Bariatric surgery is considered the most effective treatment for severe obesity. However, it is not clear if patients with diabetes mellitus or insulin resistance have the same response than patients without those conditions. Our objective was to evaluate association between pre-surgical HOMA-IR index and percentage of excess weight loss (EWL%) one year after bariatric surgery using sleeve gastrectomy.
MethodsRetrospective cohort including patients ≥18 years old with BMI ≥ 35 Kg/m2, who underwent primary sleeve gastrectomy between 2014-2017 at the Avendaño Medical Center, Peru. Only patients with Type 2 Diabetes, Hypertension, or Dyslipidemia were included. EWL% ≥60% one year after surgery was considered satisfactory. Crude and adjusted Lineal and Poisson regression with robustness was used to assess statistical associations with EWL%.
ResultsNinety-one patients were included with a median of 34 years, and 57.1% were women. 85.7% had insulin resistance as per HOMA-IR. One year after surgery, 76.9% had a satisfactory EWL%. The lineal model showed 0.29% less EWL% per each extra year of life (P = .019), and 0.93% more EWL% per each extra HOMA-IR point (P = .004). The adjusted Poisson model showed 2% lower risk of having a satisfactory EWL% per each additional year of life (P = .050), and 2% more chance of success per each additional HOMA-IR point (P = .038).
ConclusionsThere was association between a higher pre-surgical HOMA-IR index and increased EWL% one year after surgery. It is possible that insulin resistance does not affect negatively sleeve gastrectomy outcomes.
La cirugía bariátrica es considerada la herramienta más efectiva para el tratamiento de obesidad severa. No es claro si los pacientes con resistencia a la insulina responden igual que otras personas. Nuestro objetivo es evaluar asociación entre el índice HOMA-IR pre-quirúrgico con el porcentaje de sobrepeso perdido (%PSP) un año post-cirugía bariátrica usando la técnica gastrectomía vertical (GV).
MétodosCohorte retrospectiva incluyendo pacientes ≥18 años con IMC ≥ 35 Kg/m2 intervenidos por primera vez con GV entre 2014–2017 en la Clínica Avendaño, Perú. Se incluyó solo aquellos con diabetes mellitus tipo 2, hipertensión arterial o dislipidemia. Un %PSP al año ≥60% fue considerado satisfactorio. Se usó regresión Lineal y de Poisson con varianza robusta en forma cruda y ajustada para evaluar asociaciones con %PSP.
ResultadosLa muestra de 91 pacientes tuvo una mediana de 34 años y 57,1% fueron mujeres. 85,7% presentaron resistencia a la insulina según HOMA-IR. Al año post cirugía, 76,9% tuvo un %PSP satisfactorio. En el modelo lineal ajustado, por cada año de edad adicional hubo 0,29% menos %PSP (p = 0,019), y por cada punto extra del HOMA-IR hubo 0,93% más %PSP (p = 0,004). La regresión de Poisson ajustada mostró 2% menos éxito por cada año de edad adicional (p = 0,050) y 2% más éxito por cada punto adicional de HOMA-IR (p = 0,038).
ConclusionesSe encontró asociación entre mayor valor pre-quirúrgico de HOMA-IR con un mayor %PSP al año post-cirugía. Es posible que la resistencia a insulina no afecte en forma adversa los resultados de la cirugía bariátrica GV.
Obesity, defined as a body mass index (BMI) of 30 kg/m2 or more, is a serious global health problem.1 In 2016, more than 1.9 billion adults worldwide aged ≥18 years were overweight, 650 million of which were obese.2 The World Health Organization reports that global obesity rates have almost tripled since 1975.2 In Lima, Peru, it was reported that 11.4% of people between 20-29 years of age, and 23.5% of adults between 30-59 years of age, were obese in 2010.3 Conventional treatment is usually diet and exercise.4 However, in 1991, a consensus panel from the National Institutes of Health indicated that bariatric surgery is appropriate for all patients with a BMI of ≥ 40 kg/m2, or a BMI of 35-40 kg/m2 with associated comorbidities.5 Yermilov et al. reported that even in patients with BMI < 40 kg/m2, the results of medical therapy are poor and difficult to maintain in the long term, while surgery achieves good results with a significant decrease in morbidity and mortality and few adverse events.6 Likewise, Livingston mentions that, in clinical practice, obesity centers increasingly indicate surgical treatment for patients with BMI between 30 and 35 kg/m2 when they have metabolic comorbidities such as diabetes mellitus and severe dyslipidemia.7
