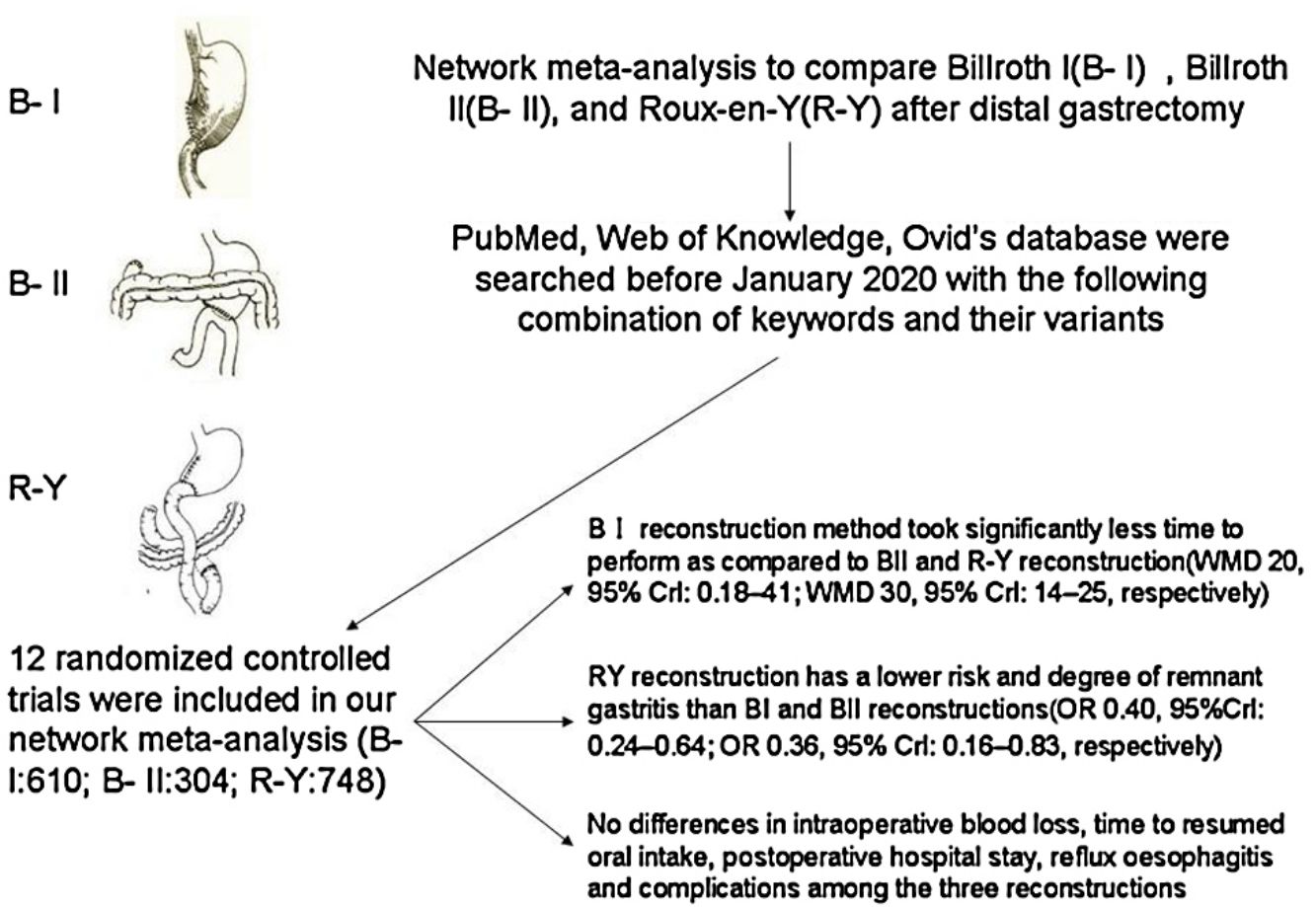
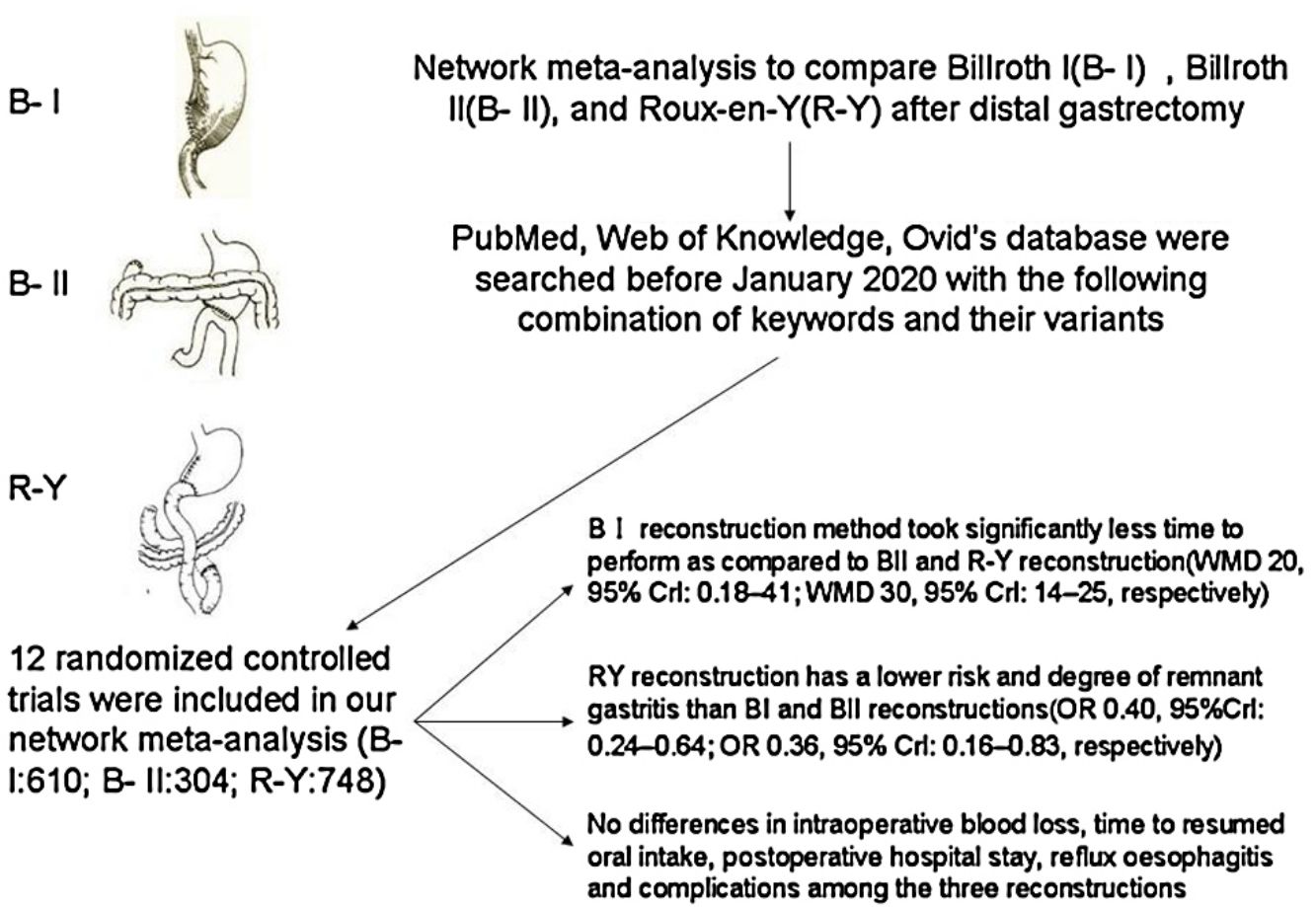
Major surgical treatment for distal gastric cancer include Billroth I (BI), Billroth II (BII), and Roux-en-Y (RY). Since the optimal reconstruction methods remains inconclusive, we aimed to compare these treatments in terms of intraoperative and postoperative course after distal gastrectomy with a systematic review and random-effects network meta-analysis. We searched PubMed, Web of Knowledge, Ovid's database for prospective, randomized, controlled trials comparing the outcomes of BI, BII, and RY reconstruction after distal gastrectomy until January 2020. From the included studies, operative time, intraoperative blood loss, postoperative hospital stay, endoscopic findings and complications were extracted as the short- and long-term outcomes of reconstructions. The network meta-analysis was performed with R 3.5.2 software as well as “gemtc” and “forestplot” packages. Twelve randomized controlled trials (RCTs) involving 1662 patients were included. RY reconstruction has a lower risk and degree of remnant gastritis than BI and BII reconstructions(OR 0.40, 95%Crl: 0.24–0.64; OR 0.36, 95% Crl: 0.16–0.83, respectively). BI reconstruction method took significantly less time to perform as compared to BII and RY reconstruction (WMD 20, 95% Crl: 0.18–41; WMD 30, 95% Crl: 14–25, respectively). No differences in intraoperative blood loss, time to resumed oral intake, postoperative hospital stay, reflux oesophagitis and complications among the three reconstructions. The RY reconstruction after distal gastrectomy was more effective in preventing remnant gastritis than Billroth I and Billroth II reconstruction, although RY reconstruction was considered as technical complexity.
El tratamiento quirúrgico principal para el cáncer gástrico distal incluye Billroth I (BI), Billroth II (BII) y Roux-en-Y (RY). Dado que los métodos de reconstrucción óptimos no son concluyentes, nuestro objetivo fue comparar estos tratamientos en términos de curso intraoperatorio y postoperatorio después de la gastrectomía distal con una revisión sistemática y un metaanálisis de red de efectos aleatorios. Se realizaron búsquedas en PubMed, Web of Knowledge, la base de datos de Ovid para ensayos prospectivos, aleatorizados y controlados que comparan los resultados de la reconstrucción de BI, BII y RY después de la gastrectomía distal hasta enero de 2020. De los estudios incluidos, tiempo operatorio, pérdida de sangre intraoperatoria, postoperatorio la estancia hospitalaria, los hallazgos endoscópicos y las complicaciones se extrajeron como resultados a corto y largo plazo de las reconstrucciones. El metaanálisis de red se realizó con el software R 3.5.2, así como con los paquetes «gemtc» y «forestplot». se incluyeron 12 ensayos controlados aleatorios (ECA) con 1.662 pacientes. La reconstrucción RY tiene un