The use of perioperative chemotherapy (CT) in patients with advanced gastric carcinoma increases their overall survival. This therapy may also increase the number of patients with R0 resection. Potential drawbacks of this therapy, besides its toxicity, include increased surgical morbidity.
MethodsWe retrospectively evaluated the records of patients undergoing gastrectomy with curative intent, for carcinoma, at our institution between January 2009 and August 2018. They were divided into two groups: direct surgery (SURG) and perioperative CT (CHEMO). Patients with other neoadjuvant therapies and cardia Siewert I and II carcinomas were excluded.
The primary objective was to evaluate the impact of perioperative CT on surgical morbidity. As secondary objectives, resection radicality and total lymph node count were compared between the two groups.
ResultsA total of 307 patients (97 direct surgery and 210 perioperative CT) were evaluated. Median age was 67 years old.
The overall major surgical morbidity (Clavien-Dindo 3–5) was 10.6% in the CHEMO group and 12.4 in the SURG group (p=0.643).
There was no statistically significant difference between the surgical radicality (R0 98% in the SURG group vs 97.5% CHEMO group (p=0.865). There was an increase in the total number of lymph nodes retrieved in the specimen in the CHEMO group (25 vs 22, p=0.001), a difference that was not maintained in the subgroup analysis as a function of the surgery performed.
ConclusionsPerioperative CT in gastric carcinoma does not increase surgical morbidity, surgical radicality and total lymph node count.
El uso de quimioterapia perioperatoria (QT) en pacientes con carcinoma gástrico avanzado aumenta su supervivencia. Esta terapia también puede aumentar el número de pacientes con resección R0. Entre los posibles inconvenientes de esta terapia, además de su toxicidad, está una mayor morbilidad quirúrgica.
El objetivo principal fue evaluar el impacto de la QT perioperatoria en la morbilidad quirúrgica. Como objetivos secundarios, la radicalidad de la resección y el recuento total de ganglios linfáticos, que se compararon entre los dos grupos.
MétodosEvaluamos retrospectivamente los registros de pacientes sometidos a gastrectomía con intención curativa para carcinoma, en nuestra institución, entre enero de 2009 y agosto de 2018. Se dividieron en dos grupos: cirugía directa (SURG) y QT perioperatoria (CHEMO).
Se evaluó un total de 307 pacientes (97 SURG y 210 CHEMO). La mediana de edad fue de 67 años.
ResultadosLa morbilidad quirúrgica mayor (Clavien-Dindo 3-5) fue de 10,6% en el grupo CHEMO y de 12,4 en el grupo SURG (p = 0,643).
No hubo diferencias estadísticamente significativas entre el radical quirúrgico (R0 98% en el grupo de SURG vs. 97,5% del grupo CHEMO (p = 0,865). Hubo un aumento en el número total de ganglios linfáticos recuperados en la muestra en el grupo CHEMO (25 vs. 22, p = 0,001), una diferencia que no se mantuvo en el análisis de subgrupos en función de la cirugía realizada.
ConclusionesLa QT perioperatoria en el carcinoma gástrico no aumenta la morbilidad quirúrgica, la radicalidad quirúrgica y el recuento total de ganglios linfáticos.
Despite its declining incidence, gastric cancer continues to be one of the most common oncological cause of death, affecting approximately 800,000 people annually.1 Despite continuous effort gastric cancer prognosis is one of the poorest with overall 5 year survival between 20 and 25%.2–3 Around 60–70% of all gastric carcinomas are in an advanced stage at time of diagnosis. The curative treatment, when possible, includes multimodal therapy with surgery and perioperative therapies (chemotherapy, chemoradiotherapy, etc.).4–5 In Europe perioperative chemotherapy (CT) according to MAGIC/FLOT trials is the most common regimen used.6–8
Oncologic gastric surgery presents postoperative morbidity between 7.7% and 57.9% and a mortality of 0–13%. The most common post-operative complications include surgical site infection (SSI) and anastomotic leak.9–15
The use of perioperative chemotherapy in patients with advanced gastric carcinoma increases their overall survival. This therapy may increase the number of patients with R0 resection.7,15 Potential drawbacks of this therapy include increased surgical morbidity and associated toxicity.16–24
Objectives: The primary objective was to evaluate the impact of perioperative CT on surgical morbidity (Clavien-Dindo 3–5). As secondary objectives, resection radicality and total lymph node count were compared between the two groups.
