. The aim of this study is to evaluate the effects on surgical and oncologic outcomes of colonic stents as a BTS for potentially resectable obstructive colorectal cancer.
MethodsWe conducted a retrospective analysis of patients operated on for potentially resectable obstructive colorectal cancer with or without distant disease between September 2002 and October 2015, comparing the patients treated with a colonic stent as a BTS (Stent group) with those directly operated on (Surgery group).
ResultsTwenty patients underwent urgent surgery, while stent placement as a BTS was attempted in 57 patients. The Stent group had more patients treated with a laparoscopic approach (64.9% vs 5%, P<.001), higher primary anastomosis rate (91.2% vs 55%, P=.001), less need for stomata (10.5% vs 50%, P=.001) and shorter postoperative hospital stay (7 vs 12 days, P=.014). Thirty-day morbidity was reduced in the Stent group, although not significantly (29.8% vs 50%, P=.104). However, 30-day mortality was significantly lower (1.8% vs 20%, P=.015). Regarding the long-term oncologic outcomes, no significant differences were found when comparing overall survival, disease-free survival, local recurrence-free survival, distant recurrence-free survival or progression-free survival.
ConclusionsColonic stenting as a BTS for potentially resectable obstructive colorectal cancer seems to offer better surgical and equal long-term oncologic outcomes when compared to those of patients directly operated on.
La seguridad y los resultados oncológicos de los pacientes tratados con stents cólicos como puente a la cirugía (PAC) son controvertidos. El objetivo de este estudio es evaluar los efectos sobre los resultados quirúrgicos y oncológicos de los stents como PAC de las neoplasias colorrectales oclusivas potencialmente resecables.
MétodosAnálisis retrospectivo de los pacientes intervenidos por neoplasia colorrectal oclusiva potencialmente resecable con o sin enfermedad a distancia entre septiembre de 2002 y octubre de 2015, comparando los pacientes tratados con stent como PAC (grupo Stent) con los intervenidos de forma urgente (grupo Cirugía).
ResultadosVeinte pacientes fueron intervenidos directamente, mientras que se intentó la colocación de un stent en 57 pacientes. En el grupo Stent hubo más intervenciones laparoscópicas (64.9 vs 5%, p<0,001), más anastomosis primarias (91,2 vs 55%, p=0,001), menos estomas (10,5 vs 50%, p=0,001) y una estancia postoperatoria más corta (7 vs 12 días, p=0,014). La morbilidad a los 30 días fue menor en el grupo Stent, pero no de forma significativa (29,8 vs 50%, p=0,104), aunque sí lo fue la mortalidad (1,8 vs 20%, p=0,015). Respecto a los resultados oncológicos, no se encontraron diferencias significativas al comparar la supervivencia global, el intervalo libre de enfermedad, la supervivencia libre de recidiva local o a distancia ni la supervivencia libre de progresión.
ConclusionesLa utilización de stents cólicos como PAC de las neoplasias colorrectales oclusivas potencialmente resecables parece proporcionar mejores resultados quirúrgicos y resultados oncológicos equiparables a los de los pacientes intervenidos directamente.
Up to 30% of colorectal neoplasms debut with symptoms of obstruction.1,2 The approach of these neoplasms has classically involved urgent laparotomy, with a low rate of primary anastomoses and high morbidity.3 In 1990, Dohmoto et al. published their technique for the palliative management of stenosing colorectal tumors, which consisted of the placement of a stent to permeabilize the rectal lumen.4 The procedure quickly became popular, and its indication extended to the treatment of colorectal cancer obstructions as a bridge to definitive surgical treatment. The main advantage of stent placement would be colonic decompression,5 thereby allowing an urgent surgery to be converted into a semi-elective surgery, with the consequent reduction in morbidity and mortality.6,7 However, the procedure is not free of risks.8,9 Success depends largely on who is performing it, and complications can be serious, the most feared of which is bowel perforation, resulting in fecal peritonitis. In fact, 3 clinical trials have been canceled due to the high rate of complications.10–12 In addition, the oncological results of patients who underwent surgery after stenting have also been questioned, since theoretically the manipulation of the neoplasm could favor tumor dissemination.13,14 In recent years, contradictory articles have been published, and while some show an increase in recurrence rates, overall survival does not seem to change.15–20 In general, these studies include few patients and short follow-up intervals. The objective of the present study was to evaluate the effects of stent placement on surgical and oncological outcomes as a bridge to surgery (BTS) in patients with obstructing colorectal cancer (OCC).
MethodsWe conducted a retrospective, observational and anonymous study of all patients operated at our hospital for colorectal cancer from September 2002 to October 2015, after having received authorization from the Ethics Committee. All patients with obstruction symptoms who demonstrated radiological signs of colon obstruction at the time of diagnosis were selected for the analysis; presentations were either local or disseminated disease, but always considered potentially resectable. The criteria to classify a patient with clinical signs of obstruction were: bowel distension, closure and/or vomiting at the time of diagnosis. The radiological criterion for obstruction was a simple radiograph or CT scan showing radiological signs of obstruction. The disease was considered potentially resectable by the multidisciplinary committee when basically the entire disease could be resected without the need for chemotherapy or radiotherapy, regardless of whether interval treatments were finally used. Thus, we excluded patients with peritoneal carcinomatosis, extrahepatic and extrapulmonary metastatic disease or with extensive hepatic or pulmonary disease (considered unresectable due to volume or location and relationship to vascular structures, considered resectable but requiring preoperative hepatic modulation or extreme liver surgery, or with mediastinal lymph node involvement or insufficient postoperative pulmonary reserve).
The decision to intervene urgently or place a stent as BTS was made by the surgical team on duty, and the stents were placed by a team of experienced endoscopists following the through-the-scope technique and under fluoroscopic control.21 The different types of stents placed were Wallstent®, Wallflex® (Boston Scientific, Natick, MA, USA), Evolution® (Cook Medical, Limerick, Ireland) and Hanarostent® (MI-Tech, Seoul, Korea). The procedure was considered a technical success when it ended with the stent placed in the colon lumen, and it was also considered a clinical success when colonic decompression was achieved and later during the surgery there were no local complications detected attributable to stent placement or migration.
