Ileoanal pouch following restorative proctocolectomy is the treatment for ulcerative colitis after failed medical treatment. Our main aim was to evaluate early and late morbidity associated with restorative proctocolectomy. The secondary aim was to assess risk factors for pouch failure.
MethodsA retrospective, observational, single-center study was performed. Patients who had undergone restorative proctocolectomy for a preoperative diagnosis of ulcerative colitis from 1983 to 2015 were included. Early (<30 days) and late (>30 days) adverse events were analyzed. Pouch failure was defined as the need for pouch excision or when ileostomy closure could not be performed. Univariate and multivariate analyses were performed to assess pouch failure risk factors.
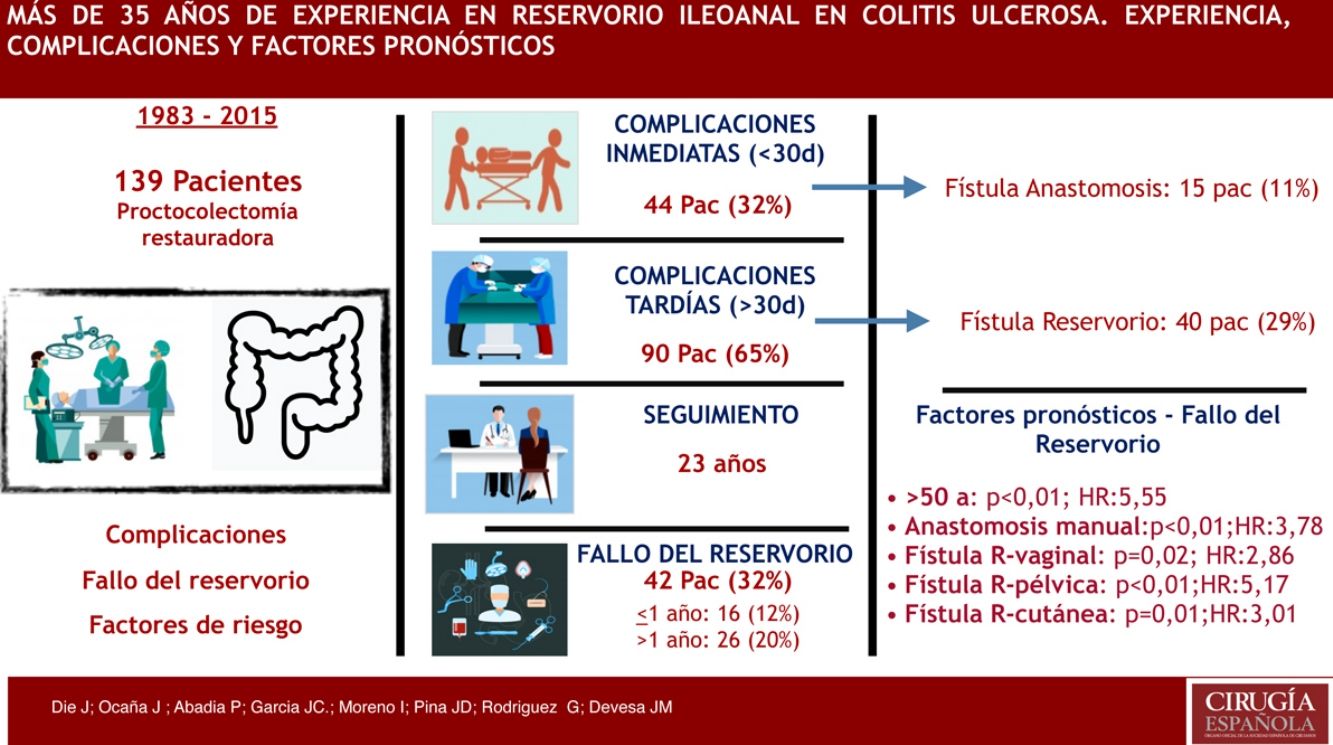
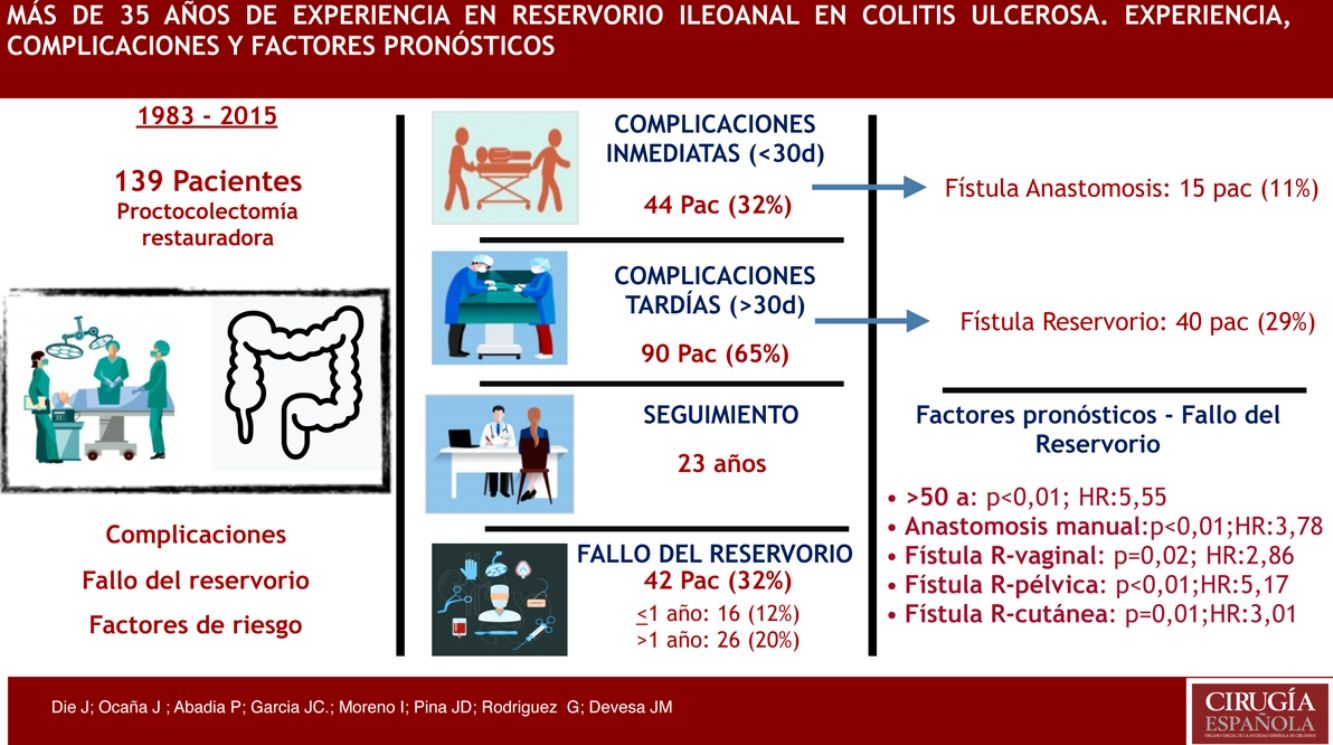
ResultsThe study included 139 patients. One patient subsequently died in the early postoperative period. Mean follow-up was 23 years. Manual anastomoses were performed in 54 patients (39%). Early adverse events were found in 44 patients (32%), 15 of which (11%) had anastomotic fistula. Late adverse events were found in 90 patients (65%), and pouchrelated fistulae (29%) were the most commonly found in this group. Pouch failure was identified in 42 patients (32%). In the multivariate analysis, age >50 years ( P < .01; HR: 5.55), handsewn anastomosis ( P < .01; HR: 3.78), pouch-vaginal ( P = .02; HR: 2.86), pelvic ( P < .01; HR: 5.17) and cutaneous P = .01; HR: 3.01) fistulae were the main pouch failure risk factors.
ConclusionRestorative proctocolectomy for a preoperative diagnosis of ulcerative colitis has high morbidity rates. Long-term outcomes could be improved if risk factors for failure are avoided.