MethodsOur hospital is an oncological reference centre that receives patients from the central and southern regions of Portugal, and the islands of Madeira and the Azores. All patients are discussed by dedicated multidisciplinary teams and decisions are made based on an institutional protocol that was written according to current guidelines, notably ESMO, NCCN and Japanese Gastric Cancer Association.6,25,26
Approximately 150 patients with gastric carcinoma are referred to our centre each year.
Inclusion criteria: We used a prospective database of patients undergoing gastrectomy with curative intent, for carcinoma, at our institution between January 2009 and August 2018. They were divided into two groups: direct surgery (SURG) and perioperative CT (CHEMO).
Exclusion criteria: patients with other neoadjuvant therapies, patients with previous gastric surgery, multivisceral resections and cardia Siewert I and II carcinomas were excluded. Due to the increase risk of morbidity, patients with more than 80 years old and patients presenting with haemorrhage or occlusion at diagnosis were also excluded.
All patient data were collected prospectively including patient demographics, tumour characteristics, length of and complications during post-operative stay according to the Clavien-Dindo classification.27
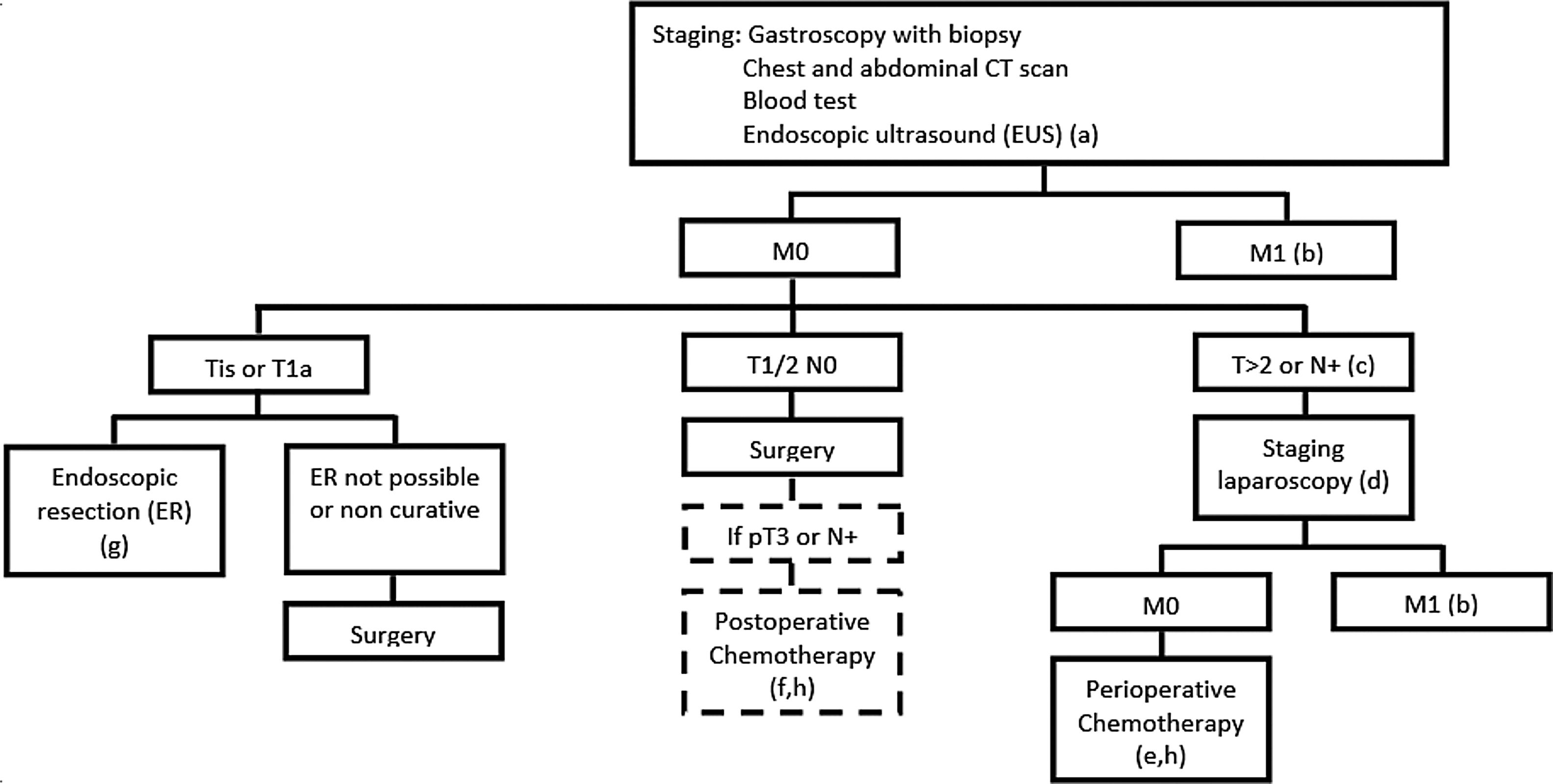
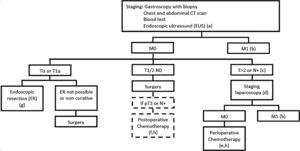
Staging protocol: patients routinely underwent upper GI endoscopy with biopsy, chest and abdominal CT scan. Endoscopic ultrasound (EUS) was used for early lesions (non-circumferential and N0 on CT scan) and diagnostic staging laparoscopy (SL) was performed in patients selected for perioperative chemotherapy.