There are restrictive, malabsorptive and mixed bariatric techniques. The restrictive methods involve reducing the capacity of the stomach, thereby restricting the passage of food. Malabsorptive techniques bypass a portion of the small intestine so that there is poor food absorption. Mixed types are a combination of both techniques mentioned.8 The most common surgery performed in Peruvian bariatric centers is sleeve gastrectomy (SG), which is a restrictive technique that is generally performed laparoscopically.4 As an exception, the duodenal switch may also be performed. This mixed technique consists of several surgical steps and involves performing a SG plus a Roux-en-Y bypass to the duodenum, with variable loop lengths.9 SG is a type of subtotal gastrectomy, where around 80% of the stomach is removed, without requiring the creation of anastomoses of any kind.5 The patient is placed in the supine position, and the surgeon is positioned between the patient’s legs or on the right side. Pneumoperitoneum is created, and 4-5 trocars are placed in the superior hemiabdomen. Previously, a gastric tube is inserted to define the size of the residual gastric cavity, with a gauge between 32-34 Fr. The short vessels are divided from an area close to the pylorus until completing the angle of His. The current trend is to initiate the gastrectomy closer to the pylorus, starting the dissection 3 cm away, it in order to further enhance the restrictive component of the procedure.10 Regardless of the diameter of the tube, the final diameter of the stomach depends on several intraoperative technical factors. Larrad mentions that indicators for success include achieving excess weight loss of more than 50% after 5 years of follow-up, maintaining a good quality of life (without repeated vomiting or permanent diarrhea), and minimal side effects on organs or systems.11
Bariatric surgery is not only aimed at losing excess weight, but it can also contribute to the management of other metabolic problems. Schauer et al. showed that bariatric surgery plus intensive medical therapy was effective in reducing, or in some cases resolving, hyperglycemia.12 It has been found that metabolic status not only improves with surgery, but it can also affect its results. Dixon et al. found that a lower rate of excess weight loss in the 12 months after bariatric surgery was associated with older age, higher initial BMI and high fasting plasma insulin levels.13 In this way, it is important to determine the metabolic state of patients before bariatric surgery. One way is the evaluation of the homeostatic model assessment for insulin resistance (HOMA-IR), developed by Matthews et al. and widely used in research.14,15 For these reasons, the objective of the present study is to demonstrate whether there is an association between the preoperative HOMA-IR index with the percentage of weight loss one year after the sleeve gastrectomy (SG) bariatric surgery in patients with BMI ≥ 35 kg/m2 who also have metabolic alterations.
MethodsA retrospective cohort study was conducted based on the review of medical records. The population consisted of patients ≥ 18 years of age with a BMI ≥ 35 kg/m2, who had undergone bariatric surgery using the SG technique for the first time from January 2014 to June 2017 at the Avendaño Day Clinic in Lima, Peru. We only included patients with type 2 diabetes mellitus (T2DM), hypertension or dyslipidemia who had preoperative glucose and insulin studies as well as a postoperative follow-up of their BMI for 12 months. We excluded patients who had been reoperated with any other bariatric technique. The sample size was calculated based on the study by Dixon et al., who found a negative correlation (r – 0.36, P < .01) between preoperative fasting insulin with the percentage of excess weight loss one year after bariatric surgery.13 Considering these values with an alpha of 0.05, a power of 90% and a 20% loss of participants due to incomplete data, a minimum sample size of 96 patients was obtained.
The outcome variable of the study was the percentage of excess weight loss (%EWL) one year after surgery, calculated as 100 × (initial weight – current weight)/(initial weight – ideal weight).11 The ideal weight was defined as 50 kg + ([height in centimeters – 150] × 0.921) for men, and 45.5 kg + ([height in centimeters – 150] × 0.921) for women.16 Likewise, the %EWL variable was dichotomized using the cut-off point suggested by Ortega et al., who considered a loss ≥ 60% as satisfactory.17 The weight variation was also evaluated using the percentage of total weight loss one year after surgery, calculated as 100 × (initial weight – current weight)/(initial weight).18,19
The main independent variable was the value of the HOMA-IR index before surgery, calculated as the fasting insulin level (mU/L) × fasting blood glucose (nmoL/L)/22.5.14 The diagnosis of insulin resistance was established in patients with a HOMA-IR index ≥ 2.6.20 Preoperative patient characteristics were included as adjustment variables: age, sex, weight, height, BMI, type of obesity (class II: 35-39.9 kg/m2, class III: ≥ 40 kg/m2), ideal weight, systolic and diastolic pressure and diagnosis of T2DM. Hypertensive patients were those with a systolic pressure ≥ 130 mmHg or a diastolic pressure ≥ 80 mmHg when they did not report this diagnosis.21 Dyslipidemia was defined by total cholesterol values ≥ 200 mg/dL, LDL cholesterol ≥ 130 mg/dL, HDL cholesterol ≤ 40 mg/dL in men and ≤ 45 mg/dL in women,22 and/or triglycerides ≥ 150 mg/dL.23 All patients were evaluated by a multidisciplinary bariatric team before surgery, obtaining a lipid profile, blood glucose and insulin levels. The patients were given a standard program for diet and physical activity after surgery, and they were checked monthly.