La proctocolectomía restauradora con reservorio ileoanal es el tratamiento de elección en gran parte de los pacientes con colitis ulcerosa tras el fracaso del tratamiento médico. Nuestro objetivo principal fue analizar la morbilidad asociada a este procedimiento y la viabilidad del reservorio a corto y largo plazo. Como objetivo secundario identificamos los factores de riesgo asociados al fallo del reservorio.
MétodosEstudio retrospectivo observacional unicéntrico donde se analizan pacientes intervenidos de proctocolectomía total restauradora con reservorio ileoanal tras el diagnóstico de colitis ulcerosa entre los años 1983 y 2015. Se identificaron y analizaron las complicaciones tempranas (< 30 días) y tardías (> 30 días). Se consideró fallo del reservorio la necesidad de extirpación del reservorio o la imposibilidad para reconstruir el tránsito. Se llevó a cabo un análisis univariante y multivariante para identificar los factores asociados al fallo del reservorio ileoanal.
ResultadosHubo 139 pacientes analizados. Un paciente falleció en el postoperatorio. La mediana de seguimiento fue de 12 años. En 54 pacientes (39%) se realizó anastomosis manual. Presentaron complicaciones inmediatas 44 pacientes (32%), 15 pacientes (11%) con fístula anastomótica. Complicaciones tardías fueron diagnosticadas en 90 pacientes (65%), las más frecuentes fueron las fístulas asociadas al reservorio (29%). Hubo 42 pacientes (32%) con fallo del reservorio. La edad > 50 años (p < 0,01; HR: 5,55), la anastomosis manual (p < 0,01; HR: 3,78), la fístula del reservorio vaginal (p = 0,02; HR: 2,86), la pélvica (p < 0,01; HR: 5,17) y la cutánea (p = 0,01; HR: 3,01) fueron los principales factores de riesgo asociados al fallo del reservorio encontrados en el análisis multivariante.