Treatment protocol: Our institutional protocol is based on the 2018 ESMO and NCCN guidelines. Patients without metastatic disease are referred to endoscopic resection (cTis/T1a N0), direct surgery (cT1b-2N0/older than 80 years old/symptomatic) or perioperative chemotherapy (cT>2 or N+). Fig. 1 resumes our protocol.
IPOLFG gastric cancer protocol (a-T1/2N0; b-palliative treatment; c-patients with haemorrhage or occlusion are selected to surgery; d-if SL contraindicated patients are selected to preop CT; e-periop CT according to MAGIC/FLOT trials; f-post op CT according to CLASSIC trial; g-successful ER: T1aM2/3+no vascular invasion+moderate/well differentiated+en-bloc removal+free margins).
Perioperative chemotherapy: patients selected to chemotherapy received different regimens, mainly ECF (Epirubicin, Cisplatin, Fluorouracil), ECX (Epirubicin, Cisplatin, Capecitabine), EOX (Epirubicin, Oxaliplatin, Capecitabine) or FLOT (5FU, Folinic acid, Oxaliplatin, Docetaxel).
Surgical procedure: All receive a preoperative single-shot antibiotic prophylaxis. The procedures were started with a supraumbilical midline laparotomy. A complete D2 lymphadenectomy was performed in all patients according to the Japanese Gastric Cancer Association guidelines. Distal carcinomas were managed by a subtotal gastrectomy (STG) and proximal tumours by a total gastrectomy (TG), independently of their histology. Reconstruction of the gastrointestinal passage was performed according to the local standard using a long Roux-en-Y loop for total gastrectomy and a Billroth II gastrojejunostomy for subtotal gastrectomy. Esophagojejunostomy was performed with a 25mm circular stapler, and gastrojejunostomy was hand sewn, single layer with a monofilament (Monosyn® 3/0). All specimens were submitted to an extemporaneous examination by the pathologist, allowing for wider resections if the margins were positive.
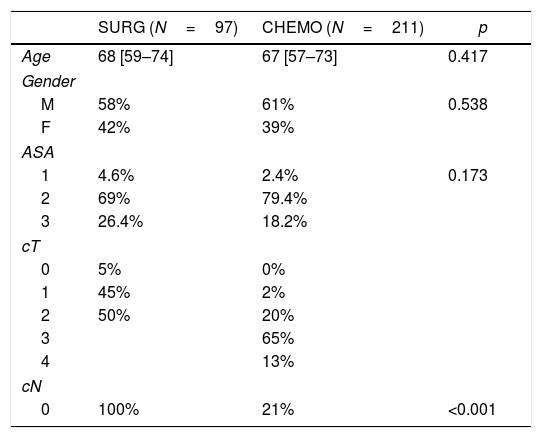
Patients and group demography: a total of 307 patients (97 direct surgery and 210 perioperative CT) were evaluated after exclusion of 133 patients (older than 80 years old and/or symptomatic at diagnosis). The median age was 68 in the direct surgery group vs 67 in the CT group (p=0.417). ASA score distribution was similar in both groups with most patients classified as ASA II (69% SURG vs 79.4% CHEMO; p=0.173). Patients demographics are summarized in Table 1.
As expected, tumour staging was higher in the CT group (p<0.001). Preoperative staging is summarized in Table 1.