Patient follow-up was carried out following the clinical guidelines of our hospital, with clinical, analytical and radiological studies every 3 months during the first 2 years, and then every 6 months. For the overall survival analysis, we used the time from surgery until death for any reason. Disease-free survival was calculated using the time that patients without residual disease after the intervention (R0) took to develop any type of recurrence. Similarly, we defined local recurrence-free survival (patients without local disease after the intervention), distant relapse-free survival (patients without distant disease after the intervention) and progression-free survival (patients with residual disease after the R1 or R2 intervention).
Continuous variables are presented as mean (standard deviation) when they followed a normal distribution (verified by the Kolmogorov–Smirnov test), while those that did not were presented as median (interquartile range). For the statistical analysis, the SPSS® software package version 20.0.0 was used (IBM, Armonk, NY, USA). The categorical variables were compared using the Pearson's test or Fisher's exact test depending on the expected frequencies. The continuous variables were compared using the Student's t test for those that presented normal distribution or the Mann–Whitney U test for those that did not. For the survival analysis, the Kaplan–Meier method was used, comparing groups with the log-rank test. In all cases, statistical significance was defined by a P<.05.
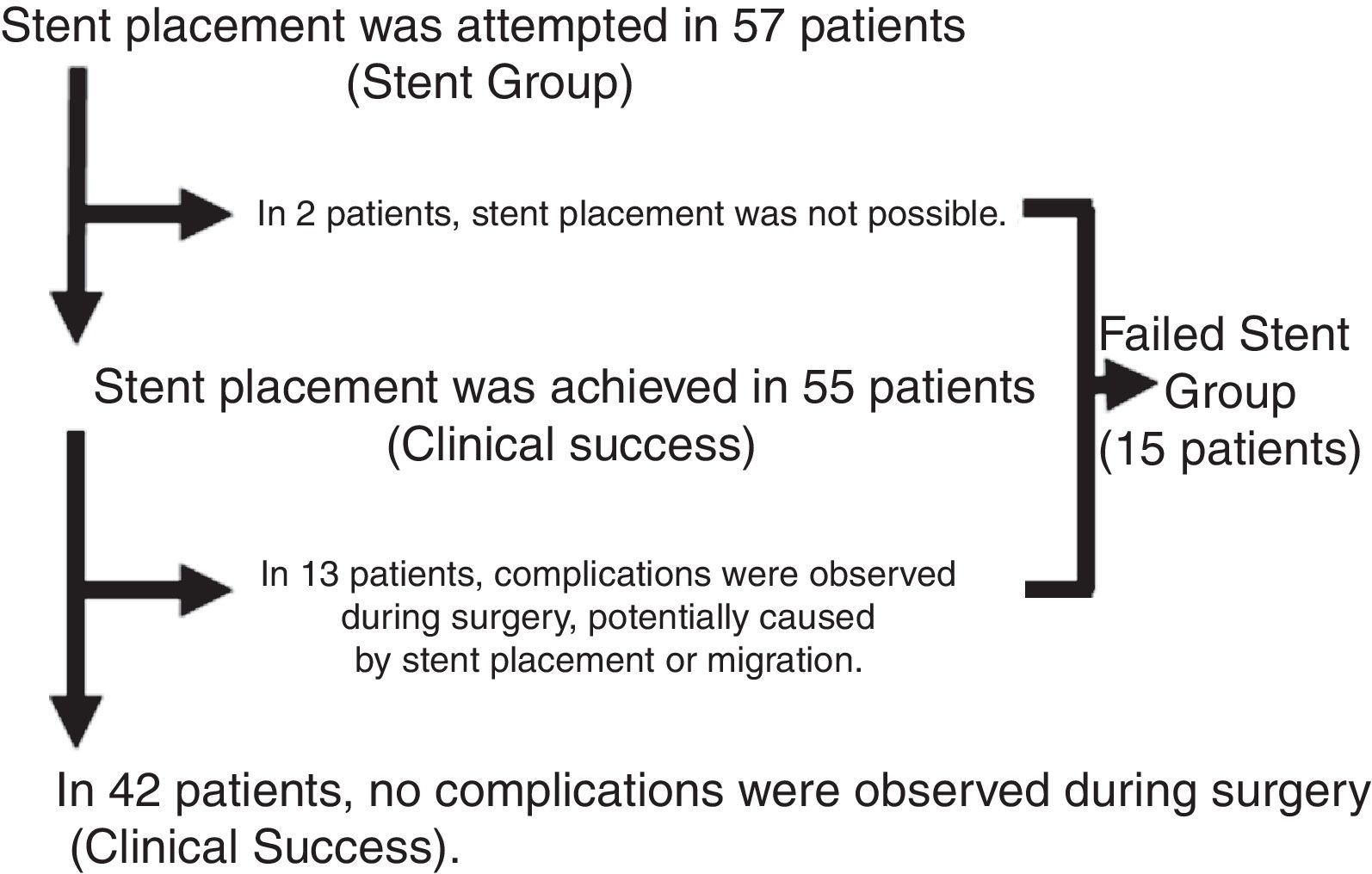
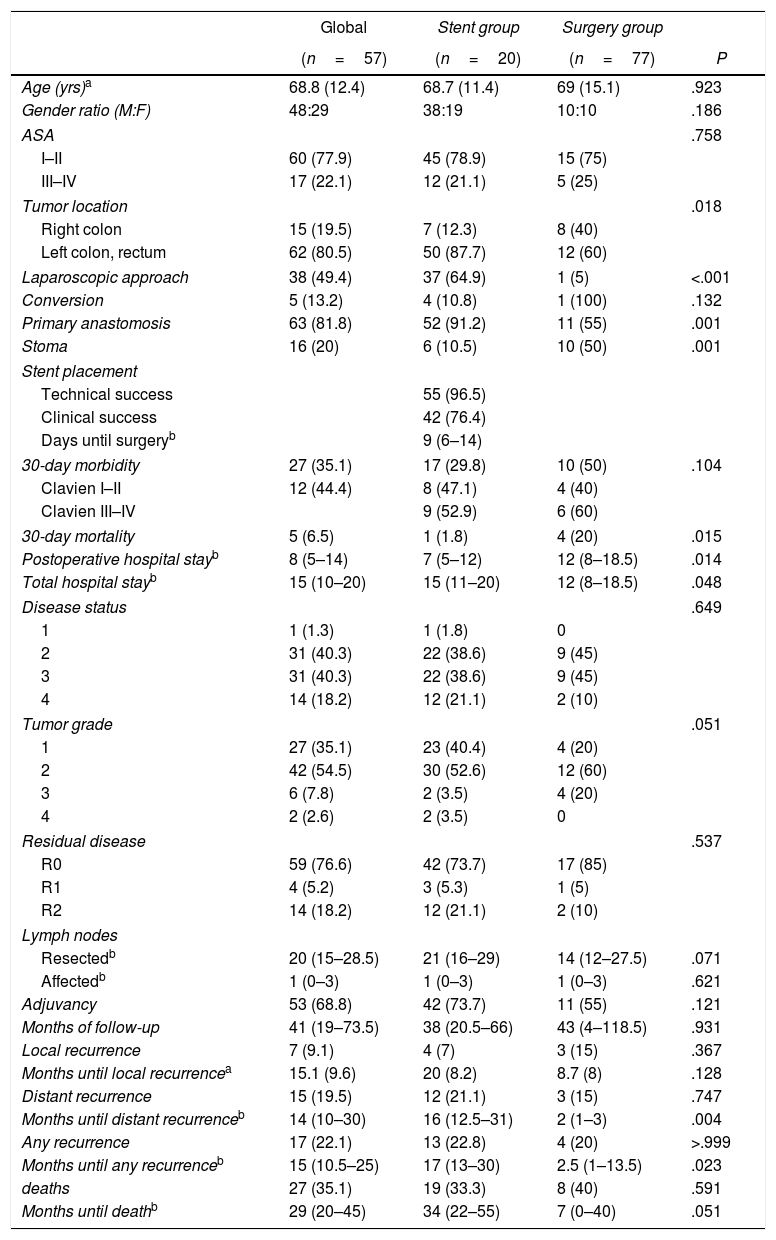
ResultsIn accordance with the inclusion criteria, we identified 77 patients who underwent surgery for potentially resectable OCC. Twenty of these patients (26%) were operated on directly (Surgery Group), while in 57 (74%, Stent Group) stent placement was attempted as BTS. The characteristics of the patients and the surgical results are summarized in Table 1. A greater proportion of patients in the Surgery Group had OCC that affected the right colon, and most of the patients with OCC of the left colon were treated with stent placement as BTS (80.6%). In the Stent Group, placement was not achieved in 2 patients (in one because the guide could not pass and in another because a perforation was found), so surgical treatment was urgent. However, since the study design is an intention-to-treat analysis, for the statistical analysis these cases remained in the Stent Group. The median (interquartile range) number of days from stent placement to