Esophageal cancer is the sixth most common cause of cancer-related mortality worldwide. Despite advances in diagnostic modalities and treatment options, five-year survival rates are below 20%. Esophagectomy with extended lymph node dissection is the mainstay of treatment. More than 50% of patients experience recurrence within 1-3 years postoperatively. Recurrent disease may present locoregionally at the site of anastomosis or as recurrence through lymphatic spread in lymph node basins, as hematogenic metastasis, or as a combination of these. The standard treatment of recurrence is currently predicated on systemic chemotherapy and/or radiotherapy. Recent evidence suggests that surgical treatment of metachronous oligometastatic disease may be prognostically advantageous over medical management alone. Given the considerably low response rates to chemoradiotherapy, many institutions have adopted surgical treatment strategies for oligo-recurrent disease on a case-by-case basis. The aim of this article is to review the current evidence on the role of surgical treatment for metachronous oligometastases from esophageal cancer.
El cáncer de esófago es la sexta causa más frequente de mortalidad relacionada con el cáncer en todo el mundo. A pesar de los avances en los medios de diagnóstico y las opciones de tratamiento, la tasa de supervivencia a los cinco años es menor del 20%. La esofagectomía con la disección de ganglios linfáticos extendida es la base del tratamiento. Más del 50% de los pacientes experimentan recurrencia dentro de 1-3 años después de la operación. La enfermedad recurrente puede presentarse como recidiva locorregional en el sitio de la anastomosis o como recidiva a través de diseminación en los ganglios linfáticos, como metástasis hematológicas o como una combinación de todos ellos. El tratamiento estándar de la recidiva se basa actualmente en la quimioterapia sistémica y/o radioterapia. La evidencia reciente sugiere que el tratamiento quirúrgico de la enfermedad oligometastásica metacrónica puede tener ventajas pronósticas superiores al tratamiento exclusivamente médico. Dada la tasa de respuesta considerablemente baja a la quimiorradioterapia, muchas instituciones han adoptado estrategias de tratamiento quirúrgico para la enfermedad oligorrecurrente caso por caso. El objetivo de este artículo es revisar la evidencia actual sobre el papel del tratamiento quirúrgico de las oligometástasis metacrónicas del cáncer de esófago.
Esophageal cancer (EC) is the sixth most deadly solid organ malignancy, accounting for nearly 400,000 deaths worldwide in annual basis.1–3 The most common histologic types are squamous cell carcinoma (SCC) and adenocarcinoma, with the former type being the most commonly seen type in Eastern countries.2 Esophageal surgery with extended lymphadenectomy is considered the cornerstone of treatment.2 Over the last decades, several treatment modalities have emerged, including the introduction of chemotherapy and the refinement of surgical techniques, but five-year survival is still below 20%.4 Despite these improvements the prognosis of EC remains poor, since this malignancy is frequently complicated by regional relapse or metastasis.5 Neoadjuvant chemotherapy with or without radiotherapy followed by esophageal surgery is the cornerstone of treatment for locally advanced EC.6 On the other hand, patients exhibiting metastatic or recurrent disease most commonly follow systemic chemotherapy with a five-year survival not surpassing 4–6%.7,8
Relapse may present locoregionally at the site of anastomosis or as recurrence through lymphatic spread in lymph node basins, as hematogenic metastasis, or as a combination of these. Patients with esophageal carcinomas frequently develop lymphatic, hepatic, cerebral, pulmonary, and osseous metastases.9 Of note, recent data support that the surgical treatment of oligo-recurrent disease may be implemented in selected subgroup of patients with isolated EC recurrence, who can be treated with curative intent.10,11 Oligometastatic disease is defined as a limited number of metastases in distant organs or lymph nodes for which local therapies may be implemented.12 Most studies agree that the number of metastatic lesions in oligometastatic disease may range from one to five in various fields.1,13 Oligometastasis can be synchronous, which is found during the initial EC diagnosis, or metachronous, which occurs after treating the primary lesion.4,14 However, no broadly accepted EC recurrence classification has yet been defined.5,15
Recent data have shown that operating on oligometastatic disease may substantially enhance patient prognosis,13 as seen in other types of malignancies, including non-small cell lung cancer16 and colorectal metastasis.13 Currently, there are no international or societal guidelines for the treatment of EC oligo-recurrence.17 Taking into consideration that response rates to chemoradiotherapy remain low for recurrent EC, many institutions have adopted to individualize surgical treatment strategies for disease recurrence.10 Therefore, in the present study, we sought to systematically review all available data assessing the outcomes of surgery for metachronous oligometastases from esophageal cancer.