This study follows the principles of the Helsinki declaration and has been approved by the Ethics Committee of the Health Sciences College of the Peruvian University of Applied Sciences. First, we requested all the records of the patients who underwent SG during the study period. Only those who met the selection criteria were included. The information was double digitized in a database and exported to Stata 14.0 format (College Station, TX). The categorical variables were described using absolute frequencies and percentages. Numerical variables were described using median and interquartile range (IQR) when distribution was not normal. The outcome variable %EWL was compared dichotomously (≥ 60% and < 60%) with the rest of the variables using the Chi squared test when they were categorical, or the Mann-Whitney U test if they were numerical. P values ≤.05 were considered statistically significant. Linear regression analyses were then performed to evaluate the association between %EWL (in numerical form) and the study variables, reporting beta coefficients. Robust variances were used in anticipation of lack of normality. Poisson generalized linear models were also constructed with robust variances to evaluate the association with %EWL dichotomously, obtaining relative risks. Regression analyses were crude and then multivariate, including sex, age, initial BMI, presence of T2DM, preoperative HOMA-IR, and any other variable that had statistical significance ≤.20 in the crude analysis. These additional variables were removed from the final models in case their adjusted P values were >.05.
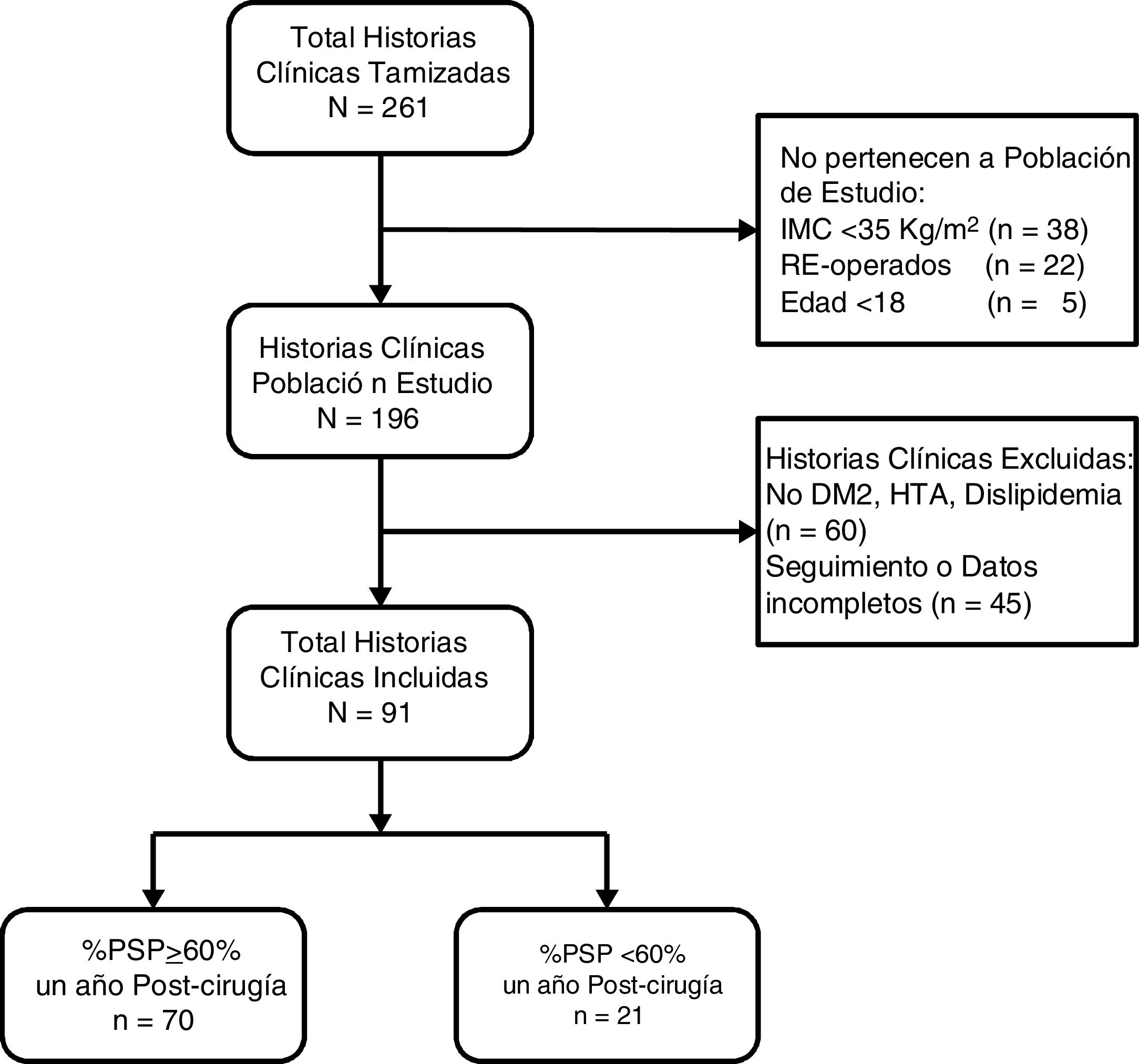
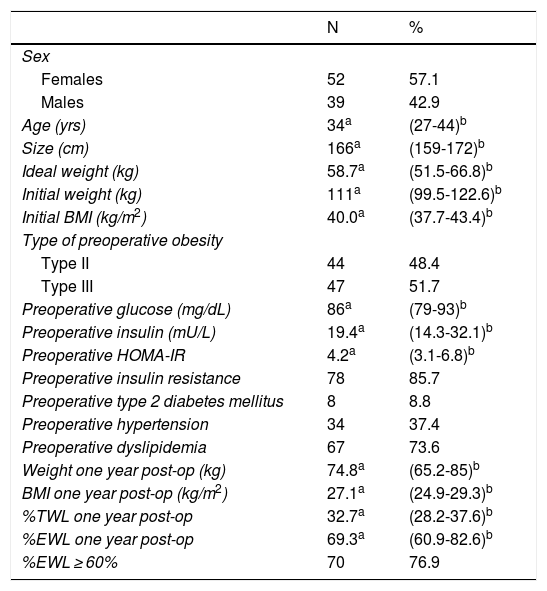
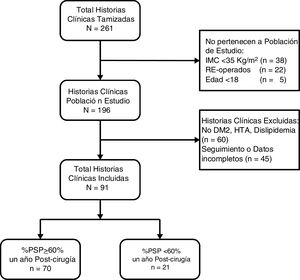
ResultsA total of 261 medical records were reviewed, 196 of which were of patients who underwent surgery for the first time with BMI ≥ 35 kg/m2. We excluded 60 patients because they did not present comorbidities and 45 because they did not have follow-up or complete data (Fig. 1). The remaining 91 patients had a median age of 34 years (IQR: 27-44), and 52 subjects were women (57.14%). Before surgery, the patients had a median BMI of 40.0 kg/m2 (IQR: 37.7-43.4) and 51.65% had type III obesity. Furthermore, 8.79% had T2DM, 37.36% HTN and 