elective intervention was 9 (6–14). During surgery, complications were observed in 13 of the 55 patients that could be attributed to colon manipulation during or after stent placement (inflammation, ulceration, perforation) or migration (clinical success rate 76.4%). Almost half of the patients in our study were treated laparoscopically, with a significantly higher proportion in the Stent Group (64.9% vs 5%, P<.001), with an overall conversion rate to laparotomy of 13.2% (100% in the Surgery Group). Half of the patients in the Surgery Group required a terminal or lateral stoma, and primary anastomosis was able to be performed in 55% of the patients. In contrast, only 10.5% of the patients in the Stent Group required a stoma, and primary anastomosis was performed in 91.2% (P=.001 in both cases). Thirty-day morbidity was higher in the Surgery Group, although without reaching statistical significance and with a similar distribution of complications according to the Clavien-Dindo scale.22 Thirty-day mortality was significantly higher in the Surgery Group (20% vs 1.8%, P=.015). The overall hospital stay was higher in the Stent Group, unlike the postoperative stay (15 vs 12 days, P=.048 and 7 vs 12 days, P=.014, respectively). All these interventions were limited to the treatment of local disease.
Patient Characteristics, Surgical Results, Pathology Characteristics and Oncology Results.
| Global | Stent group | Surgery group | ||
|---|---|---|---|---|
| (n=57) | (n=20) | (n=77) | P | |
| Age (yrs)a | 68.8 (12.4) | 68.7 (11.4) | 69 (15.1) | .923 |
| Gender ratio (M:F) | 48:29 | 38:19 | 10:10 | .186 |
| ASA | .758 | |||
| I–II | 60 (77.9) | 45 (78.9) | 15 (75) | |
| III–IV | 17 (22.1) | 12 (21.1) | 5 (25) | |
| Tumor location | .018 | |||
| Right colon | 15 (19.5) | 7 (12.3) | 8 (40) | |
| Left colon, rectum | 62 (80.5) | 50 (87.7) | 12 (60) | |
| Laparoscopic approach | 38 (49.4) | 37 (64.9) | 1 (5) | <.001 |
| Conversion | 5 (13.2) | 4 (10.8) | 1 (100) | .132 |
| Primary anastomosis | 63 (81.8) | 52 (91.2) | 11 (55) | .001 |
| Stoma | 16 (20) | 6 (10.5) | 10 (50) | .001 |
| Stent placement | ||||
| Technical success | 55 (96.5) | |||
| Clinical success | 42 (76.4) | |||
| Days until surgeryb | 9 (6–14) | |||
| 30-day morbidity | 27 (35.1) | 17 (29.8) | 10 (50) | .104 |
| Clavien I–II | 12 (44.4) | 8 (47.1) | 4 (40) | |
| Clavien III–IV | 9 (52.9) | 6 (60) | ||
| 30-day mortality | 5 (6.5) | 1 (1.8) | 4 (20) | .015 |
| Postoperative hospital stayb | 8 (5–14) | 7 (5–12) | 12 (8–18.5) | .014 |