ConclusiónLa proctocolectomía restauradora es una técnica con elevada morbilidad a corto y largo plazo. Controlando los factores de riesgo del fallo del reservorio se podrían mejorar los resultados a largo plazo.
Restorative proctocolectomy is the surgical treatment of choice in certain patients with ulcerative colitis when medical treatment is not effective. Studies have demonstrated that it is a safe and effective procedure.1,2 Since the beginnings of restorative proctocolectomy with ileoanal pouch in Spain in the 1980s, there has been great development and diffusion of the technique. It is currently performed in almost all the hospitals of our country, although no results have been recently published in the literature. Even though satisfactory functional and quality-of-life results have been published after restorative proctocolectomy,3 several studies show limited long-term results4,5 and progressive deterioration of the results over time. Since patients with ileoanal pouch are generally under the age of 60, it is necessary to carefully analyze the long-term outcomes and risk factors associated with pouch failure.
The main objective of this study was to analyze the short- and long-term surgical complications and viability of the ileoanal pouch after restorative proctocolectomy in patients diagnosed with ulcerative colitis. The secondary objective was to identify the risk factors associated with ileoanal pouch failure.
MethodsWe retrospectively identified patients who had undergone total proctocolectomy with ileoanal J-pouch surgery after an initial diagnosis of ulcerative colitis between 1983 and 2015 using a prospective database from a single institution. Inclusion criteria were: 1) patients diagnosed with ulcerative colitis at the time of surgery, based on compatible clinical symptoms and histological findings after colonoscopy suggestive of ulcerative colitis;6,7 and 2) elective total proctocolectomy with ileoanal J-pouch. Exclusion criteria were: Previous diagnosis of indeterminate colitis, familial adenomatous polyposis (FAP), chronic constipation, Crohn’s disease or colorectal cancer.
The creation of the pouch was carried out in one, two or three stages in patients treated with proctocolectomy. In one stage, the pouch was constructed during the same operation as the proctocolectomy, without a protective stoma. In 2 stages, the construction of the pouch was performed after the proctocolectomy in the same operation, with subsequent closure of the ileostomy. And in 3 stages, the construction of the pouch and the proctocolectomy were carried out in different surgical stages, with subsequent reconstruction of the tract or ileostomy closure. In all cases, J-pouches were constructed. The proctectomy was transmesorectal, using the abdominal approach.
Failure of the pouch was determined by the following: removal of the pouch was necessary; ileostomy was performed without removal of the pouch; closure of the protective ileostomy, if done, was not possible at any time during the follow-up. Demographic variables and surgical technique variables were collected and analyzed. Immediate complications were defined as those occurring during the first 30 days after surgery. Late-onset complications were those that occurred after the first 30 postoperative days until the final date of follow-up.
Statistical AnalysisIn the descriptive statistical analysis, medians and ranges were used for continuous variables, and frequencies and proportions were used for categorical variables. All were calculated for dependent and independent variables. The Student’s t test and the Mann-Whitney U test were used, as appropriate, for the comparison of means of the continuous variables. We used the chi-squared test and Fisher’s exact test for the analysis of categorical variables. Long-term complications, including pouch failure, were analyzed as time until the event studied. The cumulative probability for patients having either no complications or pouch failure was estimated using the Kaplan-Meier method. Survival curves were compared using the log-rank test. For the study of prognostic factors associated with pouch failure, all clinically relevant variables with a P < .20 in the univariate analysis were included in the multivariate logistic regression analysis.
A P < .05 was considered significant. Confidence intervals were estimated with a 95% interval. The statistical analysis was performed with SPSS Statistics® v.22.
ResultsOne hundred thirty-nine patients were analyzed retrospectively using data from a prospective database of a single hospital. Seven patients were lost to follow-up after hospital discharge.
Out of the 132 patients with complete follow-up, 2 (1%) died: one in the immediate postoperative period due to sepsis secondary to H1N1 influenza-related pneumonia in a patient previously treated surgically for a pancoast tumor with resection of the ribcage; the second patient died 12 years after completion of the ileal pouch due to postoperative sepsis after reoperation for a pouch-bladder fistula.
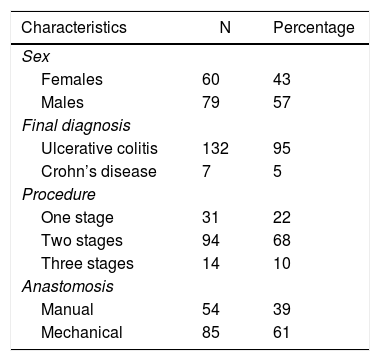
Mean follow-up was 12 years (range: 1–29). Mean patient age at the time of surgery was 35 years (range: 15–78). The demographic variables, final diagnosis during follow-up, surgical times for the construction of the pouch and types of anastomoses are shown in Table 1.
