Among the strategies designed to optimize the number of existing liver grafts for transplantation, the implementation of the graft assessment process is one of the least explored. The main objective is to identify the risk factors presented by liver donors for «NO validity». Secondly, we analyzed the coincidence between the surgeon's assessment and that of the anatomo-pathologist in the invalid donors.
Material and methodRetrospective study conducted from a prospective database that analyzes 190 liver donors, 95 valid and 95 NOT valid. The variables of each of them corresponding to the donation protocol of the National Transplant Organization are studied. Through a multivariate study we determine the independent risk factors of NO validity. We checked the causes of NO validity argued with the histopathological findings of these grafts.
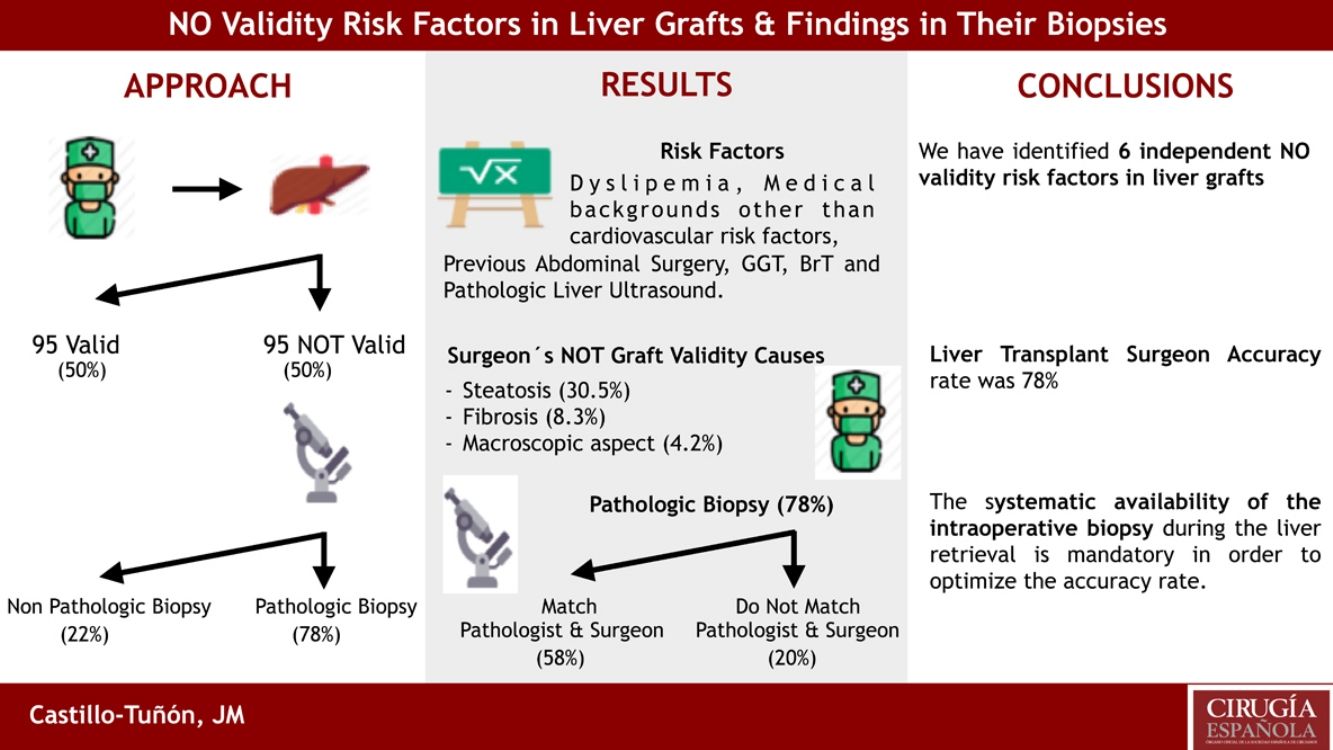
ResultsThe independent risk factors of non-validity in the multivariate study (P<.05) were: dyslipidemia, personal medical history other than cardiovascular and abdominal surgical risk factors, GGT, BrT, and the result of previous liver ultrasound. The 3 most frequent causes of NO validity were: steatosis, fibrosis and macroscopic appearance of the organ. 78% of the biopsies confirmed the NO validity of the graft (in 57.9% of the cases the histological findings coincided with those described by the surgeon). The 22.1% of the biopsies hadńt pathological findings.