| Total hospital stayb | 15 (10–20) | 15 (11–20) | 12 (8–18.5) | .048 |
| Disease status | .649 | |||
| 1 | 1 (1.3) | 1 (1.8) | 0 | |
| 2 | 31 (40.3) | 22 (38.6) | 9 (45) | |
| 3 | 31 (40.3) | 22 (38.6) | 9 (45) | |
| 4 | 14 (18.2) | 12 (21.1) | 2 (10) | |
| Tumor grade | .051 | |||
| 1 | 27 (35.1) | 23 (40.4) | 4 (20) | |
| 2 | 42 (54.5) | 30 (52.6) | 12 (60) | |
| 3 | 6 (7.8) | 2 (3.5) | 4 (20) | |
| 4 | 2 (2.6) | 2 (3.5) | 0 | |
| Residual disease | .537 | |||
| R0 | 59 (76.6) | 42 (73.7) | 17 (85) | |
| R1 | 4 (5.2) | 3 (5.3) | 1 (5) | |
| R2 | 14 (18.2) | 12 (21.1) | 2 (10) | |
| Lymph nodes | ||||
| Resectedb | 20 (15–28.5) | 21 (16–29) | 14 (12–27.5) | .071 |
| Affectedb | 1 (0–3) | 1 (0–3) | 1 (0–3) | .621 |
| Adjuvancy | 53 (68.8) | 42 (73.7) | 11 (55) | .121 |
| Months of follow-up | 41 (19–73.5) | 38 (20.5–66) | 43 (4–118.5) | .931 |
| Local recurrence | 7 (9.1) | 4 (7) | 3 (15) | .367 |
| Months until local recurrencea | 15.1 (9.6) | 20 (8.2) | 8.7 (8) | .128 |
| Distant recurrence | 15 (19.5) | 12 (21.1) | 3 (15) | .747 |
| Months until distant recurrenceb | 14 (10–30) | 16 (12.5–31) | 2 (1–3) | .004 |
| Any recurrence | 17 (22.1) | 13 (22.8) | 4 (20) | >.999 |
| Months until any recurrenceb | 15 (10.5–25) | 17 (13–30) | 2.5 (1–13.5) | .023 |
| deaths | 27 (35.1) | 19 (33.3) | 8 (40) | .591 |
| Months until deathb | 29 (20–45) | 34 (22–55) | 7 (0–40) | .051 |
ASA: American Society of Anesthesiologists; F: female; M: male.
The values in parentheses are percentages, except:
Regarding the pathology characteristics of the resected tumors (Table 1), no differences were found in staging or tumor grade. The distribution of patients with R0, R1 and R2 residual disease was similar in both groups. During the follow-up period (Table 1), 17 patients presented some type of recurrence: 2 patients only local, 10 patients only distant and 5 patients both, these recurrences being earlier in the Surgery Group.
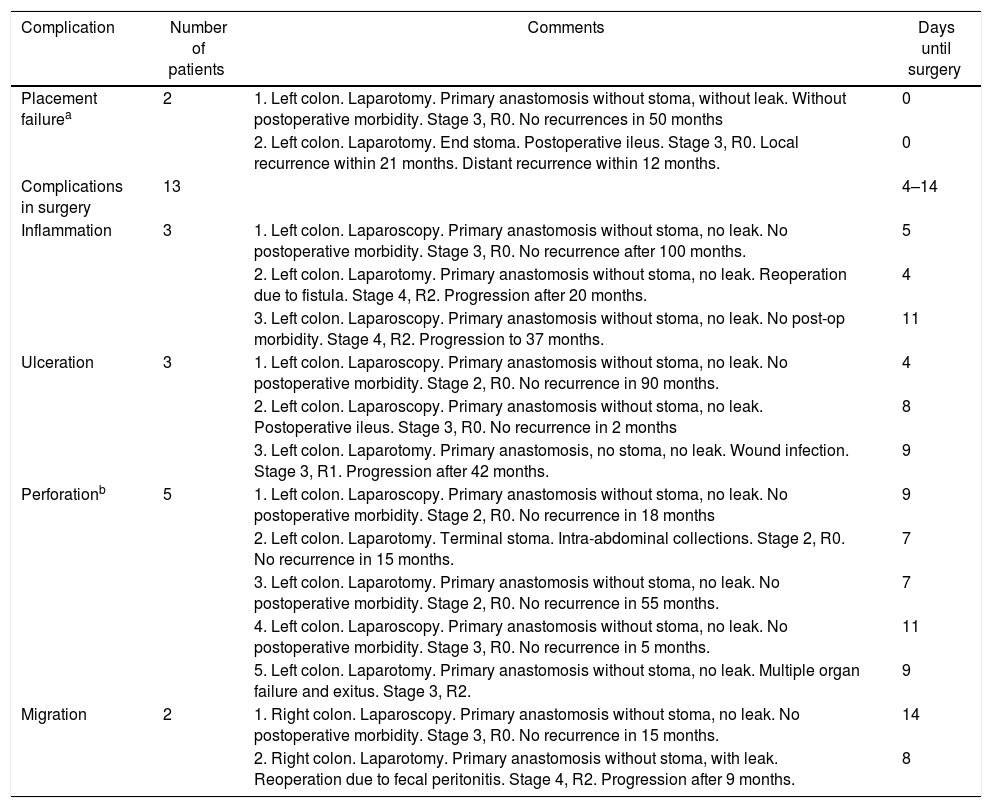
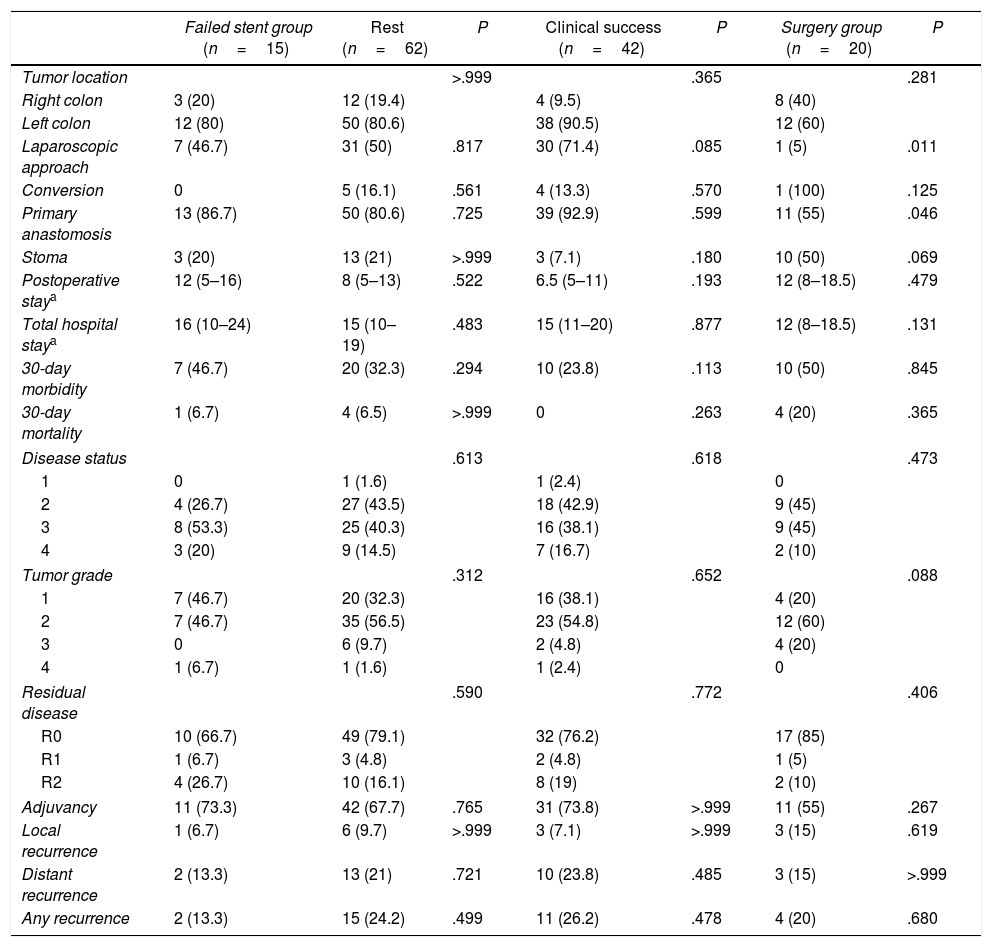
Fig. 1 shows the results of the stent placement procedures and the different subgroups generated. After the previous analysis, a new group of patients was defined, the Failed Stent Group (n=15), comprised of patients in whom stent placement either was not or had been placed but in whom complications were found attributable to stent placement or migration. The characteristics of these patients are summarized in Table 2. This group was compared with the remaining 62 patients, with the 42 considered as clinical stent-placement successes and with the 20 of the Surgery Group (Table 3). The results of this second analysis are discussed below.