Immediate complications were diagnosed in 44 patients (32%). Anastomotic fistula was observed in 15 patients (11%), while a fistula between the pouch and the vagina was found in 6 patients (4%). Enterocutaneous fistulae were diagnosed in 2 patients (1%). Evisceration, intestinal obstruction and stenosis of the pouch were analyzed, as observed in 2 patients (1%), 5 (3%) and one (1%), respectively. Five patients (4%) presented hematochezia during the immediate postoperative period, while 2 patients (1%) had deep infection of the surgical site, manifesting as an intra-abdominal abscess. Two patients (1%) had surgical wound infection. Three patients (2%) were diagnosed with moderate or severe respiratory problems in the immediate postoperative period, and only one (1%) presented perianal fistula.
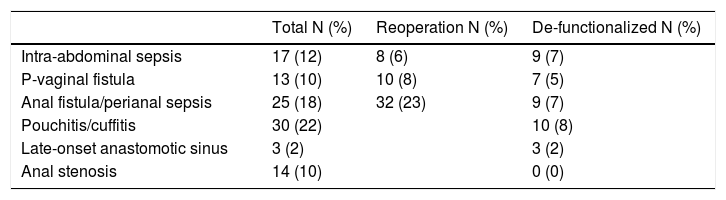
Late-onset complications were observed in 90 patients (65%). The most frequent late complications were fistulae associated with the pouch in 40 patients (29%): 11 patients (8%) with fistula between the pouch and the vagina, 27 patients (14%) with another type of pouch fistula (mainly pouch-anal), and 2 patients (1%) with enterocutaneous fistula. Other late-onset complications found included pouch dysfunction in 12 patients (9%), intestinal obstruction in 12 patients (9%), pouchitis in 15 patients (11%) and pouch stenosis in 8 patients (6%). Only 53 patients (40%) with pouches analyzed in this time period have remained completely asymptomatic. All complications associated with the procedure are shown in Table 2.
Complications Associated With the Procedure.
| Total N (%) | Reoperation N (%) | De-functionalized N (%) | |
|---|---|---|---|
| Intra-abdominal sepsis | 17 (12) | 8 (6) | 9 (7) |
| P-vaginal fistula | 13 (10) | 10 (8) | 7 (5) |
| Anal fistula/perianal sepsis | 25 (18) | 32 (23) | 9 (7) |
| Pouchitis/cuffitis | 30 (22) | 10 (8) | |
| Late-onset anastomotic sinus | 3 (2) | 3 (2) | |
| Anal stenosis | 14 (10) | 0 (0) |
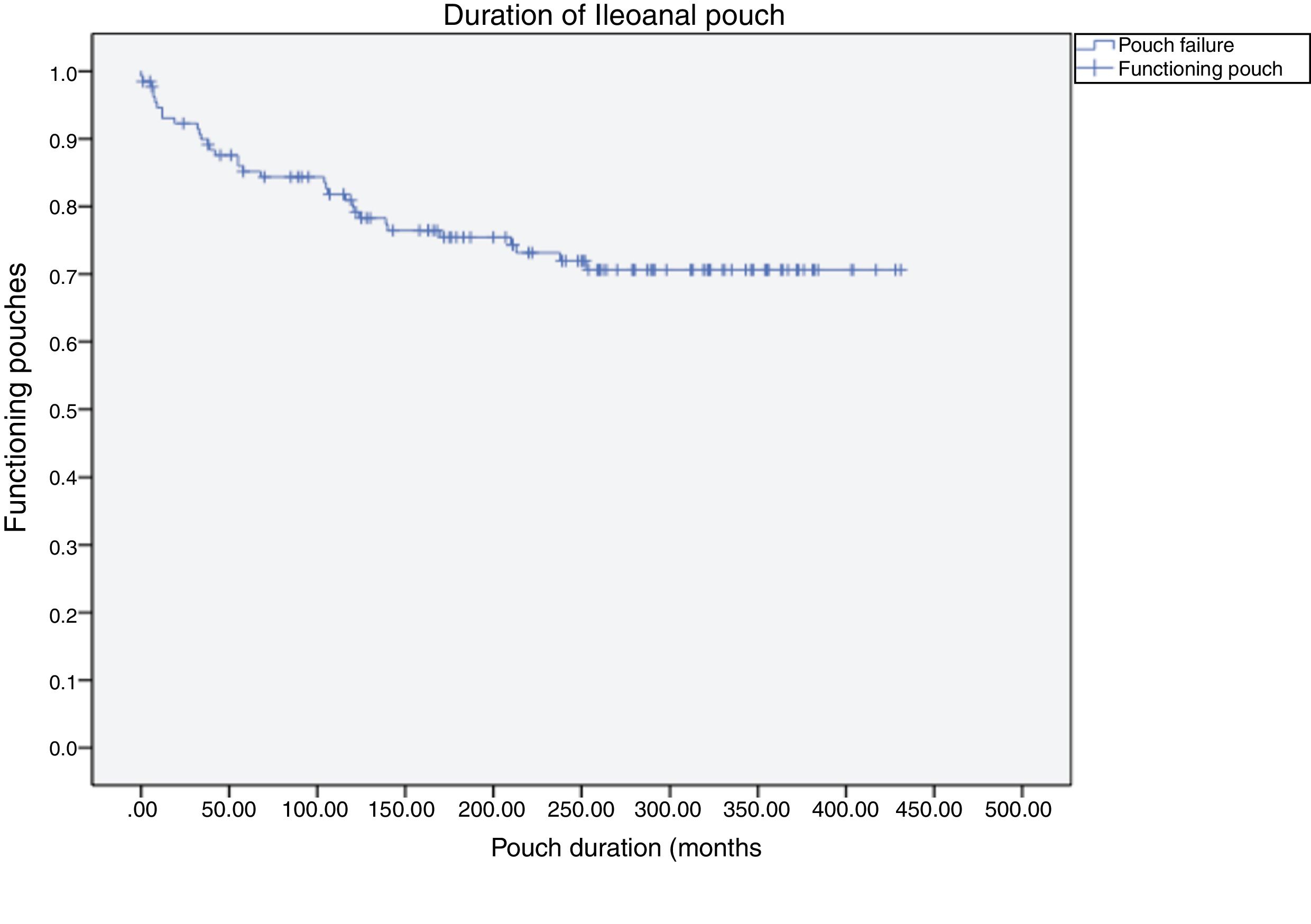
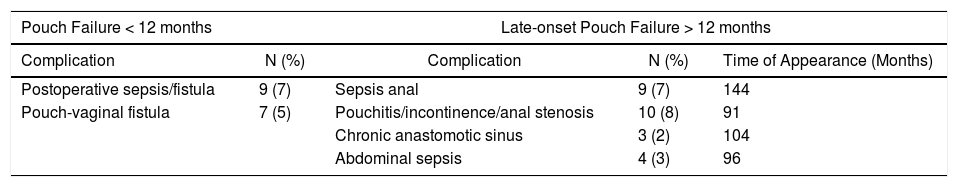