menor riesgo y grado de gastritis remanente que las reconstrucciones BI y BII (OR: 0,40; 95% Crl: 0,24-0,64; OR: 0,36; 95% Crl: 0,16-0,83, respectivamente). El método de reconstrucción BI tardó significativamente menos tiempo en realizarse en comparación con la reconstrucción BII y RY (WMD 20; 95% Crl: 0,18-41; WMD 30; 95% Crl: 14-25, respectivamente). No hay diferencias en la pérdida de sangre intraoperatoria, el tiempo para reanudar la ingesta oral, la estancia hospitalaria postoperatoria, la esofagitis por reflujo y las complicaciones entre las 3 reconstrucciones. La reconstrucción RY después de la gastrectomía distal fue más efectiva para prevenir la gastritis remanente que la reconstrucción BI y BII, aunque la reconstrucción RY se consideró de mayor complejidad técnica.
Gastric cancer is the fifth most common cancer and the third most common cause of death from cancer.1 It is responsible for over 1,000,000 new cases in 2018, with an estimated 783,000 deaths worldwide.1
Complete surgical resection is the main method of curative treatment. Billroth I (BI), Billroth II (BII), and Roux-en-Y (RY) are all acceptable options.2 The BI reconstruction has been commonly performed, because of its technical simplicity, with only one anastomotic site and maintaining physiological intestinal continuity.3,4 Billroth II reconstruction solves the problem of anastomotic tension, but it may increase the incidence of postoperative alkaline reflux gastritis, esophagitis, and anastomotic ulcers because of the changes in normal physiological pathways.5,6 RY reconstruction was chosen to prevent postoperative alkaline reflux gastritis and esophagitis of the remnant stomach after distal gastrectomy(DG).7,8 However, RY also has complications, such as Roux limb stasis, internal hernia, and intestinal obstruction.9 Thus, the choice of reconstruction after distal gastrectomy remains controversial.
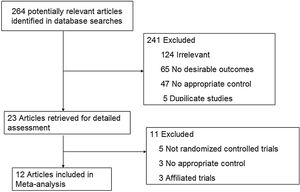
In our meta-analysis, we included trials that compared two or three reconstructions after distal gastrectomy. We excluded studies if they contained only one or none of the three strategies or did not use randomization for treatment allocation.
Materials and methodsSearch strategyPubMed, Web of Knowledge, Ovid's database were searched before January 2020 with the following combination of keywords and their variants: “Roux-en-Y”, “Billroth I”, “Billroth II”, “distal gastrectomy” and “randomized clinical trial”. The reference lists of relevant studies were checked manually to locate any missing studies.
Study selectionIdentified studies were assessed for eligibility for inclusion in the review by scrutinizing the titles, abstracts and keywords of every record retrieved. Studies were restricted to those published in English and Chinese. Clinical studies concerning comparisons of any aspects between the BI, BII and RY for DG were also included.