73.63% dyslipidemia. Only 3 patients (3.3%) had all 3 comorbidities. The preoperative HOMA-IR had a median of 4.2 (IQR: 3.1-6.8), and 85.71% had insulin resistance. One year after surgery, the median BMI was 27.1 kg/m2 (IQR: 24.9-29.3), the median percentage of total weight loss was 32.7 (IQR: 28.2-37.6), the median %EWL was 69.3 (IQR: 60.9-82.6) and the proportion of patients with a %EWL ≥ 60% was 76.9% (Table 1).
Characteristics of Patients With BMI ≥ 35 kg/m2 Treated With Sleeve Gastrectomy (n = 91)
| N | % | |
|---|---|---|
| Sex | ||
| Females | 52 | 57.1 |
| Males | 39 | 42.9 |
| Age (yrs) | 34a | (27-44)b |
| Size (cm) | 166a | (159-172)b |
| Ideal weight (kg) | 58.7a | (51.5-66.8)b |
| Initial weight (kg) | 111a | (99.5-122.6)b |
| Initial BMI (kg/m2) | 40.0a | (37.7-43.4)b |
| Type of preoperative obesity | ||
| Type II | 44 | 48.4 |
| Type III | 47 | 51.7 |
| Preoperative glucose (mg/dL) | 86a | (79-93)b |
| Preoperative insulin (mU/L) | 19.4a | (14.3-32.1)b |
| Preoperative HOMA-IR | 4.2a | (3.1-6.8)b |
| Preoperative insulin resistance | 78 | 85.7 |
| Preoperative type 2 diabetes mellitus | 8 | 8.8 |
| Preoperative hypertension | 34 | 37.4 |
| Preoperative dyslipidemia | 67 | 73.6 |
| Weight one year post-op (kg) | 74.8a | (65.2-85)b |
| BMI one year post-op (kg/m2) | 27.1a | (24.9-29.3)b |
| %TWL one year post-op | 32.7a | (28.2-37.6)b |
| %EWL one year post-op | 69.3a | (60.9-82.6)b |
| %EWL ≥ 60% | 70 | 76.9 |
%EWL: percentage of excess weight loss; %TWL: percentage of total weight loss
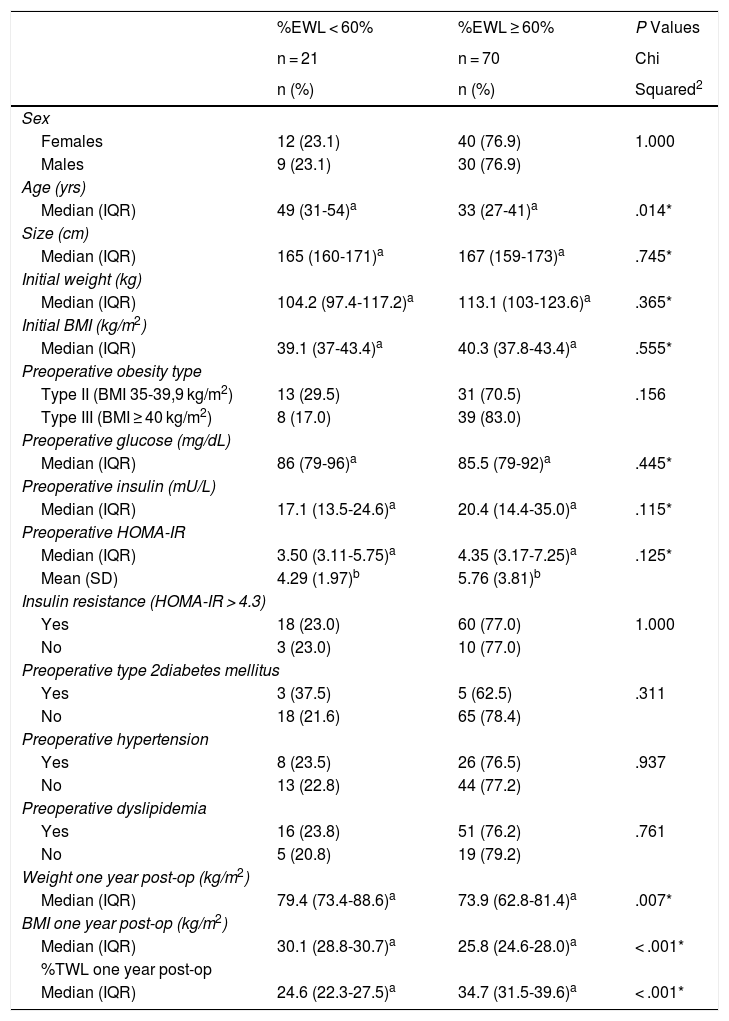
No differences in sex, initial weight, initial BMI, ideal weight or obesity type were found between people who lost < 60% of excess weight and those who lost ≥ 60%. There were also no differences in preoperative glycemia, T2DM, hypertension, or dyslipidemia. We did observe that the patients who lost ≥ 60% of excess weight were younger (median age 33 vs. 49 years), which was a significant difference (P = .014). Patients who lost ≥ 60% excess weight had a higher median of preoperative HOMA-IR (4.35 vs. 3.50), as well as preoperative insulin (20.4 vs. 17.1), although the differences were not significant (Table 2). These differences were maintained for the subgroup of diabetic patients, both for preoperative HOMA-IR (7.89 vs. 3.37) and for preoperative insulin (23.3 vs. 14.5). The presence of a higher preoperative HOMA-IR in patients who had a %EWL ≥ 60% was maintained independently in patients with BMI < 40 kg/m2 and in those with BMI ≥ 40 kg/m2.