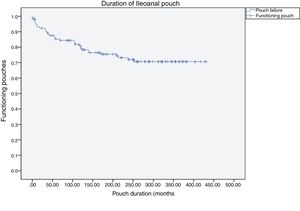
Pouch failure (Fig. 1) was identified in 42 patients (32%), while during follow-up some 90 patients (69%) continued to have a functioning pouch. Out of these 42 pouch failures, 16 (38%) occurred within the first year, while 26 (62%) occurred after the first year, at an average of 117 months (almost 10 years) after the creation of the pouch. Therefore, in the first year of the postoperative period, 16 patients (12%) experienced pouch failure. The causes of pouch failure are described in Table 3 and mainly include: perianal sepsis in 9 cases (7%) with presentation at an average of 12 years after pouch creation; pouchitis, incontinence or anal stenosis in 10 cases (8%), at an average 7 years and 7 months after pouch creation; chronic anastomotic sinus in 3 (2%) at an average of 8 years after the creation of the pouch; and late-onset abdominal sepsis in 4 cases (3%) at an average of 8 years after the creation of the pouch.
Causes of Pouch Failure and Time of Appearance.
| Pouch Failure < 12 months | Late-onset Pouch Failure > 12 months | |||
|---|---|---|---|---|
| Complication | N (%) | Complication | N (%) | Time of Appearance (Months) |
| Postoperative sepsis/fistula | 9 (7) | Sepsis anal | 9 (7) | 144 |
| Pouch-vaginal fistula | 7 (5) | Pouchitis/incontinence/anal stenosis | 10 (8) | 91 |
| Chronic anastomotic sinus | 3 (2) | 104 | ||
| Abdominal sepsis | 4 (3) | 96 | ||
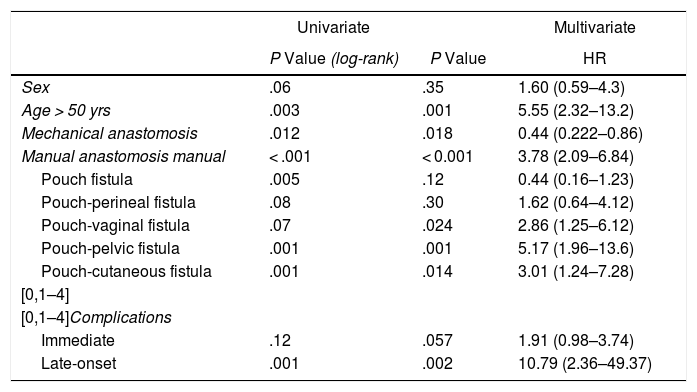
In the multivariate analysis (Table 4 and Fig. 2), risk factors for pouch failure included: age > 50 years, P < .01, HR: 5.55 (2.32–13.2); manual anastomosis, P < .01, HR: 3.78 (2.09–6.84); pouch-vaginal fistula, P = .02, HR: 2.86 (1.25–6.12); pouch-pelvic fistula, P < .01, HR: 5.17 (1.96–13.6); pouch-cutaneous fistula, P = .01, HR: 3.01 (1.24–7.26) and the development of late-onset complications P < .01, HR: 10.79 (2.36–49.37). Meanwhile, mechanical anastomosis P = .01, HR: 0.44 (0.22-0.86) was considered a good prognostic factor for pouch maintenance over time.