Data extractionTwo coauthors (LY and JH) independently selected studies for inclusion and exclusion and reached consensus when they did not agree in the initial assignment. The following variables were recorded: authors, journal and year of publication, number of patients, age, operation time, blood loss, postoperative hospital stay, time to resumed oral intake, reflux oesophagitis, remnant gastritis and total complications. If necessary, the corresponding authors of studies were contacted to obtain supplementary information.
Quality assessmentThe quality of the trials was assessed in the light of Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0.10 The scale consists of three items, randomization, blinding, and description of the withdrawals and dropouts. Studies with a score of 3–5 were considered to be of high quality.
In view of that the trials which did not cover the outcomes of completely randomized patients were regarded as suffering from bias because of incomplete outcome data and thus they were excluded from further analysis.
Statistical analysisThe network meta-analyses using the Bayesian Methods11 was performed in Stata 15 (Stata Corp), JAGS and R (version x64 3.5.2) with the gemtc package (version: 0.8–2) and rjags package (version: 4–6) with a random-effect model. The inconsistency of our results was confirmed by the node-splitting method and its Bayesian p value,12 comparing the direct and the indirect estimates for each comparison. p-value <0.05 indicates a significant inconsistency. For categorical data, treatment effects were expressed as odds ratios (ORs) and 95% confidence intervals (95%CIs). For continuous data, treatment effects were expressed as standardized mean differences (SMDs) and 95%CIs. Surface under the cumulative ranking curve (SUCRA) was used to calculate the hierarchy of treatments for each intervention. The SUCRA value ranges from 0 to 1, and a higher SUCRA value indicates a better efficacy.13
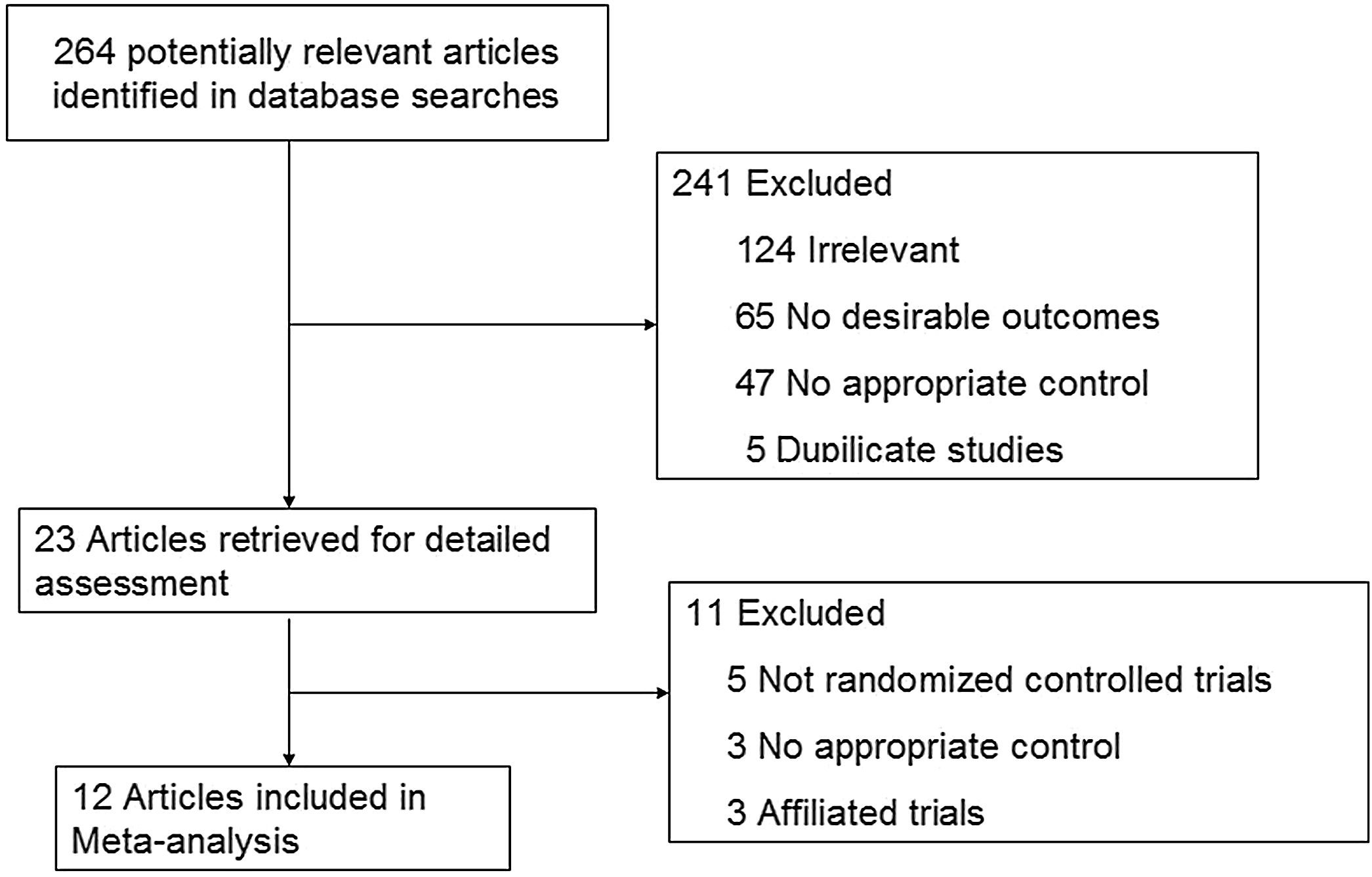
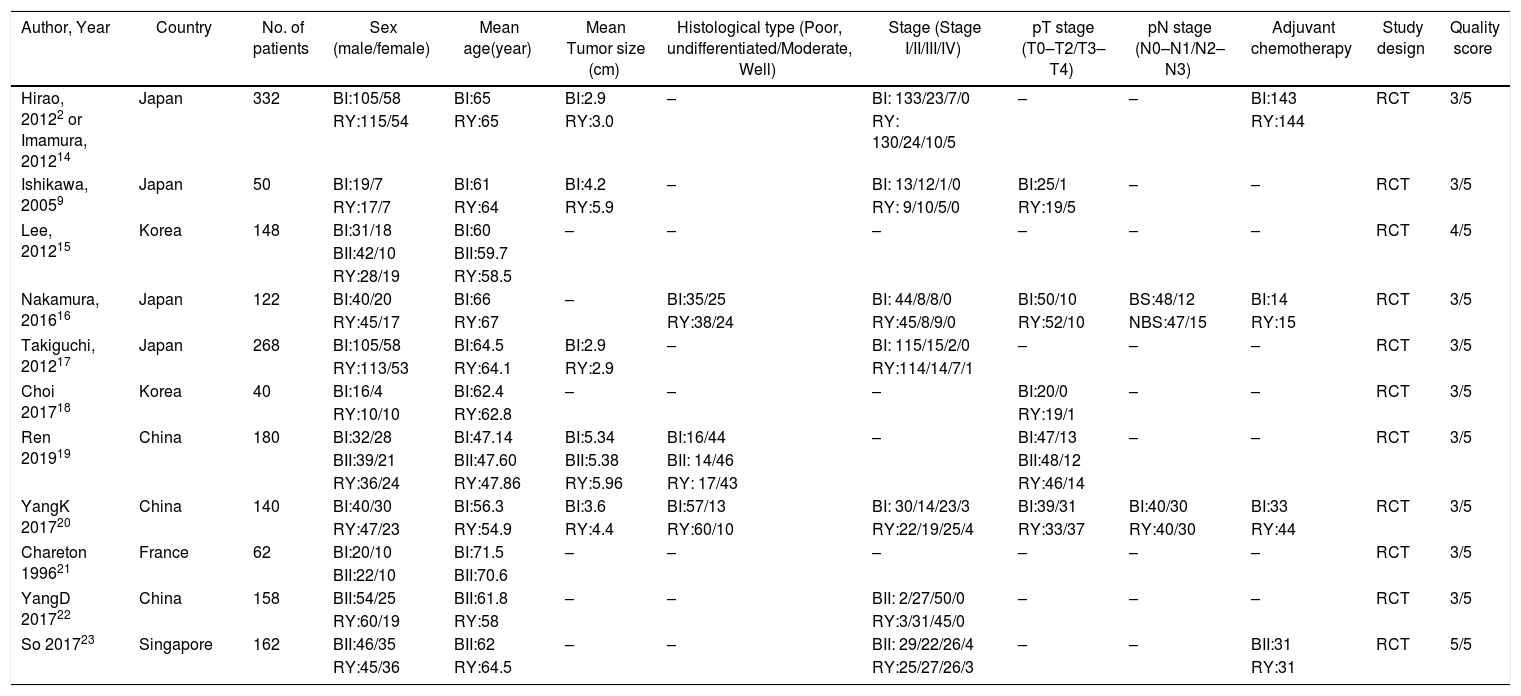
ResultsStudy characteristicsAfter a comprehensive inspection, 12 randomized controlled trials (RCTs)2,9,14–23 were included in our network meta-analysis(NMA) (Fig. 1). Among them, two studies2,14 have same study populations. Two studies simultaneously compared BI, BII, and RY anastomoses; one study compared BI and BII anastomoses; two studies compared BII and RY anastomoses; and six studies compared BI and RY anastomoses. In total, 1662 patients were included in our analysis: 610 were treated with BI anastomosis; 304 were treated with BII anastomosis; and 748 were treated with RY anastomosis. Patient demographics for the 12 studies are presented in Table 1.