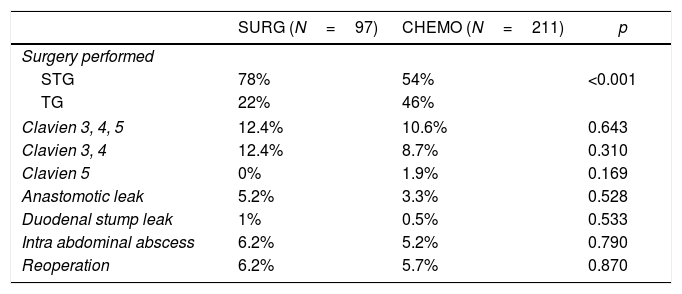
CHEMO patients had higher rate of total gastrectomies than the SURG group (46 vs 22%) (p<0.001) – Table 2. In the CHEMO group the median time from the fist day of the last chemotherapy cycle to surgery was 35 days.
Surgery performed and surgical morbidity.
| SURG (N=97) | CHEMO (N=211) | p | |
|---|---|---|---|
| Surgery performed | |||
| STG | 78% | 54% | <0.001 |
| TG | 22% | 46% | |
| Clavien 3, 4, 5 | 12.4% | 10.6% | 0.643 |
| Clavien 3, 4 | 12.4% | 8.7% | 0.310 |
| Clavien 5 | 0% | 1.9% | 0.169 |
| Anastomotic leak | 5.2% | 3.3% | 0.528 |
| Duodenal stump leak | 1% | 0.5% | 0.533 |
| Intra abdominal abscess | 6.2% | 5.2% | 0.790 |
| Reoperation | 6.2% | 5.7% | 0.870 |
Patients were evaluated as a whole group and considering the two groups: direct surgery (SURG) and perioperative CT (CHEMO). An exploratory analysis was carried out for all variables. Categorical data were presented as frequencies and percentages, and continuous variables as mean and standard deviation (SD), or median and inter-quartile range [25th percentile; 75th percentile], when deviations to normal distribution was found. Nonparametric chi-square test or the extension of the Fisher's exact test were used for qualitative variables and for continuous variables the Mann Whitney Wilcoxon test was applied.
To study the association between the total lymph node count and age the Spearman's linear correlation coefficient was estimated.
To analyze the association between the total lymph nodes and the several variables, Log- linear Poisson regression models were used. Confidence intervals (95% CI) for the change in incidence rates were also calculated. The level of significance α=0.05 was considered. All data were analyzed using SPSS 22.0 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp) and R software (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria, 2014).
ResultsThe overall major surgical morbidity (Clavien-Dindo 3–5) was 10.6% in the CHEMO group and 12.4 in the SURG group (p=0.643). Postoperative mortality was 1.9% in the CHEMO group and 0% on the SURG group (p=0.169). Table 2 details the complications registered.
There was no statistically significant difference between the median days of hospitalization (7 days in both groups, p=0.833).
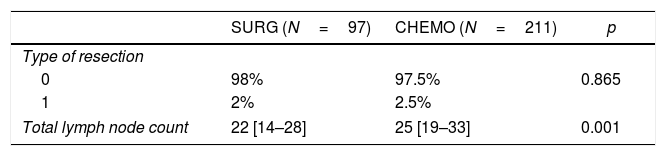
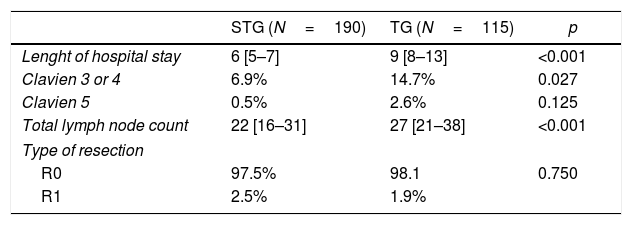
Complete resection (R0) achieved was 97.5% in the CHEMO group and 98% in the SURG group (p=0.865). There was an increase in the number of total lymph nodes (LN) retrieved favouring the CHEMO group (25 vs 22, p=0.001) (Table 3). This asymmetry was not maintained in the subgroup analysis as a function of the surgery performed (TG with a median of 27 LN and STG with a 22 median LN count, p<0.001).