Failed Stent Group.
| Complication | Number of patients | Comments | Days until surgery |
|---|---|---|---|
| Placement failurea | 2 | 1. Left colon. Laparotomy. Primary anastomosis without stoma, without leak. Without postoperative morbidity. Stage 3, R0. No recurrences in 50 months | 0 |
| 2. Left colon. Laparotomy. End stoma. Postoperative ileus. Stage 3, R0. Local recurrence within 21 months. Distant recurrence within 12 months. | 0 | ||
| Complications in surgery | 13 | 4–14 | |
| Inflammation | 3 | 1. Left colon. Laparoscopy. Primary anastomosis without stoma, no leak. No postoperative morbidity. Stage 3, R0. No recurrence after 100 months. | 5 |
| 2. Left colon. Laparotomy. Primary anastomosis without stoma, no leak. Reoperation due to fistula. Stage 4, R2. Progression after 20 months. | 4 | ||
| 3. Left colon. Laparoscopy. Primary anastomosis without stoma, no leak. No post-op morbidity. Stage 4, R2. Progression to 37 months. | 11 | ||
| Ulceration | 3 | 1. Left colon. Laparoscopy. Primary anastomosis without stoma, no leak. No postoperative morbidity. Stage 2, R0. No recurrence in 90 months. | 4 |
| 2. Left colon. Laparoscopy. Primary anastomosis without stoma, no leak. Postoperative ileus. Stage 3, R0. No recurrence in 2 months | 8 | ||
| 3. Left colon. Laparotomy. Primary anastomosis, no stoma, no leak. Wound infection. Stage 3, R1. Progression after 42 months. | 9 | ||
| Perforationb | 5 | 1. Left colon. Laparoscopy. Primary anastomosis without stoma, no leak. No postoperative morbidity. Stage 2, R0. No recurrence in 18 months | 9 |
| 2. Left colon. Laparotomy. Terminal stoma. Intra-abdominal collections. Stage 2, R0. No recurrence in 15 months. | 7 | ||
| 3. Left colon. Laparotomy. Primary anastomosis without stoma, no leak. No postoperative morbidity. Stage 2, R0. No recurrence in 55 months. | 7 | ||
| 4. Left colon. Laparoscopy. Primary anastomosis without stoma, no leak. No postoperative morbidity. Stage 3, R0. No recurrence in 5 months. | 11 | ||
| 5. Left colon. Laparotomy. Primary anastomosis without stoma, no leak. Multiple organ failure and exitus. Stage 3, R2. | 9 | ||
| Migration | 2 | 1. Right colon. Laparoscopy. Primary anastomosis without stoma, no leak. No postoperative morbidity. Stage 3, R0. No recurrence in 15 months. | 14 |
| 2. Right colon. Laparotomy. Primary anastomosis without stoma, with leak. Reoperation due to fecal peritonitis. Stage 4, R2. Progression after 9 months. | 8 |
Comparison of the Failed Stent Group.