Materials and methodsOur systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement. According to our predefined protocol, eligible studies were (i) randomized controlled trials, (ii) non-randomized cohort or prospective case series, (iii) retrospective studies, which were (iv) published in English and reported on the surgical management of metachronous esophageal oligometastases.
From the present analysis, we excluded reviews and meta-analyses, animal and in vitro studies, comments, editorials and letters providing no primary patient data, as well as published abstracts with no available full text online. No study sample restrictions were adopted in our search. For our review, we queried MEDLINE (through PubMed), Cochrane database, Scopus or Embase from inception up to June 15th, 2020. We used Boolean operators in combination with the following keywords: “surgery”, “surgical treatment”, “metastasectomy”, “esophageal”, “oesophageal”, “cancer”, “carcinoma”, “metastasis”, “metastases”, “recurrence”. We also hand-searched the reference lists of the articles that fulfilled the inclusion criteria to identify studies that might have been missed by the algorithm. The titles/abstracts of all articles were initially screened by two researchers independently (M.V. and M.S.) and any potential conflicts were resolved by a third researcher via discussion (D.S.). Then the full-texts of the eligible studies were retrieved and evaluated independently by two researchers (M.V. and M.S.), while discrepancies were similarly resolved through discussion with a third author (D.S.) when needed.
A total of 31 studies were deemed eligible based on our inclusion criteria (Fig. 1). Due to the high heterogeneity of the eligible articles, no quantitative pooling of the individual study results could be performed. Instead, the results are presented qualitatively by anatomic site.
Lymph nodesSolitary lymph node recurrence (SLNR) may reflect tumors with more favorable biological behavior, which may benefit from an aggressive surgical treatment.18,19 Based on a recent study, for patients with upper thoracic SCC lesions, lymph node metastases at the time of diagnosis are most frequent along the right recurrent nerve (60%) and cervical paraesophageal lymph nodes. For the group of patients with a middle thoracic tumor, the prevalence is highest along the right recurrent nerve (23%), right cervical paraesophageal lymph nodes (24%), and middle thoracic paraesophageal lymph nodes (23%). The lymph nodes along the left gastric artery (28%) and lower thoracic esophagus (23%) showed the highest prevalence of lymph node metastases for tumors in the lower thoracic esophagus.20 For adenocarcinoma and tumors of distal esophagus and GEJ, a prevalence of 30% in the periesophageal lymph nodes, 37% in the paracardial lymph nodes, 35% in the perigastric lymph nodes, and 14% in the celiac axis was reported.20
In patients with carcinoma of the upper esophagus, recurrence is frequently observed in the region of the neck. Conversely, in cases with carcinoma of the lower third of esophagus, intraabdominal lymph node recurrence is prevalent, when compared with patients with carcinoma in other parts of the esophagus.21 More specifically, several studies of the patterns of lymph node recurrence after radical surgery indicated that the nodes of the mediastinum was the most common site of recurrence (52.7% to 80.2%), whereas the incidence of intraabdominal lymph node recurrence was 12.2% to 14.1%; however the incidence of supraclavicular lymph node recurrence (SCLN) was controversial (range, 33.3% to 75.0%), with some studies reporting the latter as the most prevalent site of metachronous recurrence.22 The patterns of regional lymph node recurrence seem to be related to the tumor location and pN stage.22
There is also ongoing debate about the extent of lymphadenectomy based on different locations of lymph nodes recurrences. The AJCC TNM classification defines metastasis to lymph nodes other than regional lymph nodes, particularly supraclavicular nodes, as M1. However, many researchers think that supraclavicular nodes should be reevaluated as regional nodes, due to survival rates in patients with solitary metastases in this lymph node basin. Thus, they believe that in the upper thoracic EC patients, SCLNs appear to be regional nodes, and if there is no distant organ metastasis, the three-field lymph node dissection can initially be used. On the contrary, in patients with middle and lower thoracic tumors, SCLNs should be defined as distant metastasis (M1), and neoadjuvant therapy followed by surgery might be an available treatment method.23 The same concept seems to be validated in EC patients presenting with metachronous solitary supraclavicular lymph node metastases.