Summary and comparison of baseline characteristics between BI, BII and RY.
| Author, Year | Country | No. of patients | Sex (male/female) | Mean age(year) | Mean Tumor size (cm) | Histological type (Poor, undifferentiated/Moderate, Well) | Stage (Stage I/II/III/IV) | pT stage (T0–T2/T3–T4) | pN stage (N0–N1/N2–N3) | Adjuvant chemotherapy | Study design | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hirao, 20122 or Imamura, 201214 | Japan | 332 | BI:105/58 | BI:65 | BI:2.9 | – | BI: 133/23/7/0 | – | – | BI:143 | RCT | 3/5 |
| RY:115/54 | RY:65 | RY:3.0 | RY: 130/24/10/5 | RY:144 | ||||||||
| Ishikawa, 20059 | Japan | 50 | BI:19/7 | BI:61 | BI:4.2 | – | BI: 13/12/1/0 | BI:25/1 | – | – | RCT | 3/5 |
| RY:17/7 | RY:64 | RY:5.9 | RY: 9/10/5/0 | RY:19/5 | ||||||||
| Lee, 201215 | Korea | 148 | BI:31/18 | BI:60 | – | – | – | – | – | – | RCT | 4/5 |
| BII:42/10 | BII:59.7 | |||||||||||
| RY:28/19 | RY:58.5 | |||||||||||
| Nakamura, 201616 | Japan | 122 | BI:40/20 | BI:66 | – | BI:35/25 | BI: 44/8/8/0 | BI:50/10 | BS:48/12 | BI:14 | RCT | 3/5 |
| RY:45/17 | RY:67 | RY:38/24 | RY:45/8/9/0 | RY:52/10 | NBS:47/15 | RY:15 | ||||||
| Takiguchi, 201217 | Japan | 268 | BI:105/58 | BI:64.5 | BI:2.9 | – | BI: 115/15/2/0 | – | – | – | RCT | 3/5 |
| RY:113/53 | RY:64.1 | RY:2.9 | RY:114/14/7/1 | |||||||||
| Choi 201718 | Korea | 40 | BI:16/4 | BI:62.4 | – | – | – | BI:20/0 | – | – | RCT | 3/5 |
| RY:10/10 | RY:62.8 | RY:19/1 | ||||||||||
| Ren 201919 | China | 180 | BI:32/28 | BI:47.14 | BI:5.34 | BI:16/44 | – | BI:47/13 | – | – | RCT | 3/5 |
| BII:39/21 | BII:47.60 | BII:5.38 | BII: 14/46 | BII:48/12 | ||||||||
| RY:36/24 | RY:47.86 | RY:5.96 | RY: 17/43 | RY:46/14 | ||||||||
| YangK 201720 | China | 140 | BI:40/30 | BI:56.3 | BI:3.6 | BI:57/13 | BI: 30/14/23/3 | BI:39/31 | BI:40/30 | BI:33 | RCT | 3/5 |
| RY:47/23 | RY:54.9 | RY:4.4 | RY:60/10 | RY:22/19/25/4 | RY:33/37 | RY:40/30 | RY:44 | |||||
| Chareton 199621 | France | 62 | BI:20/10 | BI:71.5 | – | – | – | – | – | – | RCT | 3/5 |
| BII:22/10 | BII:70.6 | |||||||||||
| YangD 201722 | China | 158 | BII:54/25 | BII:61.8 | – | – | BII: 2/27/50/0 | – | – | – | RCT | 3/5 |
| RY:60/19 | RY:58 | RY:3/31/45/0 | ||||||||||
| So 201723 | Singapore | 162 | BII:46/35 | BII:62 | – | – | BII: 29/22/26/4 | – | – | BII:31 | RCT | 5/5 |
| RY:45/36 | RY:64.5 | RY:25/27/26/3 | RY:31 |
Three nodes were compared in the network graph, in which the size of the nodes was associated with the number of patients undergoing a certain type of anastomosis, and the thickness of the lines was related to the number of direct comparisons between 2 reconstruction methods.