| Failed stent group (n=15) | Rest (n=62) | P | Clinical success (n=42) | P | Surgery group (n=20) | P | |
|---|---|---|---|---|---|---|---|
| Tumor location | >.999 | .365 | .281 | ||||
| Right colon | 3 (20) | 12 (19.4) | 4 (9.5) | 8 (40) | |||
| Left colon | 12 (80) | 50 (80.6) | 38 (90.5) | 12 (60) | |||
| Laparoscopic approach | 7 (46.7) | 31 (50) | .817 | 30 (71.4) | .085 | 1 (5) | .011 |
| Conversion | 0 | 5 (16.1) | .561 | 4 (13.3) | .570 | 1 (100) | .125 |
| Primary anastomosis | 13 (86.7) | 50 (80.6) | .725 | 39 (92.9) | .599 | 11 (55) | .046 |
| Stoma | 3 (20) | 13 (21) | >.999 | 3 (7.1) | .180 | 10 (50) | .069 |
| Postoperative staya | 12 (5–16) | 8 (5–13) | .522 | 6.5 (5–11) | .193 | 12 (8–18.5) | .479 |
| Total hospital staya | 16 (10–24) | 15 (10–19) | .483 | 15 (11–20) | .877 | 12 (8–18.5) | .131 |
| 30-day morbidity | 7 (46.7) | 20 (32.3) | .294 | 10 (23.8) | .113 | 10 (50) | .845 |
| 30-day mortality | 1 (6.7) | 4 (6.5) | >.999 | 0 | .263 | 4 (20) | .365 |
| Disease status | .613 | .618 | .473 | ||||
| 1 | 0 | 1 (1.6) | 1 (2.4) | 0 | |||
| 2 | 4 (26.7) | 27 (43.5) | 18 (42.9) | 9 (45) | |||
| 3 | 8 (53.3) | 25 (40.3) | 16 (38.1) | 9 (45) | |||
| 4 | 3 (20) | 9 (14.5) | 7 (16.7) | 2 (10) | |||
| Tumor grade | .312 | .652 | .088 | ||||
| 1 | 7 (46.7) | 20 (32.3) | 16 (38.1) | 4 (20) | |||
| 2 | 7 (46.7) | 35 (56.5) | 23 (54.8) | 12 (60) | |||
| 3 | 0 | 6 (9.7) | 2 (4.8) | 4 (20) | |||
| 4 | 1 (6.7) | 1 (1.6) | 1 (2.4) | 0 | |||
| Residual disease | .590 | .772 | .406 | ||||
| R0 | 10 (66.7) | 49 (79.1) | 32 (76.2) | 17 (85) | |||
| R1 | 1 (6.7) | 3 (4.8) | 2 (4.8) | 1 (5) | |||
| R2 | 4 (26.7) | 10 (16.1) | 8 (19) | 2 (10) | |||
| Adjuvancy | 11 (73.3) | 42 (67.7) | .765 | 31 (73.8) | >.999 | 11 (55) | .267 |
| Local recurrence | 1 (6.7) | 6 (9.7) | >.999 | 3 (7.1) | >.999 | 3 (15) | .619 |
| Distant recurrence | 2 (13.3) | 13 (21) | .721 | 10 (23.8) | .485 | 3 (15) | >.999 |
| Any recurrence | 2 (13.3) | 15 (24.2) | .499 | 11 (26.2) | .478 | 4 (20) | .680 |
The values in parentheses are percentages, except:
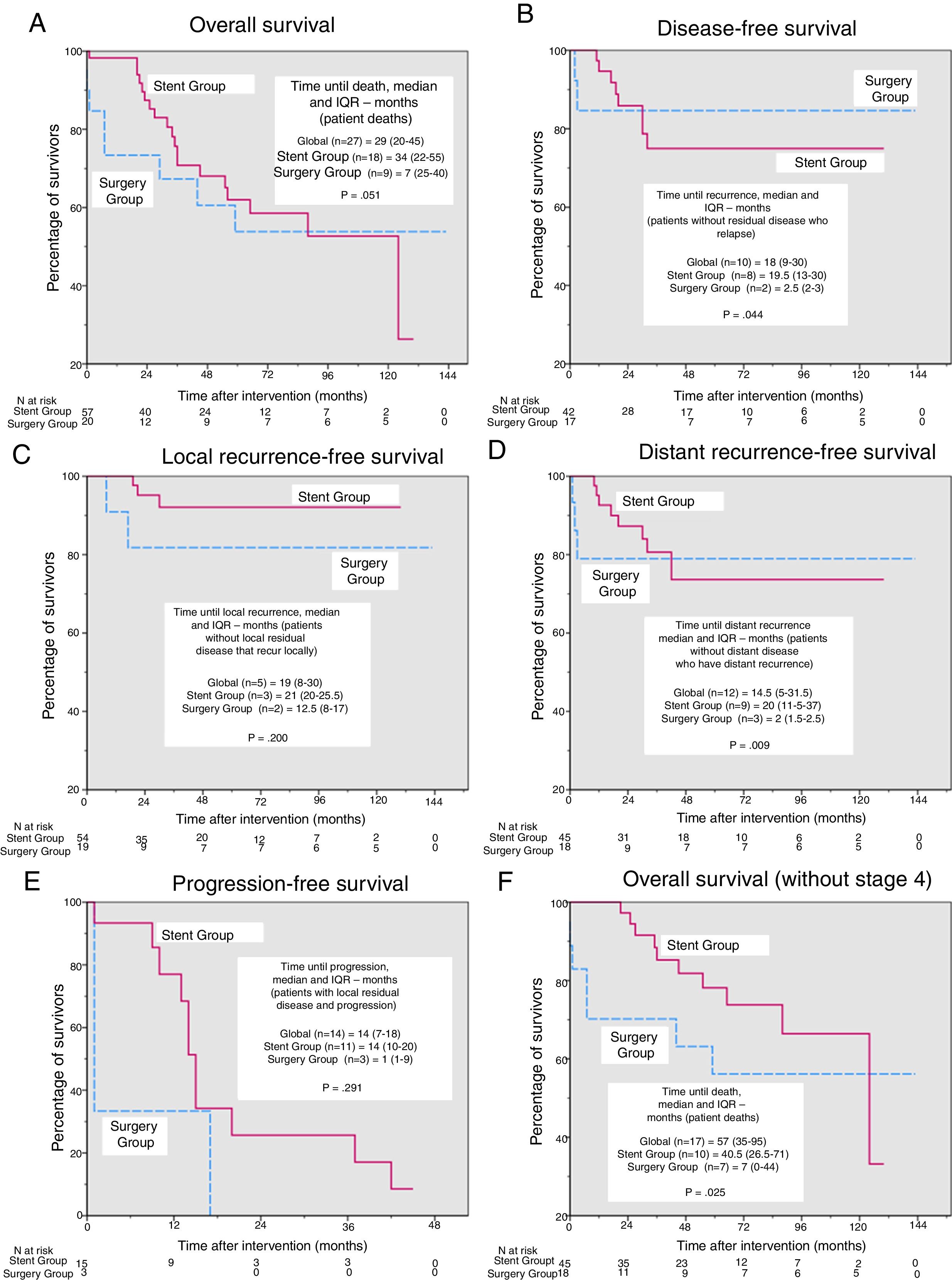
In the survival analysis, we found no significant differences in overall survival (Fig. 2A), disease-free survival (Fig. 2B), survival free of local or distant recurrence (Fig. 2C and D) or progression-free survival (Fig. 2E) after a median (interquartile range) follow-up of 41 months (19–73.5). However, the time to each type of event (Fig. 2A–E) was shorter in the Surgery Group, with significant differences in time to any type of recurrence and time to distant recurrence (2.5 vs 19.5 months, P=.044 and 2 vs 20 months, P=.009, respectively). For overall survival, after excluding patients in stage 4 (Fig. 2F), the time to death became significantly shorter in the Surgery Group (7 vs 40.5 months, P=.025).