ConclusionsThe determination of the risk factors of NO validity will contribute to the design of future assessment scores that are useful tools in the process of liver graft assessment.)
Entre las estrategias diseñadas para optimizar el número de injertos hepáticos existentes para trasplante, la implementación del proceso de valoración de injertos constituye una de las menos exploradas. El objetivo principal es identificar los factores de riesgo que presentan los donantes hepáticos para la «NO validez». Secundariamente analizamos la coincidencia entre la valoración del cirujano y la del anatomopatólogo en los donantes NO válidos.
Material y métodoEstudio retrospectivo realizado a partir de una base de datos prospectiva que analiza 190 donantes hepáticos, 95 válidos y 95 NO válidos. Se estudian las variables de cada uno de ellos correspondientes al protocolo de donación de la Organización Nacional de Trasplantes. Mediante el estudio multivariante determinamos los factores de riesgo independientes de NO validez. Cotejamos las causas de NO validez argumentadas con los hallazgos histopatológicos de dichos injertos.
ResultadosLos factores de riesgo independientes de NO validez en el estudio multivariante (p<0,05) fueron: dislipemia, antecedentes personales médicos distintos a factores de riesgo cardiovascular y quirúrgicos abdominales, GGT, BrT, y el resultado de la ecografía hepática previa. Las dos causas más frecuentes de NO validez fueron: esteatosis y fibrosis. El 78% de las biopsias confirmaron la NO validez del injerto (en 57,9% del total coincidían los hallazgos histológicos con los descritos por el cirujano). El 22% restante de las biopsias no presentaban hallazgos patológicos.
ConclusionesLa determinación de los factores de riesgo de NO validez contribuirá al diseño de futuros scores de valoración que constituyan herramientas útiles en el proceso de valoración de injertos hepáticos.
Since Bethesda Concensus Conference in 1983, Liver Trasplantation (LT) is considered the treatment of choice for terminal liver diseases.1 Currently, it associates excellent survival rates for both, the graft and the recipient. As a consequence, the number of candidates and the indications of LT have increased. This, added to the change in the profile of donors, has generated an imbalance between grafts offered and candidates on the waiting list.1
Currently donors with all or almost all “ideal” parameters; age less tan 50 years, hemodynamic stability with minimal vasopressor requirement and abscense of abdominal trauma, systemic infection, malignancy, or chronic disease are rare.2
In order to increase the number of donors, different strategies have been developed to obtain grafts; living donor, split, domino trasplant and donor with expanded criteria (DEC).3 DEC are those that are more likely to have primary graft failure or primary graft dysfuction, or associated a lower survival rate of the graft and/or recipient when compared to the ideal donor.4–6
The evaluation of liver grafts for donation should be a rigorous process with as few subjective elements as posible, guaranteeing máximum use of available organs. This assessment, is carried out on numerous occasions without the possibility of a histological study that may or may not confirm the validity of the organ. It is the experience of transplant surgeon, the main criterion we have.
The main objective of this study is to identify the risk factors presented by hepatic donor for “No validity”. Secondly, we analise the coincidence between the surgeońs assesment and that of the anatomopathologist in the case of NO valid donors.
Material and methodRetrospective study base don a prospective database. We analized 190 liver donors (95 considered valid and 95 considered NO valid) for the 2012-2016 period. In all cases, the grafts were evaluated by the same LT surgeons. They had at that time more tah 5 years of experience in liver donation in a high volumen national center. They share the same criteria for on-site evaluation of the donor.
The variables studied are those included in the donation protocol of the National Trasplant Organisation (N.T.O.), an oficial document that must be complied with by the coordination that generates the offer and that serves to asses the suitability of the graft on the part of surgical team (Table 1).