Uni/multivariate Analysis About Risk Factors for Pouch Failure.
| Univariate | Multivariate | ||
|---|---|---|---|
| P Value (log-rank) | P Value | HR | |
| Sex | .06 | .35 | 1.60 (0.59–4.3) |
| Age > 50 yrs | .003 | .001 | 5.55 (2.32–13.2) |
| Mechanical anastomosis | .012 | .018 | 0.44 (0.222–0.86) |
| Manual anastomosis manual | < .001 | < 0.001 | 3.78 (2.09–6.84) |
| Pouch fistula | .005 | .12 | 0.44 (0.16–1.23) |
| Pouch-perineal fistula | .08 | .30 | 1.62 (0.64–4.12) |
| Pouch-vaginal fistula | .07 | .024 | 2.86 (1.25–6.12) |
| Pouch-pelvic fistula | .001 | .001 | 5.17 (1.96–13.6) |
| Pouch-cutaneous fistula | .001 | .014 | 3.01 (1.24–7.28) |
| [0,1–4] | |||
| [0,1–4]Complications | |||
| Immediate | .12 | .057 | 1.91 (0.98–3.74) |
| Late-onset | .001 | .002 | 10.79 (2.36–49.37) |
HR: hazard ratio.
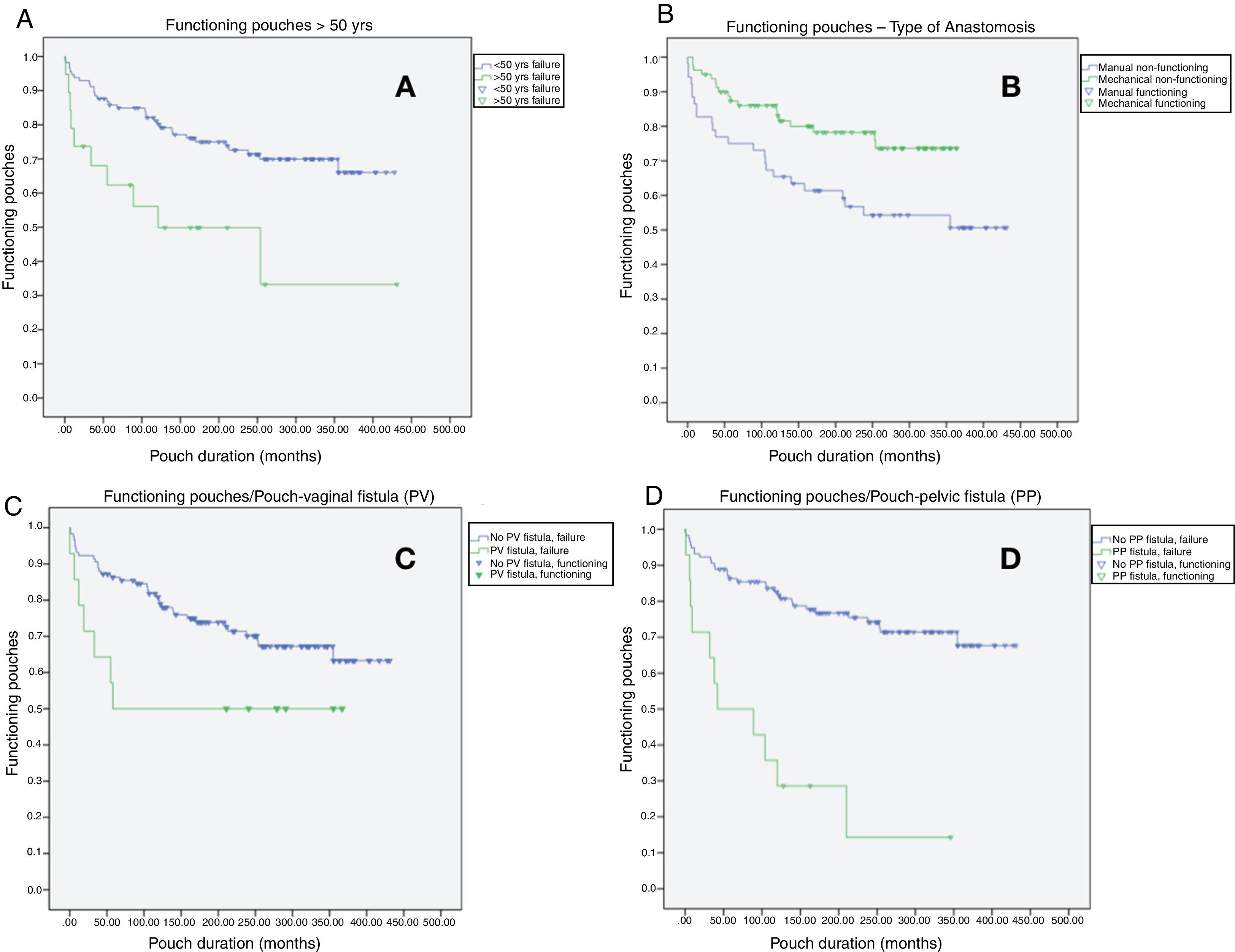
Risk factors and their impact on pouch failure: A) functioning pouches in patients over the age of 50 (P < .01; HR: 5.55 [2.32–13.2]); B) functioning pouches according to the type of anastomosis (P < .01; HR: 3.78 [2.09–6.84]); C) functioning pouches in patients with pouch-vaginal fistula (P < .02; HR: 2.86 [1.25–6.12]); D) functioning pouches in patients with pouch-pelvic fistula (P < .01; HR: 5.17 [1.96–13.6]).
The 2 longest series published in our country (the Hospital de Bellvitge study8 published in 2002 and this present study) do not exceed 150 patients, the vast majority of which were treated in the 1990s.4 Many of the patients treated at our hospital had come from other autonomous communities, which in our current context is unthinkable. There are not many hospitals in Spain that perform more than 10 procedures of this type per year.
Therefore, the experience of Spanish hospitals in constructing ileoanal pouches is not comparable to any reference medical center in other European countries, where there is greater centralization of these processes.9–12
In a historical series such as this, we should be reminded that the passage of time affects the learning curve of surgeons.9,13 Likewise, results are also influenced by the improved technical quality of the material used and improvements in perioperative patient management, which have reduced the annual pouch failure rate to 8%.9