Kaplan–Meier curves and comparisons of time until each event. Except for in overall survival, deaths were considered censored cases. (A) Overall survival, P=.736 (log-rank test). Additionally, for patients who died during the study period, time until death (median and interquartile range [IQR]) were compared using the Mann–Whitney U. (B) Disease-free survival, P=.780 (log-rank test). Additionally, for the patients without residual disease who presented recurrence during the study period, the time until death (median and IQR) were compared using the Mann–Whitney U. (C) Local recurrence-free survival, P=.233 (log-rank test). Additionally, for the patients who were not local disease free and presented local recurrence during the study period, time until death (median and IQR) were compared using the Mann–Whitney U. (D) distant recurrence-free survival, P=.859 (log-rank test). Additionally, for the patients who had distant residual disease and presented distance recurrence during the study period, time until death (median and IQR) were compared using the Mann–Whitney U. (E) Progression-free survival, P=.155 (log-rank test). Additionally, for the patients who had residual disease and progressed during the study period, the time until death (median and IQR) were compared using the Mann–Whitney U. (F) Overall survival, excluding patients in stage 4, P=.287 (log-rank test). Additionally, for patients who died during the study period, time until death (median and IQR) was compared using the Mann–Whitney U.
The present study demonstrates a clear superiority of postoperative results in the group of patients treated with colonic stents as BTS, while survival and long-term recurrences remained similar in both groups.
Since the introduction of the colonic decompression technique using stent placement and the publication of surprising initial results that were probably too optimistic,23 the evaluation of this treatment in recent years has gone from enthusiasm to skepticism due to reports of possible complications secondary to the procedure and, more recently, rejection with the appearance of studies that show poor long-term oncological results.24,25
In our series, 57 patients out of a total of 77 were treated with stents as BTS. This difference in the size of the groups is mainly representative of the personal preferences of surgeons at our hospital, probably because there is no perception of a high rate of complications with the placement of the stents, contrary to the findings in the literature and which has been the reason for cancelation of several clinical trials.10–12
Patients treated with stents could avoid urgent surgery. In the case of colonic obstruction, urgent surgery usually involves performing a wide laparotomy and, especially in tumors that affect the left colon and rectum, the impossibility of performing a primary anastomosis. These urgent laparotomies have high associated morbidity and mortality rates, and many stomata created will never be reconstructed.26 The majority of patients in the Stent Group underwent laparoscopic surgery, with more primary anastomoses, and their postoperative hospital stay was significantly shorter. However, when hospitalization days are added from stent placement to surgical intervention, the hospital stay advantage is reversed. In spite of this, we must consider that half of the patients in the Surgery Group would need at least another admission for intestinal tract reconstruction, increasing hospital stay and without being free of possible complications, leading to increased costs for the entire process.27–31
Given that primary anastomosis is not usually contraindicated in right colon obstruction, fewer potential advantages are attributed to resolving these occlusions by stenting,32,33 and this is probably the reason why we found more patients with right colon neoplasms in the Surgery Group. However, the benefits shown here, avoiding urgent surgery and enabling the laparoscopic approach, would also justify the treatment of those with stents.
It should be emphasized that, even when the stent placement or function were considered unsuccessful due to local complications observed during the surgical intervention, simply the presence of the stent and, therefore, the resolution of the obstruction made the laparoscopic approach possible (although in a lower percentage than in the group of patients with clinical success) and increased the rate of primary anastomosis compared to that of patients without stents. Other advantages observed in the Stent Group when compared to the Surgery Group that can be attributed to the type of approach are less evident in the Stent Failed group.
If the complications observed during stent placement (n=1, since the inability to place the stent is not considered a complication) and during the interventions (n=13) are counted as complications of the procedure, then morbidity would be 24.6%, with a perforation rate of 10.5%. The postoperative morbidity and mortality of the Failed Stent group were 46.7% and 6.7%, respectively. Although they seem high, these percentages are lower than those observed in the Surgery Group and, therefore, even when the stent placement was not considered successful, the care process of these patients was safer than those who had been treated with direct surgery.
The main reason for developing this study was to evaluate the effect of colon stent placement on long-term oncological results. Although some authors have published an increase in both local and distant recurrence rates in patients treated with stents, this effect has not been observed in our patients, in which both rates of recurrence are similar to those of the Surgery Group. As for the survival analysis, we did not find significant differences between the different Kaplan–Meier curves (Fig. 2). Levels of circulating tumor RNA increase after placement of a colonic stent,34 although it has not been possible to demonstrate that this entails a worse prognosis. Similarly, a recent experiment in mice has shown that the placement of colonic stents causes metastatic dissemination,35 although the authors admit to important limitations in the design and methodology of the study that prevent extrapolation of the results to clinical practice in humans. Some published studies conclude that there is a higher rate of recurrence and that these recurrences occur earlier in patients treated with stents, without this translating to a reduction in overall survival. To date, there is only one meta-analysis evaluating the long-term oncological results of patients treated with colonic stenting,36 which concludes that stenting as BTS does not adversely affect overall survival, disease-free interval or recurrence rates compared to patients treated with urgent surgery. The results presented here agree with this meta-analysis and further support the use of stents before surgery, since short-term results are better and patients treated directly with surgery present recurrences earlier, although no differences are seen in global survival.