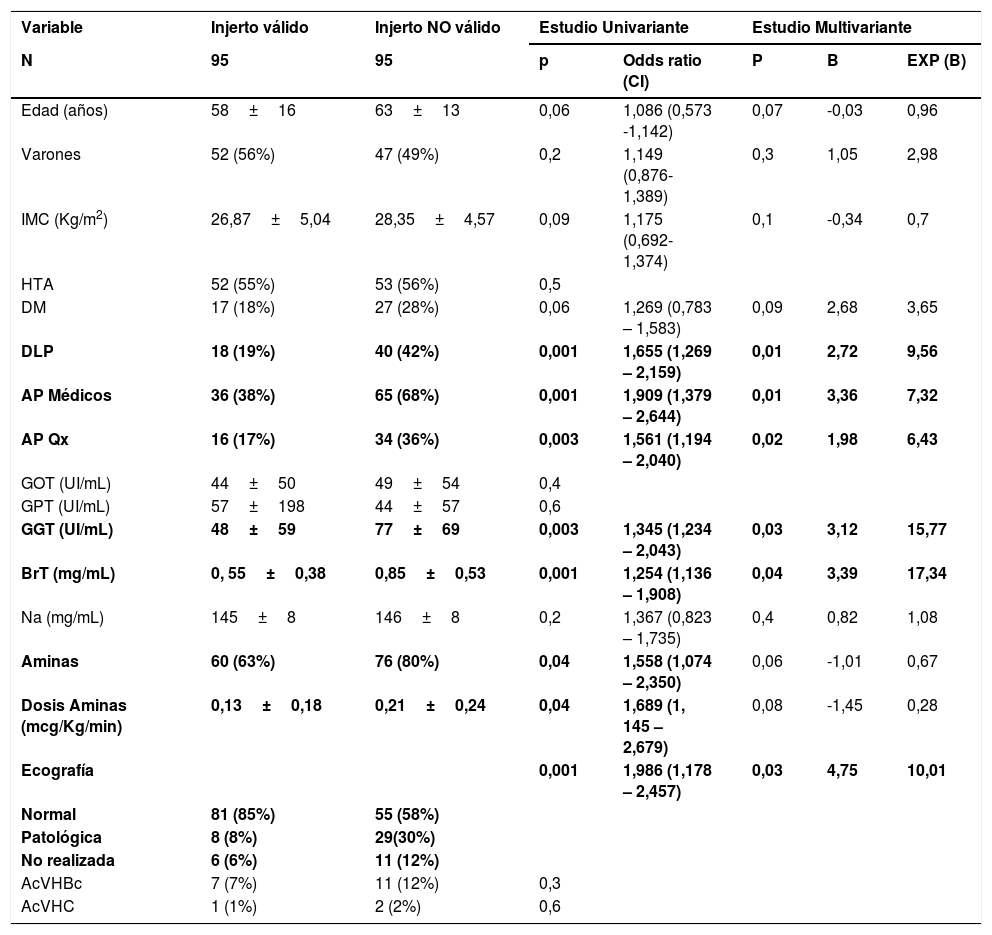
Estudio uni- y multivariante sobre los factores de riesgo de NO validez.
| Variable | Injerto válido | Injerto NO válido | Estudio Univariante | Estudio Multivariante | |||
|---|---|---|---|---|---|---|---|
| N | 95 | 95 | p | Odds ratio (CI) | P | B | EXP (B) |
| Edad (años) | 58±16 | 63±13 | 0,06 | 1,086 (0,573 -1,142) | 0,07 | -0,03 | 0,96 |
| Varones | 52 (56%) | 47 (49%) | 0,2 | 1,149 (0,876-1,389) | 0,3 | 1,05 | 2,98 |
| IMC (Kg/m2) | 26,87±5,04 | 28,35±4,57 | 0,09 | 1,175 (0,692-1,374) | 0,1 | -0,34 | 0,7 |
| HTA | 52 (55%) | 53 (56%) | 0,5 | ||||
| DM | 17 (18%) | 27 (28%) | 0,06 | 1,269 (0,783 – 1,583) | 0,09 | 2,68 | 3,65 |
| DLP | 18 (19%) | 40 (42%) | 0,001 | 1,655 (1,269 – 2,159) | 0,01 | 2,72 | 9,56 |
| AP Médicos | 36 (38%) | 65 (68%) | 0,001 | 1,909 (1,379 – 2,644) | 0,01 | 3,36 | 7,32 |
| AP Qx | 16 (17%) | 34 (36%) | 0,003 | 1,561 (1,194 – 2,040) | 0,02 | 1,98 | 6,43 |
| GOT (UI/mL) | 44±50 | 49±54 | 0,4 | ||||
| GPT (UI/mL) | 57±198 | 44±57 | 0,6 | ||||
| GGT (UI/mL) | 48±59 | 77±69 | 0,003 | 1,345 (1,234 – 2,043) | 0,03 | 3,12 | 15,77 |
| BrT (mg/mL) | 0, 55±0,38 | 0,85±0,53 | 0,001 | 1,254 (1,136 – 1,908) | 0,04 | 3,39 | 17,34 |
| Na (mg/mL) | 145±8 | 146±8 | 0,2 | 1,367 (0,823 – 1,735) | 0,4 | 0,82 | 1,08 |
| Aminas | 60 (63%) | 76 (80%) | 0,04 | 1,558 (1,074 – 2,350) | 0,06 | -1,01 | 0,67 |
| Dosis Aminas (mcg/Kg/min) | 0,13±0,18 | 0,21±0,24 | 0,04 | 1,689 (1, 145 – 2,679) | 0,08 | -1,45 | 0,28 |
| Ecografía | 0,001 | 1,986 (1,178 – 2,457) | 0,03 | 4,75 | 10,01 | ||
| Normal | 81 (85%) | 55 (58%) | |||||
| Patológica | 8 (8%) | 29(30%) | |||||
| No realizada | 6 (6%) | 11 (12%) | |||||
| AcVHBc | 7 (7%) | 11 (12%) | 0,3 | ||||
| AcVHC | 1 (1%) | 2 (2%) | 0,6 | ||||