Characteristics of Patients With BMI ≥ 35 kg/m2 Who Have Undergone Bariatric Surgery With Sleeve Gastrectomy According to Percentage of Excess Weight Loss (%EWL) One Year After Surgery (n = 91)
| %EWL < 60% | %EWL ≥ 60% | P Values | |
|---|---|---|---|
| n = 21 | n = 70 | Chi | |
| n (%) | n (%) | Squared2 | |
| Sex | |||
| Females | 12 (23.1) | 40 (76.9) | 1.000 |
| Males | 9 (23.1) | 30 (76.9) | |
| Age (yrs) | |||
| Median (IQR) | 49 (31-54)a | 33 (27-41)a | .014* |
| Size (cm) | |||
| Median (IQR) | 165 (160-171)a | 167 (159-173)a | .745* |
| Initial weight (kg) | |||
| Median (IQR) | 104.2 (97.4-117.2)a | 113.1 (103-123.6)a | .365* |
| Initial BMI (kg/m2) | |||
| Median (IQR) | 39.1 (37-43.4)a | 40.3 (37.8-43.4)a | .555* |
| Preoperative obesity type | |||
| Type II (BMI 35-39,9 kg/m2) | 13 (29.5) | 31 (70.5) | .156 |
| Type III (BMI ≥ 40 kg/m2) | 8 (17.0) | 39 (83.0) | |
| Preoperative glucose (mg/dL) | |||
| Median (IQR) | 86 (79-96)a | 85.5 (79-92)a | .445* |
| Preoperative insulin (mU/L) | |||
| Median (IQR) | 17.1 (13.5-24.6)a | 20.4 (14.4-35.0)a | .115* |
| Preoperative HOMA-IR | |||
| Median (IQR) | 3.50 (3.11-5.75)a | 4.35 (3.17-7.25)a | .125* |
| Mean (SD) | 4.29 (1.97)b | 5.76 (3.81)b | |
| Insulin resistance (HOMA-IR > 4.3) | |||
| Yes | 18 (23.0) | 60 (77.0) | 1.000 |
| No | 3 (23.0) | 10 (77.0) | |
| Preoperative type 2diabetes mellitus | |||
| Yes | 3 (37.5) | 5 (62.5) | .311 |
| No | 18 (21.6) | 65 (78.4) | |
| Preoperative hypertension | |||
| Yes | 8 (23.5) | 26 (76.5) | .937 |
| No | 13 (22.8) | 44 (77.2) | |
| Preoperative dyslipidemia | |||
| Yes | 16 (23.8) | 51 (76.2) | .761 |
| No | 5 (20.8) | 19 (79.2) | |
| Weight one year post-op (kg/m2) | |||
| Median (IQR) | 79.4 (73.4-88.6)a | 73.9 (62.8-81.4)a | .007* |
| BMI one year post-op (kg/m2) | |||
| Median (IQR) | 30.1 (28.8-30.7)a | 25.8 (24.6-28.0)a | < .001* |
| %TWL one year post-op | |||
| Median (IQR) | 24.6 (22.3-27.5)a | 34.7 (31.5-39.6)a | < .001* |
%EWL: percentage excess weight loss; %TWL: percentage total weight loss. *Mann-Whitney U.