As far as studies reporting outcomes after surgical management of lymph node recurrence are concerned, Makino et al.,24 reported that the five-year survival rates of 24 patients with SLNR and 124 patients with non-SLNR were 37.8% versus 2.5%, respectively (P=0.0002). Ma et al.,25 in a sample of nearly 80 patients with cervical lymph node disease recurrence, compared survival rates of those who underwent surgery for lymph node recurrence with those who followed salvage radiotherapy and/or chemotherapy. Five-year survival rate was 50.1% and 12.6%, respectively, showing a statistically significant difference between the two studied arms.25 Likewise, Watanabe et al.,26 showed that overall survival of 17 EC patients who had resections of lymph node recurrence after esophageal surgery was 75.5%.26 Additional data suggest that patients with recurrence in cervical lymph node region exhibit superior survival when compared to patients with abdominal or mediastinal lymph node recurrence (P=0.0097).26 Wang et al.,27 studied a group of 66 patients with recurrent disease in the cervical nodes with SLNR or multiple lymph node involvement and found those patients who proceeded to salvage lymphadenectomy had superior prognosis vs those who underwent reduction surgery (P=0.004).27 Motoyama et al.,28 described ten patients treated with lymphadenectomy followed by radiotherapy and/or chemotherapy and demonstrated an average survival of 20 months following diagnosis of cervical lymph node relapse.
LiverOverall, 5–25% of EC patients will develop liver metastasis. Most commonly, these patients are referred for definitive chemotherapy.29,30 Due to the considerably low response rates to chemotherapy, many centers have started to combine chemotherapy with liver resection for low-tumor burden patients.31–33 While survival is limited to 6–8 months with chemotherapy alone, the combination with surgical extirpation for patients with up to three liver lesions seems to lead to a more favorable prognosis.30
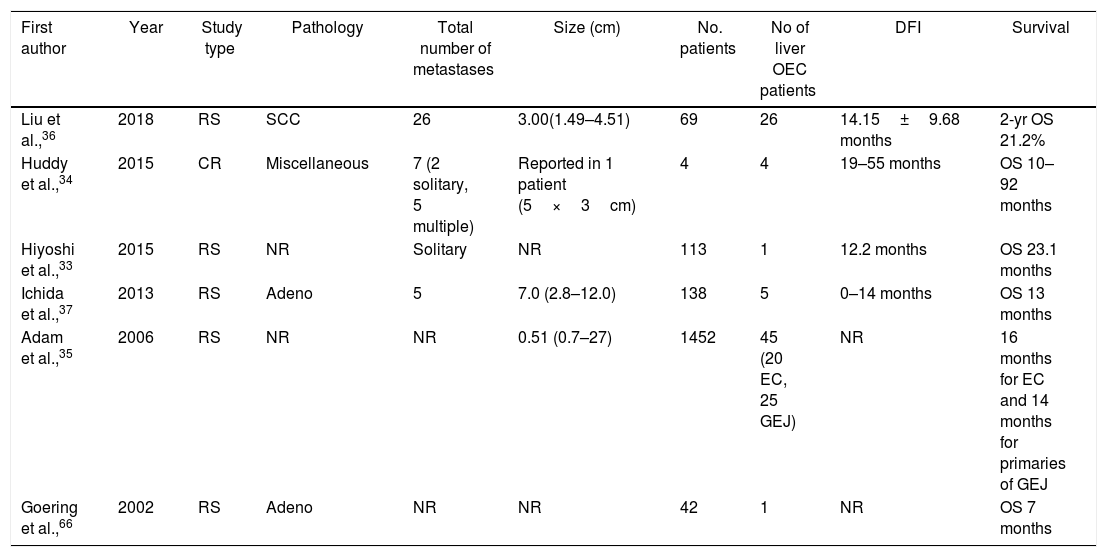
There are no available randomized control studies comparing chemotherapy alone versus chemotherapy plus liver resection for recurrent oligometastatic liver disease. Most data were drawn from small descriptive studies, which precludes safe conclusions30–34 (Table 1). Multidisciplinary tumor boards typically approve surgical excision of metachronous liver metastases from EC in patients with favorable ASA (American Society of Anesthesiology) scores and limited disease extent. Adam et al.,35 reviewed a large group of 1452 patients treated with resection for liver metastasis with no colorectal primary origin – 20 patients with metastases from EC and 25 patients with metastatic lesions from gastroesophageal junction carcinoma – reporting a 3-year survival rate of 32% and 5-year survival of 12%, respectively.35 The largest study was published in 2018 by Liu et al.,36 who compared surgically and non-surgically treated patients with hepatic resection for post-operative solitary liver metastasis from oesophageal squamous cell carcinoma (26 patients versus 43 patients) and reported superior survival in the operative arm (1- and 2-year survival rates: 50.8% and 21.2% versus 31% and 7.1%, respectively).36 Moreover, the same group concluded that longer disease-free interval (>12 months after esophagectomy) may be used as a predictor for improved prognosis following surgery for hepatic metastasis from EC. Furthermore, the role of surgical resection for metachronous liver metastases from EC was also highlighted by Ichida et al.,37 who reported a median overall survival of 13 months in 5 surgically treated patients with liver metastases.
Oncological outcomes of patients with recurrent disease in the liver treated with resection.
| First author | Year | Study type | Pathology | Total number of metastases | Size (cm) | No. patients | No of liver OEC patients | DFI | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al.,36 | 2018 | RS | SCC | 26 | 3.00(1.49–4.51) | 69 | 26 | 14.15±9.68 months | 2-yr OS 21.2% |
| Huddy et al.,34 | 2015 | CR | Miscellaneous | 7 (2 solitary, 5 multiple) | Reported in 1 patient (5×3cm) | 4 | 4 | 19–55 months | OS 10–92 months |
| Hiyoshi et al.,33 | 2015 | RS | NR | Solitary | NR | 113 | 1 | 12.2 months | OS 23.1 months |
| Ichida et al.,37 | 2013 | RS | Adeno | 5 | 7.0 (2.8–12.0) | 138 | 5 | 0–14 months | OS 13 months |
| Adam et al.,35 | 2006 | RS | NR | NR | 0.51 (0.7–27) | 1452 | 45 (20 EC, 25 GEJ) | NR | 16 months for EC and 14 months for primaries of GEJ |
| Goering et al.,66 | 2002 | RS | Adeno | NR | NR | 42 | 1 | NR | OS 7 months |
RS: retrospective study, CR: case report, NR: not reported, DFI: Disease-free interval, EC: esophageal cancer, OEC: esophageal cancer oligometastatic disease, GEJ: gastroesophageal cancer, OS: overall survival, SCC: squamous cell carcinoma, Adeno: adenocarcinoma.