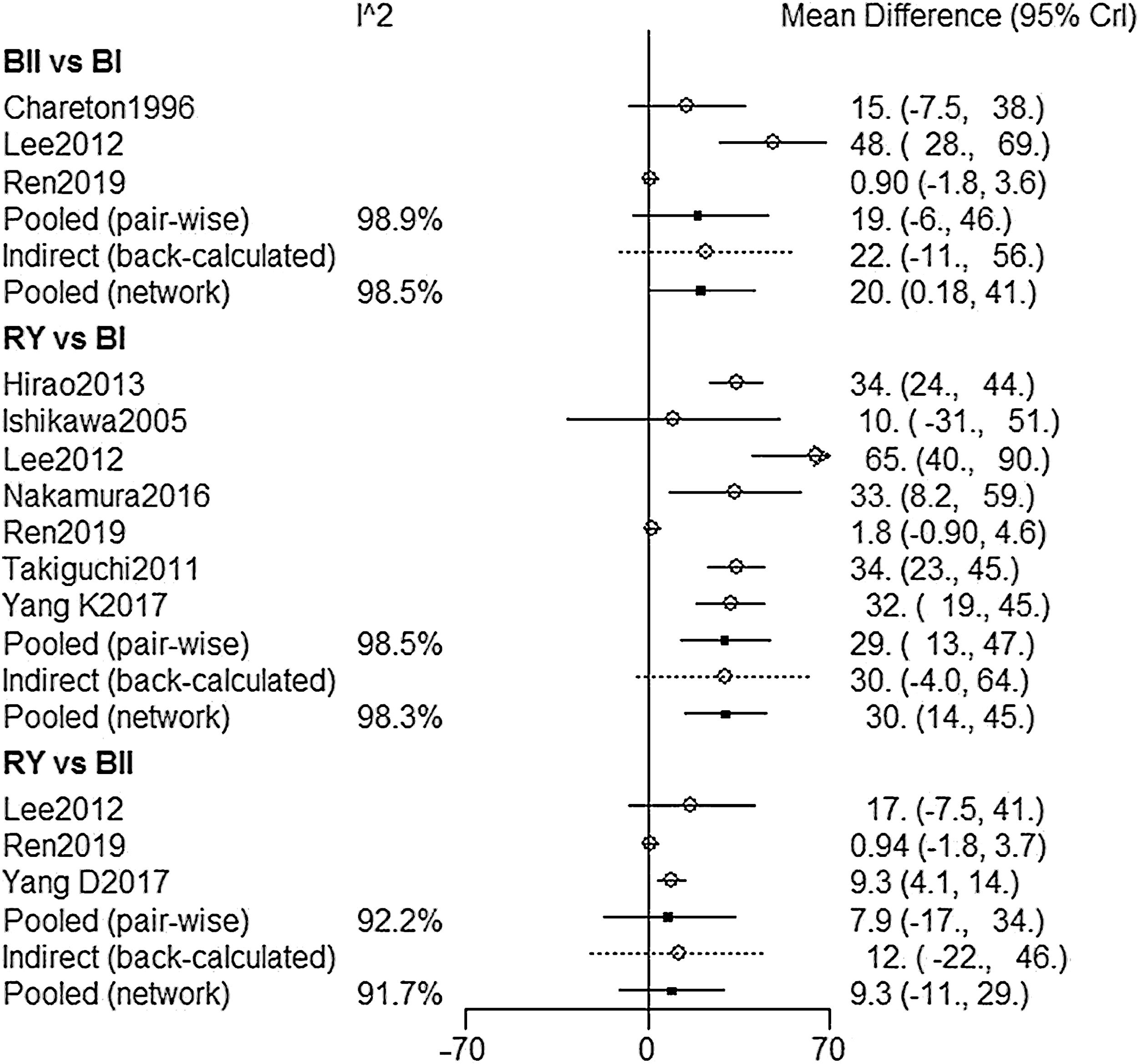
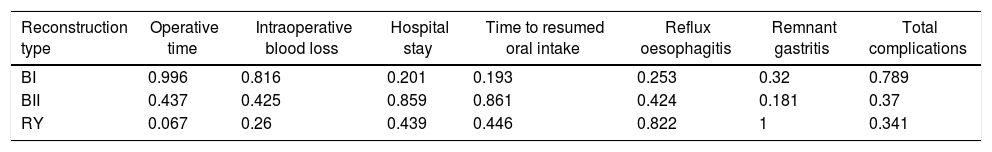
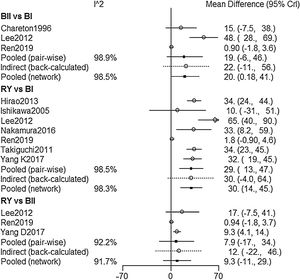
NMA resultsOperative time was extracted from 9 studies,2,9,15–17,19–22 The NMA results (Fig. 2) showed that BI reconstruction had a significantly shorter operative time than (WMD 20, 95% Crl: 0.18–41) and RY(WMD 30, 95% Crl: 14–25) reconstruction. SUCRA plots indicated that RY reconstruction had the highest probability of being the worst method for reducing operative time (SUCRA=6.7%), while BI reconstruction had the highest probability of being the best method (SUCRA=99.6%), followed by BII (SUCRA=43.7%) (Table 2). The results of comparisons of operative blood loss, hospital stay and time to resumed oral intake in our network meta-analysis suggested there were no significant differences among the 3 procedures (Table 3).
Surface under the cumulative ranking curve (SUCRA) results for all outcomes.