IMC: Índice de Masa Corporal. HTA: Hipertensión Arterial. DM: Diabetes Mellitus. DLP: Dislipemia. AP Médicos: Antecedentes Médicos distintos a factores de riesgo cardiovascular. AP Qx: Antecedentes Personales de Cirugía Abdominal. GOT: Aspartato AminoTransferasa. GPT: Alanino AminoTransferasa. GGT: GammaGlutamil Transpeptidasa. BrT: Bilirrubina Total. Na: Sodio. AcVHBc: Anticuerpo anticore del virus de la hepatitis B. AcVHC: Anticuerpo frente al virus de la hepatitis C.
We understand as valid graft both the ideal donors and DEC.7,8 The rest are considered “NO valid” grafts.
We divide our series into two groups:
- -
Valid hepatic grafts: 95 grafts that were considered valid after evaluation of the characteristic included in the donation protocol, “in situ” evaluation of the graft after laparotomy and its behaviour during perfusión. They all had biopsy in time 0 reperfusion.
- -
NO valid liver graft: 95 grafts declared NO valid after evaluation of the same ítems commented previously. We picked up the cause of NO validity given by transplant surgeon to the N.T.O.. Once the grafts was listed as NO valid, a liver biopsy was taken. All these biopsies were sent to the Pathological Anatomy Service of the Virgen del Rocio Hospital in Seville, according to protocolo, and analysed by a pathologist who is an expert in histopathology of the liver.
We compared the variables of the donors in both groups and studied wether there were statistically significant differences between them by means of a univariate study (Table 2). Those variables that presented statistical signifcance (p<0,05) in the univariate study were included in the multivariate study to determine the independent factors of NO validity.
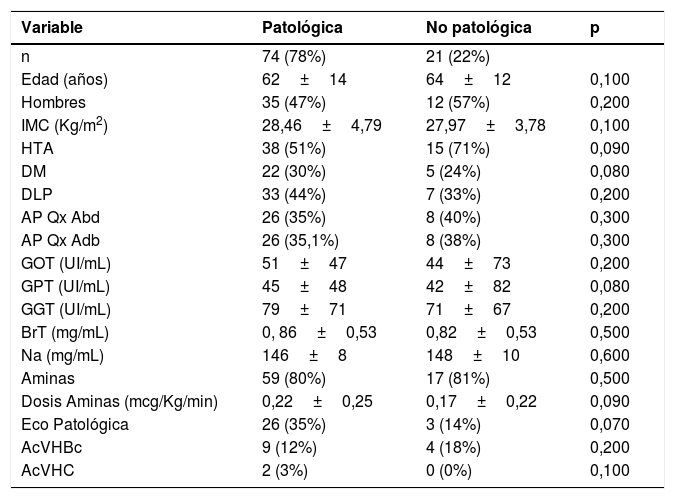
Injertos NO válidos, comparación entre subgrupo con biopsia patológica y biopsia no patológica (Estudio Univariante).