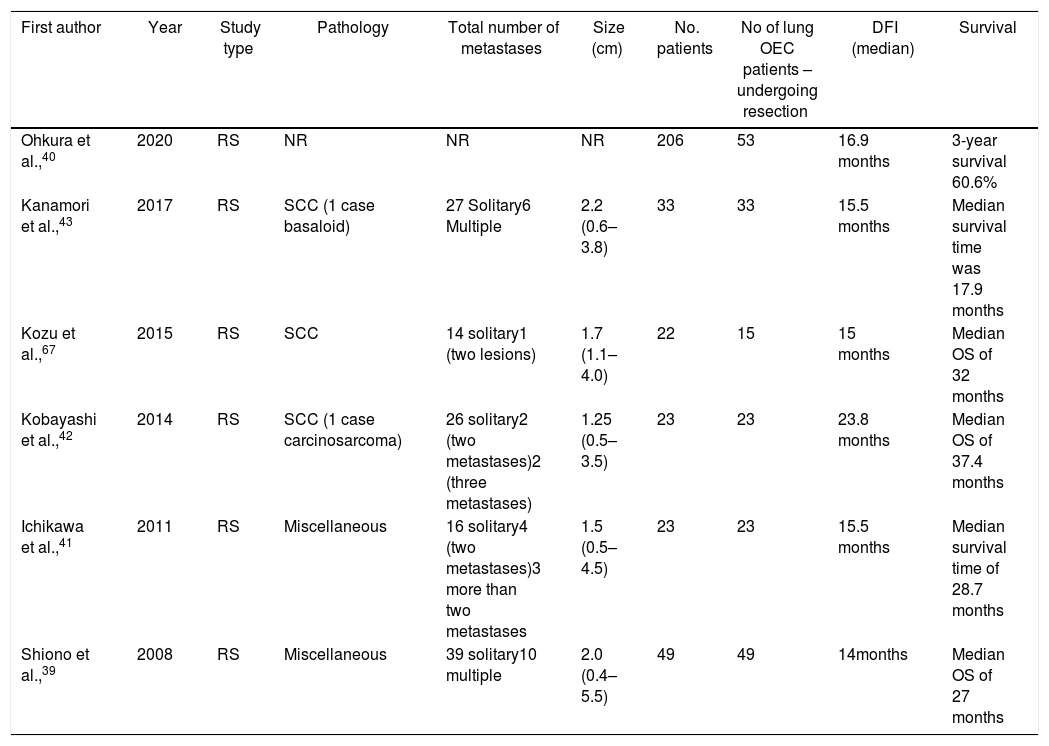
A growing body of literature has shown favorable outcomes after lung metastasectomy in metachronous lesions from EC (Table 2). According to a recent study, lung metastases are observed more frequently in SCC (57.4%) compared with adenocarcinoma patients (45.5%, P=0.059).38 One of the largest studies was conducted by Shiono et al.,39 who included patients with metastases mostly from SCC (n=48) undergoing surgical resection for metachronous lesions. The authors reported a 5-year overall survival of that reached 30%, also highlighting the worse outcomes for the group of patients with a disease-free interval shorter than 12 months.39 Similarly, Ohkura et al.,40 assessed the outcome of 53 patients reporting 3-year survival figures of 60.6, for patients undergoing metastasectomy. Ichikawa et al.,41 retrospectively reviewed a group of 23 patients with EC who were treated with surgery, reporting a median survival approaching 28.7 months and 1-, 3-, and 5-year survival rates of 73.9%, 43.5%, and 43.5% respectively.40 Kobayashi et al.,42 studied 23 consecutive patients who underwent 30 curative resections of metastatic lesions; a total of 18 patients underwent a single metastatic resection, three patients underwent 2 resections, and two patients underwent 3 or more metastasectomies. The mean survival time reported was nearly 38 months, with patients treated with repeat resection for recurrent lung metastases surviving a mean of 58 months (range 24–114 months). In this study, it was identified that development of extrapulmonary metastases before pulmonary recurrence, poorly differentiated type, and a decreased disease-free interval were unfavorable prognostic factors.42 Similar findings were reported by Kanamori et al.,43 who reviewed 33 patients undergoing metastasectomy. A total of 27 patients presented with solitary lung lesions, two patients presented with two lesions, and 4 patients had 3 or more lesions, while the overall median survival time was approximately 18 months.