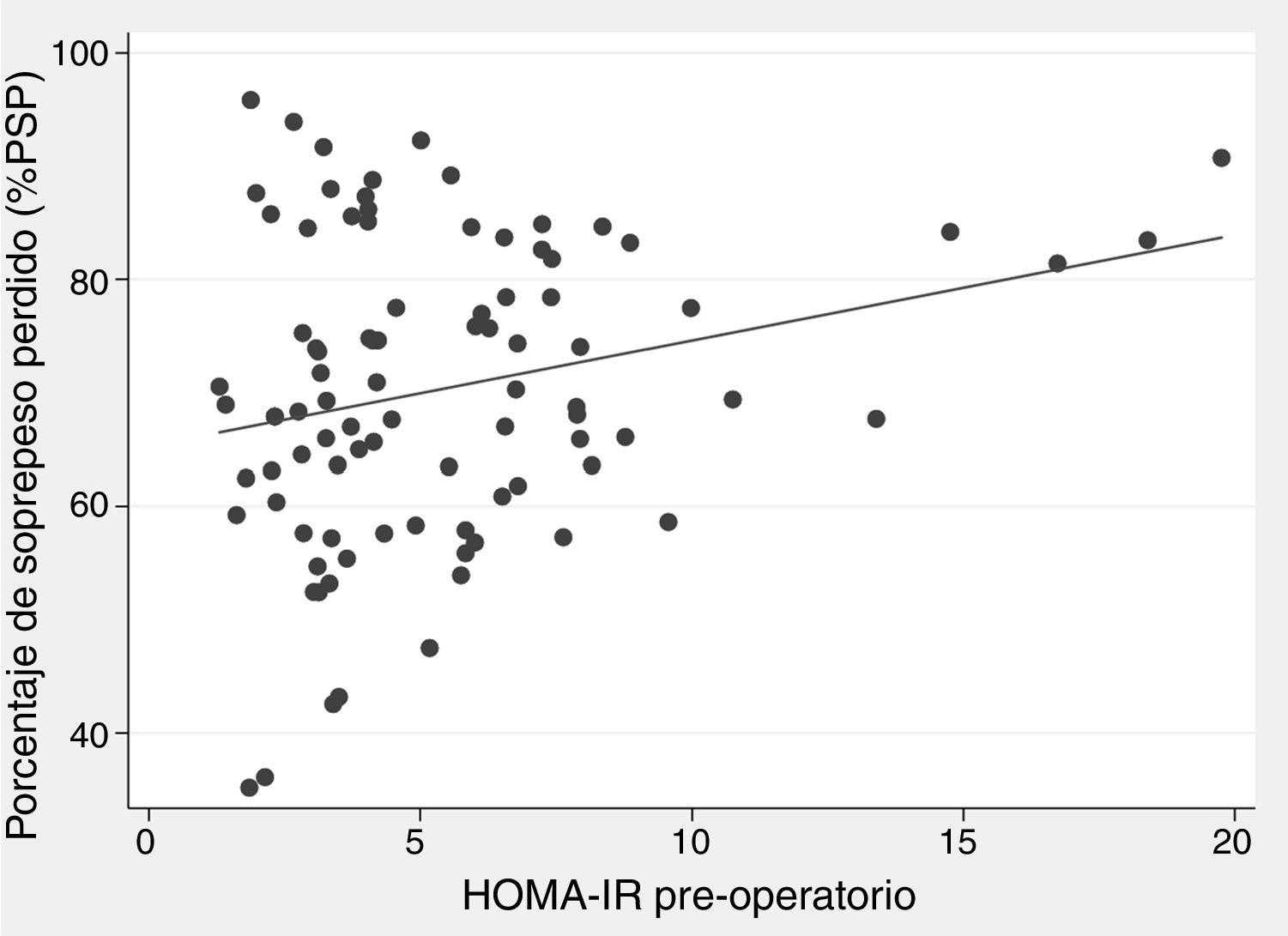
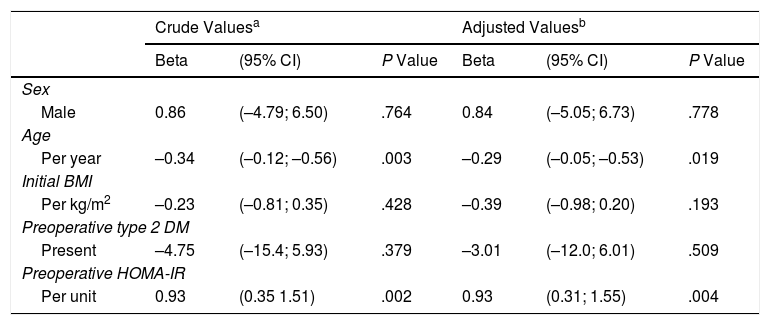
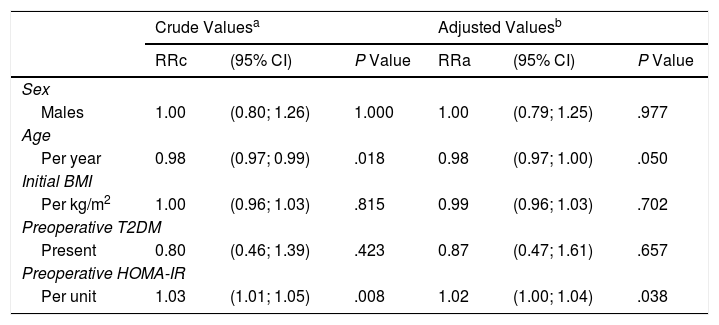
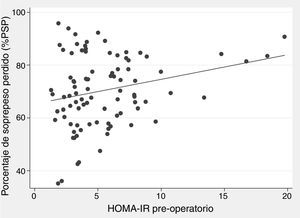
There was a positive correlation between preoperative HOMA-IR and %EWL one year after surgery, with a beta coefficient of 0.93 and P = .002 in the crude linear regression (Fig. 2), although the r was only 0.25. The initial multivariate models included sex, age, initial BMI, presence of T2DM, and preoperative HOMA-IR. Preoperative insulin was not included because it was collinear with the HOMA-IR. In the linear model adjusted for each additional year of age, there was 0.29% less excess weight loss per year (P = .019), and for each extra point of the HOMA-IR there was 0.93% more excess weight loss per year (P = .004) (Table 3). The binary model demonstrated that there is a 2% lower probability of obtaining a satisfactory %EWL after surgery for each additional year of age (P = .050), and a 2% greater probability for each additional point of preoperative HOMA-IR (P = .038) (Table 4).
Correlation Between Characteristics of Patients With BMI ≥ 35 kg/m2 Treated With Sleeve Gastrectomy and Percentage of Excess Weight Lost (%EWL) One Year After Surgery (n = 91)
| Crude Valuesa | Adjusted Valuesb | |||||
|---|---|---|---|---|---|---|
| Beta | (95% CI) | P Value | Beta | (95% CI) | P Value | |
| Sex | ||||||
| Male | 0.86 | (–4.79; 6.50) | .764 | 0.84 | (–5.05; 6.73) | .778 |