| Variable | Patológica | No patológica | p |
|---|---|---|---|
| n | 74 (78%) | 21 (22%) | |
| Edad (años) | 62±14 | 64±12 | 0,100 |
| Hombres | 35 (47%) | 12 (57%) | 0,200 |
| IMC (Kg/m2) | 28,46±4,79 | 27,97±3,78 | 0,100 |
| HTA | 38 (51%) | 15 (71%) | 0,090 |
| DM | 22 (30%) | 5 (24%) | 0,080 |
| DLP | 33 (44%) | 7 (33%) | 0,200 |
| AP Qx Abd | 26 (35%) | 8 (40%) | 0,300 |
| AP Qx Adb | 26 (35,1%) | 8 (38%) | 0,300 |
| GOT (UI/mL) | 51±47 | 44±73 | 0,200 |
| GPT (UI/mL) | 45±48 | 42±82 | 0,080 |
| GGT (UI/mL) | 79±71 | 71±67 | 0,200 |
| BrT (mg/mL) | 0, 86±0,53 | 0,82±0,53 | 0,500 |
| Na (mg/mL) | 146±8 | 148±10 | 0,600 |
| Aminas | 59 (80%) | 17 (81%) | 0,500 |
| Dosis Aminas (mcg/Kg/min) | 0,22±0,25 | 0,17±0,22 | 0,090 |
| Eco Patológica | 26 (35%) | 3 (14%) | 0,070 |
| AcVHBc | 9 (12%) | 4 (18%) | 0,200 |
| AcVHC | 2 (3%) | 0 (0%) | 0,100 |
IMC: Índice de Masa Corporal. HTA: Hipertensión Arterial. DM: Diabetes Mellitus. DLP: Dislipemia. AP Médicos: Antecedentes Médicos distintos a factores de riesgo cardiovascular. AP Qx: Antecedentes Personales de Cirugía Abdominal. GOT: Aspartato AminoTransferasa. GPT: Alanino AminoTransferasa. GGT: GammaGlutamil Transpeptidasa. BrT: Bilirrubina Total. Na: Sodio. AcVHBc: Anticuerpo anticore del virus de la hepatitis B. AcVHC: Anticuerpo frente al virus de la hepatitis C.
Since no references have been found in the Literature that reflect the effectiveness of the transplant surgeon in the evaluation of the liver graft, we compare the causes of NO validity argued during donation (Table 3) with the histopathological diagnosis of these grafts (Table 4).
Relación de causas macroscópicas de No validez argumentadas por el Citujano de TH y diagnóstico anatomo Patológico.