Oncological outcomes of patients with recurrent disease in the lung treated with resection.
| First author | Year | Study type | Pathology | Total number of metastases | Size (cm) | No. patients | No of lung OEC patients – undergoing resection | DFI (median) | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Ohkura et al.,40 | 2020 | RS | NR | NR | NR | 206 | 53 | 16.9 months | 3-year survival 60.6% |
| Kanamori et al.,43 | 2017 | RS | SCC (1 case basaloid) | 27 Solitary6 Multiple | 2.2 (0.6–3.8) | 33 | 33 | 15.5 months | Median survival time was 17.9 months |
| Kozu et al.,67 | 2015 | RS | SCC | 14 solitary1 (two lesions) | 1.7 (1.1–4.0) | 22 | 15 | 15 months | Median OS of 32 months |
| Kobayashi et al.,42 | 2014 | RS | SCC (1 case carcinosarcoma) | 26 solitary2 (two metastases)2 (three metastases) | 1.25 (0.5–3.5) | 23 | 23 | 23.8 months | Median OS of 37.4 months |
| Ichikawa et al.,41 | 2011 | RS | Miscellaneous | 16 solitary4 (two metastases)3 more than two metastases | 1.5 (0.5–4.5) | 23 | 23 | 15.5 months | Median survival time of 28.7 months |
| Shiono et al.,39 | 2008 | RS | Miscellaneous | 39 solitary10 multiple | 2.0 (0.4–5.5) | 49 | 49 | 14months | Median OS of 27 months |
RS: retrospective study, OEC: esophageal cancer oligometastatic disease, DFI: Disease-free interval, OS: overall survival, SCC: squamous cell carcinoma.
Brain metastases develop in 1–3% of esophageal cancer patients.44 Song et al.,45 retrospectively reviewed 26 patients, and brain metastases were diagnosed in 4 patients with adenocarcinoma and 22 with SCC; twelve patients presented with a single cerebral metastasis, while 14 had multiple brain involvement.45 Five patients proceeded to surgery followed by whole-brain radiation (WBRT), five qualified for stereotactic radiosurgery, 13 received WBRT, and three received systemic chemotherapy. The median survival by treatment modality was: 7 months for the surgical treated patients, 4 months for the WBRT group, and 1.8 months for the chemotherapy treated patients.45 Weinberg et al.,46 reported the outcomes of 27 patients with brain metastases. WBRT was applied in 15 patients, surgery in 10 patients (four of whom also received WBRT), and 2 patients underwent stereotactic radiosurgery.46 Better survival rates were associated with patients with single brain metastasis undergoing surgery followed by WBRT (median survival of 9.6 months), while the median survival for patients with a solitary brain lesion who underwent surgery only was 3.8 months.46 Ogawa et al.,47 described 36 patients with documented brain metastases from EC, 12 out of which underwent surgery followed by radiation, and the rest were refereed for radiation only.48 The median survival approached 9.6 months for the surgery plus radiation group versus 1.8 months for radiotherapy only group.47 Yoshida et al.,49 described 17 patients with brain metastasis from EC, two of whom were treated with WBRT alone, seven with surgical treatment alone, three with surgical treatment followed by WBRT, and four with stereotactic radiosurgery. Seven patients survived for a median of 17.7 months postoperatively, while three patients survived for a median of 65.5 months after surgical resection plus radiation.49
Adrenal glandsDespite the fact that adrenal metastasectomy has been thoroughly validated for other malignancies, such as lung cancer, there is no clear indication as of now for EC.48 Abate et al.,50 reviewed the outcome of two patients who underwent resection with or without adjuvant chemoradiotherapy with isolated adrenal metastases reporting a surprisingly median survival of 18 months. Cho et al.,51 published a case of a 70-year-old patient who underwent esophagectomy for SCC and was diagnosed with a solitary adrenal metastatic mass 8 months after surgery; the patient underwent metastasectomy and remained disease-free for 42 months postoperatively. Fumagalli et al.,52 in a case series of five patients who presented with adrenal metastatic lesions from esophagogastric junction adenocarcinoma (metachronous in four cases), reviewed the clinical course of one patient with single adrenal metastasis who underwent adrenalectomy and eventually died 15 months postoperatively. Furthermore, O'Sullivan et al.,53 described a patient who grew a solitary adrenal metastatic lesion 4 years after esophagectomy for a gastroesophageal junction adenocarcinoma and remained free of disease for over 4 years post-adrenalectomy. Bui et al.,54 described three patients suffering from esophageal adenocarcinoma treated for a resected metachronous adrenal metastasis; the first 2 patients were alive and disease-free at 20 months and 5 years postoperatively, while the third presented with recurrence in mediastinal lymph node basins 3 months postoperatively.