| Reconstruction type | Operative time | Intraoperative blood loss | Hospital stay | Time to resumed oral intake | Reflux oesophagitis | Remnant gastritis | Total complications |
|---|---|---|---|---|---|---|---|
| BI | 0.996 | 0.816 | 0.201 | 0.193 | 0.253 | 0.32 | 0.789 |
| BII | 0.437 | 0.425 | 0.859 | 0.861 | 0.424 | 0.181 | 0.37 |
| RY | 0.067 | 0.26 | 0.439 | 0.446 | 0.822 | 1 | 0.341 |
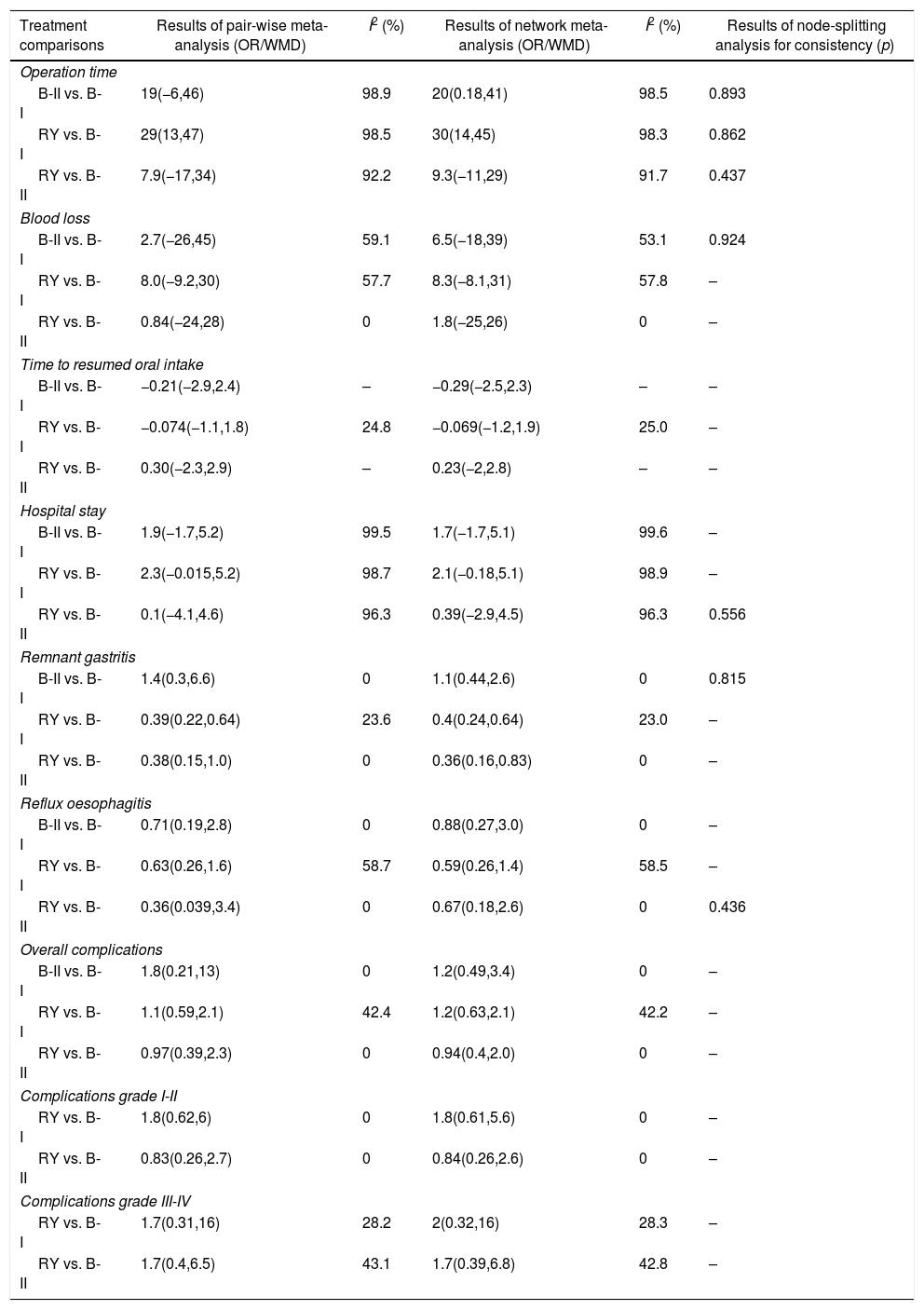
Summary of pair-wise meta-analysis and network meta-analysis results for the 3 reconstruction methods.
| Treatment comparisons | Results of pair-wise meta-analysis (OR/WMD) | I2 (%) | Results of network meta-analysis (OR/WMD) | I2 (%) | Results of node-splitting analysis for consistency (p) |
|---|---|---|---|---|---|
| Operation time | |||||
| B-II vs. B-I | 19(−6,46) | 98.9 | 20(0.18,41) | 98.5 | 0.893 |
| RY vs. B-I | 29(13,47) | 98.5 | 30(14,45) | 98.3 | 0.862 |
| RY vs. B-II | 7.9(−17,34) | 92.2 | 9.3(−11,29) | 91.7 | 0.437 |
| Blood loss | |||||
| B-II vs. B-I | 2.7(−26,45) | 59.1 | 6.5(−18,39) | 53.1 | 0.924 |
| RY vs. B-I | 8.0(−9.2,30) | 57.7 | 8.3(−8.1,31) | 57.8 | – |
| RY vs. B-II | 0.84(−24,28) | 0 | 1.8(−25,26) | 0 | – |
| Time to resumed oral intake | |||||
| B-II vs. B-I | −0.21(−2.9,2.4) | – | −0.29(−2.5,2.3) | – | – |
| RY vs. B-I | −0.074(−1.1,1.8) | 24.8 | −0.069(−1.2,1.9) | 25.0 | – |
| RY vs. B-II | 0.30(−2.3,2.9) | – | 0.23(−2,2.8) | – | – |
| Hospital stay | |||||
| B-II vs. B-I | 1.9(−1.7,5.2) | 99.5 | 1.7(−1.7,5.1) | 99.6 | – |
| RY vs. B-I | 2.3(−0.015,5.2) | 98.7 | 2.1(−0.18,5.1) | 98.9 | – |
| RY vs. B-II | 0.1(−4.1,4.6) | 96.3 | 0.39(−2.9,4.5) | 96.3 | 0.556 |
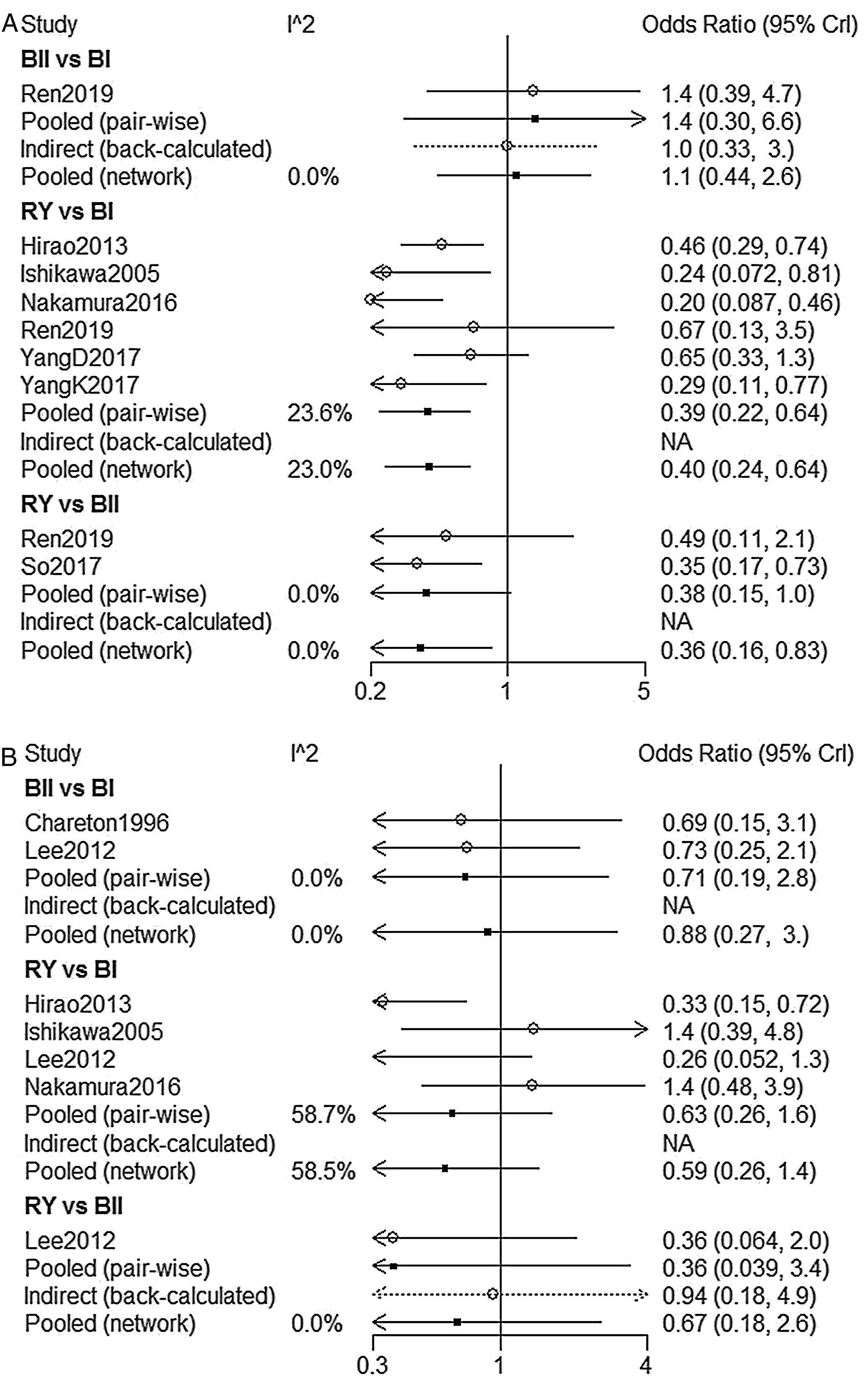
| Remnant gastritis | |||||
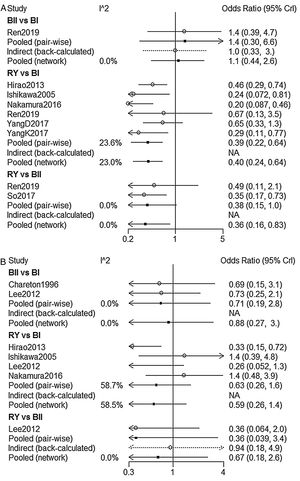
| B-II vs. B-I | 1.4(0.3,6.6) | 0 | 1.1(0.44,2.6) | 0 | 0.815 |
| RY vs. B-I | 0.39(0.22,0.64) | 23.6 | 0.4(0.24,0.64) | 23.0 | – |
| RY vs. B-II | 0.38(0.15,1.0) | 0 | 0.36(0.16,0.83) | 0 | – |
| Reflux oesophagitis | |||||
| B-II vs. B-I | 0.71(0.19,2.8) | 0 | 0.88(0.27,3.0) | 0 | – |
| RY vs. B-I | 0.63(0.26,1.6) | 58.7 | 0.59(0.26,1.4) | 58.5 | – |
| RY vs. B-II | 0.36(0.039,3.4) | 0 | 0.67(0.18,2.6) | 0 | 0.436 |
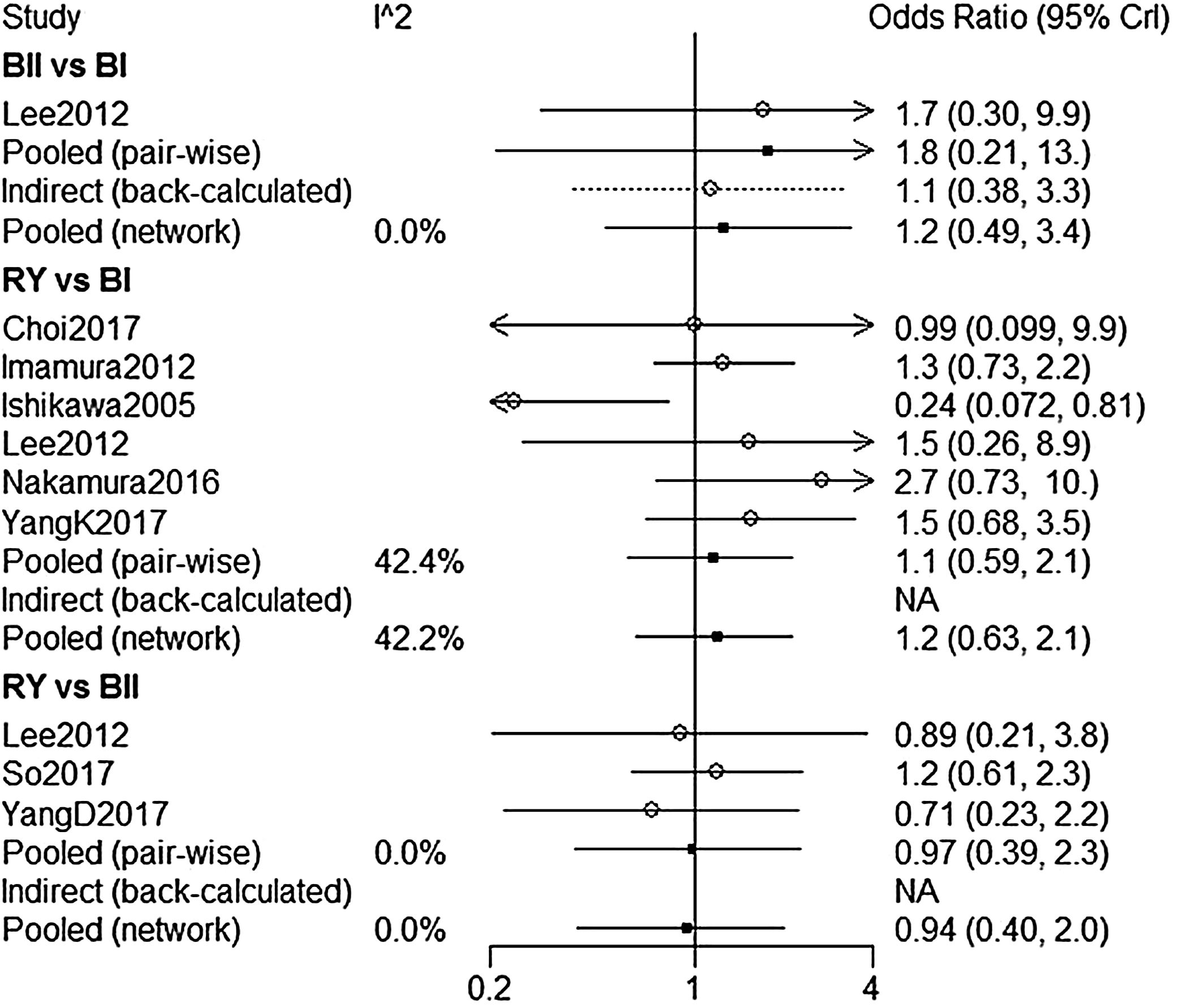
| Overall complications | |||||
| B-II vs. B-I | 1.8(0.21,13) | 0 | 1.2(0.49,3.4) | 0 | – |
| RY vs. B-I | 1.1(0.59,2.1) | 42.4 | 1.2(0.63,2.1) | 42.2 | – |
| RY vs. B-II | 0.97(0.39,2.3) | 0 | 0.94(0.4,2.0) | 0 | – |
| Complications grade I-II | |||||
| RY vs. B-I | 1.8(0.62,6) | 0 | 1.8(0.61,5.6) | 0 | – |
| RY vs. B-II | 0.83(0.26,2.7) | 0 | 0.84(0.26,2.6) | 0 | – |
| Complications grade III-IV | |||||
| RY vs. B-I | 1.7(0.31,16) | 28.2 | 2(0.32,16) | 28.3 | – |
| RY vs. B-II | 1.7(0.4,6.5) | 43.1 | 1.7(0.39,6.8) | 42.8 | – |