More than two-thirds of the patients (77%) were treated in 2 or 3 operations. Large series like the Tulchinsky et al. study reported pouch failure being reduced by half (from 16% to 8%) using protective ileostomy.9 The protection of the pouch with an ileostomy can reduce septic complications associated with corticosteroid therapy, anti-TNF or vedolizumab.14,15 Restorative proctocolectomy has a significant and severe morbidity, which in our case has led to the removal or ‘defunctionalization’ of approximately 30% of the pouches created over these 35 years. However, perioperative mortality is low11,16,17 at less than 1%, which in our series was one patient (1%) death associated with the main procedure.
In our study, we found 12% of failures in the first year, which increased to 30% during follow-up. This contrasts with more extensive series that have published a pouch failure rate of less than 30%.9,11,17 Tekkis et al.16 reported a pouch failure rate that increased progressively for 10 years, but with little increase observed in subsequent years. Their functional results were maintained, which contrasts with the results from our study, where almost 10% of failures were found after 10 years of follow-up, stabilizing in the second decade.
As seen in the multivariate analysis, pouch failures are mainly due to the appearance of septic complications in patients over 50 years of age and after performing manual anastomosis. In our series, 26 pouch failures (62%) occurred at an average of 117 months after the pouch was created. As Tulchinsky et al.9 explain, pelvic sepsis is an independent factor associated with pouch failure, which in our case was present in 16 patients (12%) (including early and late-onset pelvic sepsis); meanwhile, chronic anastomotic sinus may be the main cause of early pouch failure 40% of the time.18 Certain series, such as those by MacRae et al.19 and Fazio et al.,20 report sepsis rates associated with the pouch that are close to 40% during a follow-up of less than 5 years. In addition, Maya et al.21 report that perineal wound dehiscence is also an important cause of pouch failure. In recent years, we have seen how these complications do not necessarily lead to pouch excision,22 as surgical options have improved for saving the pouch after septic complications.23,24
The change in the diagnosis of Crohn’s disease during follow-up is one of the main causes of pouch failure.10,25 In our series, in only 7 patients (5%) was the diagnosis changed to Crohn’s disease during follow-up. This change in diagnosis should be suspected in cases of treatment-resistant pouchitis, involvement of loops proximal to the pouch or transmural inflammatory involvement, although characteristic granulomas are rarely going to be visualized.