| Causa de No Validez | Biopsia No patológica | Biopsia patológica | Total Casos (%) | Tasa de Acierto |
|---|---|---|---|---|
| n | 21 | 74 | 95 (100) | 78 |
| Esteatosis | 4 | 25 | 29 (30) | 86 |
| Fibrosis | 0 | 8 | 8 (8) | 100 |
| Aspecto Macroscopico | 2 | 2 | 4 (4) | 50 |
| Otros | 1 | 2 | 3 (4) | 67 |
| Cirrosis | 0 | 3 | 3 (4) | 100 |
| Ateromatosis | 2 | 0 | 2 (2) | 0 |
| Isquemia | 0 | 1 | 1 (1) | 100 |
| Aspecto macroscópico+Ateromatosis | 4 | 2 | 6 (6) | 33 |
| Esteatosis+Ateromatosis | 1 | 5 | 6 (6) | 83 |
| Esteatosis+Isquemia | 0 | 6 | 6 (6) | 100 |
| Esteatosis+Aspecto Macroscópico | 1 | 4 | 5 (5) | 80 |
| Esteatosis+Cirrosis | 0 | 4 | 4 (4) | 100 |
| Esteatosis+Fibrosis | 0 | 2 | 2 (2) | 100 |
| Aspecto macroscópico+Cirrosis | 0 | 2 | 2 (2) | 100 |
| Ateromatosis+Isquemia | 0 | 2 | 2 (2) | 100 |
| Aspecto Macroscopico+Isquemia | 0 | 1 | 1 (1) | 100 |
| Aspecto macroscópico+Fibrosis | 0 | 1 | 1 (1) | 100 |
| Aspecto macroscópico+Ateromatosis+Esteatosis | 1 | 2 | 3 (4) | 67 |
| Aspecto macroscópico+Fibrosis+Otros | 2 | 1 | 3 (4) | 33 |
| Aspecto macroscópico+Ateromatosis+Fibrosis | 1 | 0 | 1 (1) | 0 |
| Aspecto macroscópico+Fibrosis+Esteatosis | 1 | 0 | 1 (1) | 0 |
| Aspecto macroscópico+Ateromatosis+Cirrosis | 0 | 1 | 1 (1) | 100 |
| Fibrosis+Aspecto macroscópico+Ateromatosis+Problema quirúrgico | 1 | 0 | 1 (1) | 0 |
Tasa de acierto: (Número de Causa de NO validez con Biopsia Patológica x 100) / Total casos. Expresada en tanto porcentual (%).
We sub-divided the group of NO valid grafts according to whether they had pathological biopsy (n=74; 77,9%) or No pathological biopsy (n=21;22,1%) in order to compared the variables of the N.T.O document (Table 5), as well as the cause of NO validity given by the surgeon responsible for de donation (Table 6).
The statistical study was performed with SPSS v 22.0. We express the qualitative variables in absolute figures with the percentage (%) in brackets and compare them using the Chi-square test. The continuous variable are reflected with the mean±standar deviation and compared using the Student T test.
ResultsCharacteristics of valid Vs NO valid donors (Table 1)The mean age of valid Vs NO valid donors was 58±16 Vs 63±13 years. The gender was male in 52 (54.7%) Vs 47 (49.5%), respectively. The mean body mass index (BMI) was 26.87±5.04 Vs 28.35±4.57 Kg/m,2 respectively. Arterial Hypertension (AHT) was reported by 52 (54.7%) Vs 53 (55.8%), Mellitus Diabetes (MD) 17 (17.9%) Vs 27 (28.4%) and Dyslipemia (DL) 18 (18.9%) Vs 40 (42.1%). In relation to the presence of medical history other tan cardiovascular risk factor (MPA) were 36 (37.9%) Vs 65 (68.4%) and history of abdominal surgery (QPA) 16 (16.8%) Vs 34 (35.8%).
The mean values of GPT, GOT, GGT, Total Bilirubin (BrT) and plasma sodium (Na) in valid Vs NO valid donors were 57±19 Vs 49±54 UI/mL; 44±50 Vs 44±57 UI/mL; 48±59 Vs 77±69 UI/mL; 0, 55±0,38 Vs 0,85±0,53mg/mL y 145±8 Vs 146±8mg/mL. The serology was positive for antibodies to the Hepatitis B virus core (Ac HBVc) in 7 (7.4%) Vs 11 (11.7%), and for the anti-Hepatitis C virus (Ac HCV) in 1 (1.1%) Vs 2 (2.1%).
Ultrasound was reported as pathological in 8 (8.4%) Vs 29 (30.5%) in valid Vs NO valid donors, respectively. Amines were required for maintenance 60 (63.2%) Vs 70 (80%) with a mean dose of 0.13±0.18 Vs 0.21±0.24 mcg/Kg/min in valid Vs NO valid donors, respectively.
Risk factors for NO validity (Table 2)We obtained statiscally significant differences in the univariate study in 8 of the 18 variables studied. They had DL (OR:1,655; IC 95%: 1,269 – 2,159; p=0,001), MPA (OR:1,909; IC 95%: 1,379 – 2,644; p=0,001), QPA (OR:1,561; IC 95%: 1,194 – 2,040; p=0,003), GGT (OR:1,345; IC 95%: 1,234 – 2,043; p=0,003), BrT (OR: 1,254; IC 95%: 1,136 – 1,908; p=0,001), amines (OR:1,558; IC 95%: 1,074 – 2,354; p=0,04), dose (OR:1,986; IC 95%: 1,178 – 2,457; p=0,04) and outcome of previous liver ultrasound (OR:1,986; IC 95%: 1,178 – 2,457; p=0,001).