Despite the advances achieved in recent years in the treatment of metastatic colorectal cancer, there are inter-institutional discrepancies regarding which disease is considered resectable and curable or not. At our hospital, we define ‘resectable patients’ as those in whom complete resection of all the present disease can be achieved, both locally and remotely, without the need for conversion therapies. However, to avoid controversies, we conducted a second survival analysis excluding the 14 patients with stage 4 disease. The results obtained are practically the same as the previous ones, but their support of stent use is even stronger because the overall survival curves are further separated and the difference between groups in time to death becomes significant (Fig. 2F).
Our study has several limitations, mainly because it is observational and retrospective without any type of control or randomization. The fact that there was no established criterion according to which patients should be treated with a stent could have created a selection bias that would explain the greater number of patients treated with stents and the greater proportion of patients with right colon neoplasms in the Surgery Group. On the other hand, limiting the study to cases of potentially resectable neoplasms has markedly reduced the number of patients included, since a large number of those who start with occlusion have unresectable local or distant disease and surgery is therefore considered palliative. Even so, our numbers are similar or even greater than those of many published studies,37–39 with the added value of providing the prolonged follow-up time and the fact that, when analyzing only patients with potentially unresectable disease, the possible detrimental effects of the stents, if they exist, should be more obvious.
In conclusion, the use of stents as a BTS for potentially resectable OCC makes it possible to convert urgent to elective surgery, facilitating the minimally invasive approach and the performance of primary anastomoses. In turn, this reduces the need for stomata as well as the morbidity and mortality associated with surgery. In addition, it seems to provide long-term oncological results comparable to those of directly operated patients. Although these results are consistent with those of a recent meta-analysis, well-designed randomized prospective studies would be necessary to reach a higher level of scientific evidence.
AuthorshipAll the authors have read and approved of the manuscript, and we comply with the requirements for authorship. Antònia Crespí Mir and Juan Manuel Romero Marcos have participated in the study design, data collection, analysis and interpretation of the results, composition of the article and the approval of the final version, collaborating in the same manner in the production of the manuscript. Anabel de la Llave Serralvo has participated in data acquisition. Carlos Dolz Abadía and José Andrés Cifuentes Ródenas have participated in the analysis and interpretation of the results, critical review and approval of the final version.
Conflict of InterestsDr. Dolz participated in a study sponsored by Cook Medical in 2012. The remaining authors have no conflict of interests to declare.
The authors would like to acknowledge the staff of the Surgery and Gastroenterology departments for their contribution to the development of this study. Thanks also go to Dr. José M. Olea, Dr. Larry Hershon, Dr. Silvia Tejada and Xavier Roca for their valuable collaboration.
Please cite this article as: Crespí-Mir A, Romero-Marcos JM, de la Llave-Serralvo A, Dolz-Abadía C, Cifuentes-Ródenas JA. Impacto de la utilización de stents cólicos como puente a la cirugía de neoplasias colorrectales oclusivas potencialmente curables sobre los resultados quirúrgicos y oncológicos. Cir Esp. 2018;96:419–428.
Part of this manuscript was presented as an oral communication at the 20th National Meeting of the Spanish Association of Surgeons (Granada, October 2015).






![Kaplan–Meier curves and comparisons of time until each event. Except for in overall survival, deaths were considered censored cases. (A) Overall survival, P=.736 (log-rank test). Additionally, for patients who died during the study period, time until death (median and interquartile range [IQR]) were compared using the Mann–Whitney U. (B) Disease-free survival, P=.780 (log-rank test). Additionally, for the patients without residual disease who presented recurrence during the study period, the time until death (median and IQR) were compared using the Mann–Whitney U. (C) Local recurrence-free survival, P=.233 (log-rank test). Additionally, for the patients who were not local disease free and presented local recurrence during the study period, time until death (median and IQR) were compared using the Mann–Whitney U. (D) distant recurrence-free survival, P=.859 (log-rank test). Additionally, for the patients who had distant residual disease and presented distance recurrence during the study period, time until death (median and IQR) were compared using the Mann–Whitney U. (E) Progression-free survival, P=.155 (log-rank test). Additionally, for the patients who had residual disease and progressed during the study period, the time until death (median and IQR) were compared using the Mann–Whitney U. (F) Overall survival, excluding patients in stage 4, P=.287 (log-rank test). Additionally, for patients who died during the study period, time until death (median and IQR) was compared using the Mann–Whitney U. Kaplan–Meier curves and comparisons of time until each event. Except for in overall survival, deaths were considered censored cases. (A) Overall survival, P=.736 (log-rank test). Additionally, for patients who died during the study period, time until death (median and interquartile range [IQR]) were compared using the Mann–Whitney U. (B) Disease-free survival, P=.780 (log-rank test). Additionally, for the patients without residual disease who presented recurrence during the study period, the time until death (median and IQR) were compared using the Mann–Whitney U. (C) Local recurrence-free survival, P=.233 (log-rank test). Additionally, for the patients who were not local disease free and presented local recurrence during the study period, time until death (median and IQR) were compared using the Mann–Whitney U. (D) distant recurrence-free survival, P=.859 (log-rank test). Additionally, for the patients who had distant residual disease and presented distance recurrence during the study period, time until death (median and IQR) were compared using the Mann–Whitney U. (E) Progression-free survival, P=.155 (log-rank test). Additionally, for the patients who had residual disease and progressed during the study period, the time until death (median and IQR) were compared using the Mann–Whitney U. (F) Overall survival, excluding patients in stage 4, P=.287 (log-rank test). Additionally, for patients who died during the study period, time until death (median and IQR) was compared using the Mann–Whitney U.](https://static.elsevier.es/multimedia/21735077/0000009600000007/v1_201808180413/S2173507718301522/v1_201808180413/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