DiscussionGastric carcinoma is diagnosed in most cases in advanced stages due to the lack of associated symptomatology and lack of screening (except for Asian countries, with high prevalence, that have routine endoscopy for the entire population).3,25,26
Treatment of these patients depends on the stage of the disease. In cases of locally advanced gastric carcinoma patients are selected for (neo)adjuvant therapies in order to improve overall survival.6,25,26
The MAGIC study is of the outmost importance as it established perioperative chemotherapy with ECX/ECF (3 cycles>surgery>3 cycles) as standard of care in locally advanced gastric cancers. This study demonstrated that patients who were submitted to this treatment had prolonged disease-free and overall survival versus those who did survey only.7
The administration of neoadjuvant therapies in the oncological setting, especially in gastric carcinoma, has several advantages such as: 1. administration of chemotherapy with an intact vascular system; 2. accurate in vivo evaluation of the tumour response; 3. treatment of micrometastases; 4. Optimal/maximal treatment dose (patient without postoperative sequelae); 5. Increase R0 surgery rates. All the above may potentially lead to an increase disease-free and overall survival.7,13,14
Despite the aforementioned advantages, neoadjuvant therapy has potential deleterious effects, both associated with the drugs toxicity and their effect on the patient's performance status, immune system and scarring capacity.
Studies with neoadjuvant therapies focus on the oncological results and the adverse effects/immediate toxicities of the treatments, meanwhile neglecting the potential increased surgical morbidity.16,23,24
In animal studies the use of chemotherapy showed a decreased ability to heal influencing the rate of anastomotic dehiscences.20–22 As a result we may have an increase in postoperative complications in patients undergoing preoperative therapies.
In our study we evaluated the patients treated at our institution, an oncological reference centre in Portugal, for gastric carcinoma with curative intent between January 2009 and August 2018. We decided to exclude patients with other neoadjuvant therapies, patients with previous gastric surgeries, multivisceral resections and cardia Siewert I and II carcinomas. Patients who were selected for direct surgery by age greater than 80 years old and those who presented, at diagnosis, in occlusion or haemorrhage were also excluded. This decision took into consideration the increased surgical morbidity risk of this population and the subsequent bias to the study.
We then compared two populations: patients who underwent perioperative CT (T3/4 and/or N+) vs patients selected for direct surgery (Tis/1/2 and N0). These two groups should not differ in terms of associated pathology. All patients were discussed at a multidisciplinary weekly meeting and were divided into the two study groups according to their staging.
Post gastrectomy morbidity are frequent and multifactorial (ranges between 7.7% and 57.9% and mortality between 0.0% up to 13.0%9–15). The type of surgery performed, the performance status, age and comorbidities are factors that increase the surgical risk. The most frequent complications following gastrectomy are surgical site infections, such as wound infections and intra-abdominal abscess, as well as a leakage of the esophagojejunostomy or duodenal stump.12-15
The major morbimortality measured by the Clavien-Dindo scale 3–5 was used as the primary endpoint, which was compared between the two groups.
We obtained a total of 97 patients in the surgery group (SURG) and 210 in the perioperative chemotherapy group (CHEMO). Median age was similar in both groups (67 vs. 68 years, p=0.417). Gender and ASA distribution also did not differ (p=0.538 and p=0.173 respectively) (Table 1).
As expected, the tumours in the CHEMO group were more advanced (Table 1). Also, in this group there was a higher percentage of proximal tumours, which led to an increase in total gastrectomies (46 vs 22%, p<0.001) (Table 2).
Regarding surgical morbidity (Clavien-Dindo 3–5), there were no differences between the groups (10.6% CHEMO group and 12.4% in the SURG group, p=0.643), and these results are superposable to the literature (Table 2).
There were 98% R0 resections, with no difference in the two groups (p=0.865) (Table 3). In the literature the percentage of R1 varies between 1.8 and 20%.28 In order to have a low R1 resection rate we believe it is fundamental, besides the experience of the surgical team, the systematic use of extemporaneous examination in all surgeries.
There was a difference in the total lymph node count favouring the CHEMO group (25 vs 22, p=0.001) (Table 3). After subgroup analysis, by surgery performed, that difference is not sustained (Table 4). The explanation resides in the fact that in the CHEMO group there was a higher percentage of total gastrectomies. We routinely use at our centre, D2 lymphadenectomy in all gastric cancer patients submitted to a curative intent gastrectomy. Patients with distal tumours, in which a subtotal gastrectomy is performed, D2 lymphadenectomy omits groups 2, 4sa, 10 and 11d26 leading to a decrease in the number of lymph nodes retrieved in the surgical specimen when compared to total gastrectomy (22 vs 27, p<0.001) (Table 4).
ConclusionsIn conclusion, according to this study, perioperative chemotherapy does not increase the major morbidity of gastrectomy (Clavien-Dindo 3–5). Also, it does not affect the rate of complete resection or the total lymph node count following gastrectomy when comparing to patients who are selected to direct surgery. Therefore, perioperative chemotherapy should be considered safe and feasible in terms of surgical outcomes.
Statement of ethicsAll procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
FundingNone.
Conflicts of interestNone declared.