| Age | ||||||
| Per year | –0.34 | (–0.12; –0.56) | .003 | –0.29 | (–0.05; –0.53) | .019 |
| Initial BMI | ||||||
| Per kg/m2 | –0.23 | (–0.81; 0.35) | .428 | –0.39 | (–0.98; 0.20) | .193 |
| Preoperative type 2 DM | ||||||
| Present | –4.75 | (–15.4; 5.93) | .379 | –3.01 | (–12.0; 6.01) | .509 |
| Preoperative HOMA-IR | ||||||
| Per unit | 0.93 | (0.35 1.51) | .002 | 0.93 | (0.31; 1.55) | .004 |
beta: beta (β) regression coefficient; T2DM: type 2 diabetes mellitus; 95% CI: 95% confidence interval.
Probability of Losing ≥ 60% of the Percentage of Excess Weight Loss Within One Year of Surgery According to the Characteristics of Patients With BMI ≥ 35 kg/m2 Who Underwent Sleeve Gastrectomy (n = 91)
| Crude Valuesa | Adjusted Valuesb | |||||
|---|---|---|---|---|---|---|
| RRc | (95% CI) | P Value | RRa | (95% CI) | P Value | |
| Sex | ||||||
| Males | 1.00 | (0.80; 1.26) | 1.000 | 1.00 | (0.79; 1.25) | .977 |
| Age | ||||||
| Per year | 0.98 | (0.97; 0.99) | .018 | 0.98 | (0.97; 1.00) | .050 |
| Initial BMI | ||||||
| Per kg/m2 | 1.00 | (0.96; 1.03) | .815 | 0.99 | (0.96; 1.03) | .702 |
| Preoperative T2DM | ||||||
| Present | 0.80 | (0.46; 1.39) | .423 | 0.87 | (0.47; 1.61) | .657 |
| Preoperative HOMA-IR | ||||||
| Per unit | 1.03 | (1.01; 1.05) | .008 | 1.02 | (1.00; 1.04) | .038 |
T2DM: type 2 diabetes mellitus; 95%CI: 95% confidence level; RRa: adjusted relative risk; RRc: crude relative risk.