NMA indicated that RY reconstruction had significant superiority over B-I and B-II reconstruction in remnant gastritis (OR 0.40, 95%Crl: 0.24–0.64; OR 0.36, 95% Crl: 0.16–0.83, respectively) (Fig. 3A). SUCRA plots indicated that RY reconstruction had the highest probability of being the best method for reducing remnant gastritis (SUCRA=100%), while BII reconstruction had the highest probability of being the worst method (SUCRA=18.1%), followed by BI (SUCRA=32%) (Table 2). The results of comparisons of reflux oesophagitis (Fig. 3B) in our network meta-analysis suggested there were no significant differences among the 3 reconstructions.
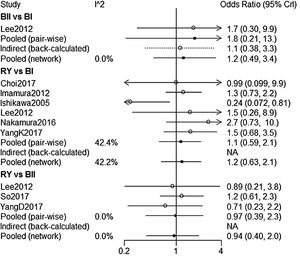
A total of 8 studies9,14–16,18,20,22,23 were included to compare the overall complications. The overall complication rates of BI, BII, and RY were 17.8%, 18.8%, and 20.4%, respectively. According to our NMA results, there were no significant difference among BI, BII and RY (Fig. 4). Five of eight studies were analyzed for the severity of complications with Clavien-Dindo Classification.24 Similar to the overall complications, there was no significant difference in grade I–II and grade III–IV complications between these three reconstructions (Table 3).
Node-spitting resultsThe node-spitting analysis was performed to confirm the consistency between direct and indirect comparisons. There was no significant difference in the comparison of BI, BII, and RY (p>0.05) in terms of consistency, and the consistency model was finally adopted (Table 3).
DiscussionUp to now, we have three main techniques for anastomosis between the residual stomach and the intestine—BI, BII and RY. The ideal gastrointestinal reconstruction surgery should minimize the incidence of postoperative complications and improve quality of life.25 To our knowledge, BI reconstruction has usually been applied after DG for gastric cancer due to its simplicity and less operating time. BII or RY have been preferred in patients with a stump stomach or a duodenum shortened by extensive resection to ensure the safety of surgical margins.
BI reconstruction was associated with a significant reduction in operation time as compared with BII and RY reconstruction. We also evaluated data regarding intraoperative blood loss, hospital stay and time to resumed oral intake, and no significant difference in either of these parameters among the three groups was found. It may be largely due to the use of gastrointestinal stapling devices and the refinement of technique.