The remaining 10 were not statistically significant: age (p=0.06), gender (p=0.2), AHT (p=0.5), MD (p=0.06), Ac HBVc+(p=0.3), Ac HCV+(p=0.6), BMI (p=0.09), GOT (p=0.4), GPT (p=0.6) and Na (p=0.2).
The multivariate study showed that 6 of the 8 variables with statistically significant differences in the univariate were independent factors for NO validity. All except use of amines and dose of amines: DL (OR:4,767; IC 95%: 1,873 – 12,134; p=0,01), MPA (OR:2,734; IC 95%: 1,227 – 6,092; p=0,01), QPA (OR:3,989; IC 95%: 1,591 – 10,001; p=0,02), GGT (OR:1,01; IC 95%: 1,004 – 1,017; p=0,03), BrT (OR: 4,963; IC 95%: 1,853 – 13,289; p=0,04) and outcome of previous liver ultrasound (OR:4,727; IC 95%: 1,714 – 13,035; p=0,03).
Description of the cause of NO validity (Table 3)The 3 most frequent causes of NO validity of the graft were: Steatosis (n=29; 30.5%), Fibrosis (n=8; 8.3%) and macroscopic aspect of the organ (n=4; 4.2%).
The combination of factors most frequent were: Atheromatosis+Macroscopic Apect (n=6; 6.4%), Steatosis+Atheromatosis (n=6; 6.4%) and Steatosis+Ischemia (n=6; 6.4%).
Results of the biopsies of NO valid grafts(Table 4): All the NO valid grafts had biopsies. In 74 cases (77.9%) the findings confirmed the No validity.
The most frequent NO valid anatomopathological diagnosis was Steatosis (n=37; 38.9%), followed by Fibrosis (n=15; 15.8%) and Cirrhosis (n=8; 8.4%). Of the 74 (77.9%) grafts NO valid with pathological biopsy, on 55 (57.9%) cases the reason given by surgeon for NO validity coincides with the histological diagnosis of the biopsy and in the remaining 19 (20%), although the histological findings ruled out validity, these were diferente from those described macroscopically by the surgeon.
There were 21 (22.1%) grafts assessed as NO valid in wich the biopsy was reported without pathological findings. In other words, they had no reason, from the pathologist́s point of view, to be considered NO valid.
Study of the subgroups of NO valid grafts (Tabla 5 and 6).We studied and analyzed the 74 NO valid grafts that were confirmed by the pathologist and the 21 NO valid in which the pathologist did not find data to contraindicate the donation. We carried aout a univariate study with the variables included in the N.T.O. donation protocol. We did not find any statistically significant differences in any of the variables studied (Table 5).
When analyzing the subgroup of NO valid donors with non-pathological biopsy (n=21; 22,1%), we observed that the most frequent isolated cause of NO validity argued by the surgeon (Table 6) was Steatosis (n=4; 19%), followed by Atheromatosis (n=2; 9.5%) and the Macroscopic Aspect of the graft (n=2; 9.5%). The most frequent combination of factors in this subgroup was Atheromatosis+Macroscopic Aspect (n=4; 19%) followed by Fibrosis+Macroscopic Aspect+Others (n=2; 9.5%).
DiscussionIt is striking that so little has been written on such a controversial subject as the assessment of liver graft during the donation process. The bibliography regarding NO valid liver donors is scarce, and even more, studies that aim to determine risk factors of NO validity.9 Perhaps the reason lies in the fact that subjective data such as the macroscopic aspecto r the perfusión of the liver2 are used to evaluated the graft and we do not currently have any tool that surpasses them. It is, precisely the subjectivity of the transplant surgeon, the limitation of the study that we present since there can be a great variability inter-observer. We retrospectively described the findings of our series of NO valid biopsied graft and compared the clinical variables with the valid consecutive graft. However, we cannot compare biopsies because we do not have a biopsy during donation, prior to cold perfusión.