Most authors define a satisfactory result as, a loss of at least 50% of preoperative excess weight.24 In contrast, our study defined a satisfactory result as, %EWL ≥ 60%, following the cut-off point used by Ortega et al.17 Using this stricter threshold, we found that 76.9% of patients reached this goal, which is a result similar to other studies.25,26 Zhu et al. found a %EWL of 77.8% in the SG group, 76.2% in the lap band group, evaluated12, months after surgery. Furthermore, they observed that a loss of more than 50% of excess weight was achieved by the ninth month27 while Van Dielen et al. observed a %EWL of 26% ± 4% within 53 ± 26, days after surgery.28
Our results showed that patients with a higher preoperative HOMA-IR value had a higher %EWL one year after surgery. In the linear model, for each extra point on the HOMA-IR there was 0.93% more %EWL one year after surgery. However, this finding is in contrast with the reports published on the subject. Papaprieto et al.29 found no association between the preoperative presence of insulin resistance with the evolution of weight after surgery; Kruljac et al.30 also found no association between preoperative HOMA-IR values with weight loss 6 or 12 months after surgery. On the other hand, Dixon et al. found an inverse relationship, with HOMA-IR being a predictor of lower %EWL one year after bariatric surgery.13 Likewise, Faria et al.31 found that patients with low levels of preoperative insulin had a better response, with weight loss percentages ≥ 80%. The reasons for the individual differences in surgically-induced weight loss are not fully understood, and no studies were found that specifically analyzed the effects of insulin resistance. In general, patients with higher plasma glucose levels are thought to produce more insulin to maintain homeostasis. This hyperinsulinemic environment could generate resistance to weight loss, since insulin inhibits lipolysis and promotes lipogenesis.31 However, we found the opposite, so we feel that more studies focused on this topic are necessary.
A possible explanation would be that the present study only analyzed patients with comorbidities (T2DM, dyslipidemia, and high blood pressure), regardless of the type of obesity. Sanchez et al.32 found that some of these factors, such as T2DM, lead to poorer weight outcomes in patients who underwent bariatric surgery. Although none of these factors was associated with the loss of excess weight in our study, it is possible that, as it only contemplates patients with metabolic comorbidities, the relationship between HOMA-IR and %EWL is different. Another possibility is that the result of the surgery depends more on how quickly the hyperinsulinemic state of postoperative patients is corrected, instead of correlating with preoperative levels. Unfortunately, we do not have postoperative HOMA-IR measurements.
We also found that, at older age, there is less loss of excess weight after SG surgery, which is similar the Nagao et al. report.33 Additionally, these authors observed that patients with an age ≥ 65 were at higher risk of mortality and morbidity after bariatric surgery. Similarly, Faria et al.31 found that patients < 50 years of age had greater success after bariatric surgery. An explanation for this inverse relationship between age and excess weight loss would be the increase in metabolic deterioration associated with increasing age, which would produce less satisfactory results after surgery. In addition, there are other predictors of excess weight loss. Andersen et al.34 found that female sex and higher BMI were predictive of less loss of excess BMI. Faria et al.31 also found the same relationship between %EWL and weight or initial BMI. However, we did not find significant associations with these factors or with the type of obesity.
A limitation of the present study is that the sample size, despite being based on an adequate calculation, was only 91 patients. This could compromise the power of the study to consider non-significant associations (P>.05) as truly non-associated. Although an attempt was made to enlarge the sample as much as possible, there was a significant loss of cases due to incomplete records, a usual fact in retrospective studies. Another limitation is that, since it is not a multicenter study, our ability to extrapolate the results to other realities is reduced. In our country, bariatric surgeries are usually only performed in highly-specialized private hospitals, so we are only studying a fraction of the entire Peruvian population with obesity. Therefore, it would be necessary to corroborate our results with studies that include a greater number of patients and in different scenarios. It would also be important to include patients who have not yet developed comorbidities. Finally, studies measuring the HOMA-IR index before and after surgery would enrich our understanding.
On the other hand, this is one of the few studies that evaluates this subject, and certainly the first in a Latin American country. The fact that the association we have found differs from other reports in the literature favors the debate on how the patient’s metabolic situation affects outcomes after bariatric surgery. If our results are confirmed in the future, this could mean that the patient’s insulin status does not negatively affect the results of the surgery and, therefore, should not prevent the patient from achieving maximum effectiveness of the surgical procedure, in this case sleeve gastrectomy.
FundingThis study has received no specific funding from public, commercial or non-profit organizations.
Conflict of InterestsLST is a surgeon at the Avendaño Day Clinic and receives a salary. The remaining authors have no conflict of interests regarding this study.
We would like to thank Dr. Gustavo Salinas Sedó, Medical Director at the Avendaño Day Clinic, for allowing us to conduct this study.
Please cite this article as: Casas-Tapia C, Araujo-Castillo RV, Saavedra-Tafur L, Bert-Dulanto A, Piscoya A, Casas-Lucich A Índice HOMA-IR como predictor de reducción de exceso de peso en pacientes con índice de masa corporal (IMC) ≥ 35 kg/m2 sometidos a gastrectomía vertical. Cir Esp. 2020;98:328–335.