The main advantage of the RY reconstruction was thought to be the prevent secretions from the biliary tract and pancreas from reaching the gastric and esophageal mucosa. Previous studies indicated that the RY reconstruction was more effective at preventing postoperative reflux esophagitis and remnant gastritis.26,27 Our NMA demonstrated that RY reconstruction was superior to BI and BII in terms of frequency of remnant gastritis, which was consistent with previous reports.28 However, there was no statistical difference in reflux esophagitis among the 3 groups, a finding that was proven to be similar with previous reports.9,15,16,29 The reason for this discrepancy may be that reflux esophagitis are caused by acid reflux rather than bile reflux. On the contrary, remnant gastritis was related with bile reflux while RY technique significantly reduced the risk of bile reflux.30,31
In the light of the Clavien-Dindo classification, the grade III–IV complications required surgical, endoscopic or radiological intervention, and even life-threatening. Although the RY reconstruction was a more complex procedure requiring additional anastomosis, the risk of overall complications and grade III–IV severe complications did not increase.
This review does have some limitations and hence the results should be interpreted with a degree of caution. First, the size of the included studies was relatively small, and the insufficiency of direct evidence might lead to inconsistency in some comparison items. Second, it was important to mention that we were unable to analyze important outcomes such as body weight change, amount of ingested food and overall survival due to a lack of original literature. Additionally, the included studies lasted for a long period and the improvement of treatment technique may influence our outcomes. We would therefore propose well-designed RCTs with adequate follow-up and emphasis on assessing important outcomes to clarify ambiguities surrounding the use of these reconstruction methods.
ConclusionsThe R-Y reconstruction was more effective in preventing remnant gastritis than BI and BII reconstruction. BI reconstruction could be considered as the substitute in consideration of technical simplicity. However, the results of the present study should be verified by long-term follow-up of these patients, and additional randomized control studies are warranted to determine the clinical efficacy of 3 reconstruction in DG.
Statement of ethicsAll analysis were based on previous published studies, thus no ethical approval and patient consent are required.
Disclosure statementThe authors disclose that there are no direct or indirect financial relationships.
Author contributionsHaitao Jiang reviewed and screened the articles included in our meta-analysis; Yujie Li performed the analysis and drafted the manuscript; Tianfei Wang designed the study and edited the manuscript.
Funding sourcesNone.
Conflict of interestThe authors declare no conflicts of interest.
This research was supported by Key Laboratory of Diagnosis and Treatment of Digestive System Tumors of Zhejiang Province.
The authors would like to thank Tianfei Wang for critically reviewing the manuscript.