On the other hand, we intend to evaluate a subjective parameter such as the validity of a graft, not the risk of primary failure of the graft as the Donor Risk Index10 does.
The main objective of this study is the identification of risk factors for NO validity of liver grafts. This is, without a doubt, something extremely interesting that associates potential future uses in the elaboration of in situ graft valuation. Scores. Recently, the N.T.O. presented in the 2017 activity report age, pathological ultrasound and history of alcohol consumption as NO valid risk factors obtaines in its multivariate study.11 In our series, the only risk factor associated with the NO validity of the graft is the ultrasound. After carrying out the multivariate study, we added DL, MPA, QPA, GGT and BrT. Those what associate a greater risk are Br T (OR:4.963) followed by DL (OR:4.767) and the result of ultrasound (OR:4.727). Althouh we did not find age as a risk factors for NO validity in the multivariate study ans yes in the Spanish series,11 it was due to the fact that in the univariate study there was no statistical significance (p=0.06). In relation to the alcohol consumption, recognized in the national simple as a risk factor, we think it could correspond to the GGT figures in our series. Remember that there is a relationship between alcohol consumption and GGT values,12 so this could be a confounding factor. The Czerwinskís et al series,9 describes a greater number of risk factors for NO validity, among wich we only share the BrT. The rest are age, BMI, days spent in critical care units, hypotension peaks, natremia, alcohol consumption, GOT, GPT and INR values.
When analyzing the causes of NO validity argued by surgeon, we observed that Steatosis was the most frequent cause of NO validity both in our series and in the national simple analyzed by N.T.O. in 2017 (30.5% and 27.05%, respetively). The second in our series was Fibrosis (8.4%), while the national series it was Macroscopic Aspect (24.4%).11 In relation to the ítem Macroscopic Aspect we think that it is an ambiguous argument since an objective cause is not determinated to discard the graft. In the national series studied,11 the cause oh NO validity is nor related to anatomopathological diagnosis. In our series, 50% of the grafts discarded for Macroscopic Aspect presented a biopsy without pathological findings. While the remaining 50% presented findings compatible with cholostasis or cirrosis. Therefore, we question the ítem Macroscopic Aspect since in up to 50% of cases these organs do not associated histological findings of NO validity.
Another objective is to asses the concordance between the surgeońs judgment and that of the anatomopathologist for NO valid donors. In our series, it coincided in 74 (77.9%) of the 95 cases studied. When evaluating these results based on the 95 NO valid grafts studied, we found a correct diagnosis of NO validity in 74 cases (77.9%), a diagnosis of NO validity with biopsy without pathological findings justifying it in 21 cases (22.1%). In this sense, Czerwiński et al,9 publish a rate of grafts NO valid with biopsy without pathological findings indicating the NO validity of 35%, higher than that obtained in our series. However, it does not publish the cause of NO validity given by the surgeon, so it does not determine the confounding in its series. In the Table 6, we can see that the main confounding factor in the surgeońs assessment was Steatosis. We ruled out 4 (19%) grafts for Steatosis with biopsy without pathological finding. It is followed by Atheromatosis and Macroscopic Aspect of the graft with 2 (9.5%) graft each. On the other hand, Fibrosis, Cirrhosis, and por perfusión-ischemia are correctly assessed by the transplant surgeon.
We found no statistically significant differences when studying subgroups of NO valid grafts (Tables 5 and 6). Czerwiński et al9 identify significant differences between the two groups; alcohol consumption, BrT and GPT values.
Finally, we considered very interesting the study of all technology that is currently being developed in relation to ex situ perfusión machines, as new posibilities are opened for the rescue of these graft.14–17
Conflict of interestsThe authors of this article declare no conflicts of interest.
Sources of financingThe present investigation has not received specific aid from public sector agencies, commercial sector or non-profit entities.
.Please cite this article as: Castillo Tuñón JM, Marín Gómez LM, Suárez Artacho G, Cepeda Franco C, Bernal Bellido C, Álamo Martínez JM, et al. Factores de riesgo para injertos hepáticos no válidos. Estudio multivariante a partir de las variables recogidas en el protocolo de donación de la Organización Nacional de Trasplantes. 2020;98:591–597.