In our series, we found that age over 50 when the pouch was created was an independent risk factor for its failure. To date, no age limit has been established for pouch procedures, even though age is a known risk factor for its failure.9,17 Furthermore, manual pouch creation was also associated with failure in our series (HR: 3.78). This is in line with other publications, such as the Helavirta et al. study, which included more than 280 patients with manual anastomosis, observing worse results for pouch duration and functional results, with a much higher stenosis rate than mechanical anastomosis.17 Our series included 38% manual anastomoses with associated mucosectomy, most of which were performed during the first years and rarely done in recent years. It is necessary to remember that the most numerous series include patients treated since the early 1980s, while circular staplers were not available until 1988.
Separate mention should be given to the 9.7% of the patients in our series who had pouch-vaginal fistulae. Although certain series describe this complication in up to 16% of patients,26 in the last 15 years we have not observed any cases. It is a devastating complication, and in 7 of the 13 patients affected it was necessary to remove the pouch. There is no gold standard treatment, as size, location and clinical symptoms must be considered.27 In our series the presence of pouch-vaginal fistula has been associated with pouch failure, as also shown in other recently published studies.28 According to Mallick et al.29 in one of the longest series published about this problem with more than 150 patients, the presence of pouch-vaginal fistula occurs in 40% of cases more than 12 months after the completion of the pouch. In our study, out of the 13 women with pouch-vaginal fistula, 2 patients previously had a fistula to the vagina that recurred after the intervention, and 10 of the 13 were re-operated. The techniques performed were very variable, mainly including transvaginal or transperineal flaps, a Martius flap in some cases, an unsuccessful Gracilis muscle interposition in one case, and in higher fistulae transabdominal approaches with repair and interposition of the omentum, redoing the pouch on one occasion. However, in 7 of the 13 patients the fistula recurred, so terminal ileostomy was performed. Therefore, it is essential to avoid including the vagina in the staple line, as well as to avoid coagulating or necrotizing over the vagina with an electric scalpel or another energy source by using vaginal separators in the dissection.
The lower rate of cuffitis in our series may be due to the fact that many of the patients underwent mucosectomy. The lower pouchitis rate may be because we have considered their clinical, endoscopic and pathological diagnoses.
For all this, we believe that restorative proctocolectomy should be a procedure that is centralized at highly specialized hospitals with multidisciplinary groups for inflammatory bowel disease management, where these patients can be offered possible solutions to the varied and abundant short- and mid-term complications that may appear. However, our study presents limitations and biases associated with the retrospective nature and the long period of time over which the patients have been analyzed, which may consequently affect the analysis of the data and drawing of conclusions.
In conclusion, the creation of an ileoanal pouch in ulcerative colitis is a surgery that requires great intraoperative technical effort. Multiple short- and long-term complications are possible, which can lead to pouch failure. This is a historical series analyzed in its entirety, with the special characteristics that this entails and the biases associated with retrospective analysis. However, it is one of the largest restorative proctocolectomy series published in our country. The improvements in the technique and in peroperative management have led to better results for pouch construction with a decrease in complications over the last decade. Nevertheless, many complications are due to the difficulty in reaching a correct preoperative diagnosis or problems in managing the disease itself that have not yet been resolved.
Therefore, we recommend that these patients be managed at hospitals with a sufficient volume of this disease and by multidisciplinary teams that are experienced in the management of these patients.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Die J, Ocaña J, Abadía P, García JC, Moreno I, Pina JD, et al. Experiencia, complicaciones y factores pronósticos con el reservorio anal en la colitis ulcerosa. Estudio observacional. Cir Esp. 2020;98:64–71.






![Risk factors and their impact on pouch failure: A) functioning pouches in patients over the age of 50 (P < .01; HR: 5.55 [2.32–13.2]); B) functioning pouches according to the type of anastomosis (P < .01; HR: 3.78 [2.09–6.84]); C) functioning pouches in patients with pouch-vaginal fistula (P < .02; HR: 2.86 [1.25–6.12]); D) functioning pouches in patients with pouch-pelvic fistula (P < .01; HR: 5.17 [1.96–13.6]). Risk factors and their impact on pouch failure: A) functioning pouches in patients over the age of 50 (P < .01; HR: 5.55 [2.32–13.2]); B) functioning pouches according to the type of anastomosis (P < .01; HR: 3.78 [2.09–6.84]); C) functioning pouches in patients with pouch-vaginal fistula (P < .02; HR: 2.86 [1.25–6.12]); D) functioning pouches in patients with pouch-pelvic fistula (P < .01; HR: 5.17 [1.96–13.6]).](https://static.elsevier.es/multimedia/21735077/0000009800000002/v1_202002210737/S2173507720300193/v1_202002210737/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