DiscussionEven though our diagnostic and therapeutic arsenal has improved substantially in recent years, recurrence is seen in more than 50% of the patients within 1–3 years postoperatively.55 The mainstay of treatment for recurrence is usually systemic chemotherapy and/or radiotherapy. However, emerging data from case series and comparative studies have recently proposed the concept of surgical treatment for disease recurrence.
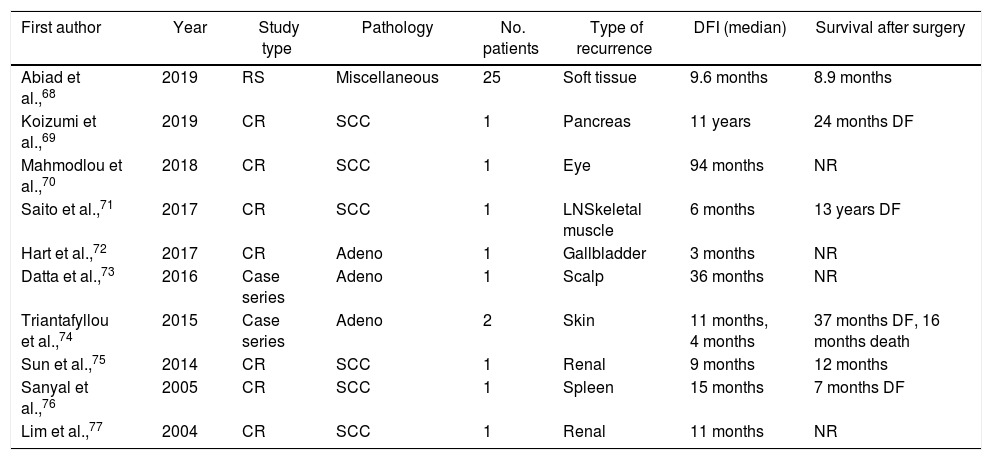
Recurrence is considered locoregional when detected at the site of the esophageal surgery and lymphadenectomy.56 On the other hand, distant recurrence occurs due to hematogenous spread to distant organs or involvement of lymph nodes other than the regional ones.4,56 Patients with esophageal carcinomas frequently develop lymphatic, hepatic, cerebral, pulmonary, and osseous metastases.4,9 Three potential pathways leading to lymph node metastasis have been described. The first is longitudinal expansion along the submucosa to locoregional and non-locoregional lymph node basins. The second pattern refers to the proliferation of tumor cells through the muscularis propria, while the third one comprises of perpendicular malignant spread through the muscularis mucosa to the thoracic duct and venous system. Moreover, venous drainage of the gastrointestinal tract via the portal vein and upper esophagus through systemic non-portal circulation promotes the migration of cancer cells in liver and lung metastases.57,58 Uncommon sites of recurrences include muscle, soft tissue, gallbladder, renal, spleen, pancreas, skin, eyes, heart, and the gastrointestinal tract48,59 (Table 3).
Oncological outcomes of patients with recurrent disease in miscellaneous sites treated with resection.
| First author | Year | Study type | Pathology | No. patients | Type of recurrence | DFI (median) | Survival after surgery |
|---|---|---|---|---|---|---|---|
| Abiad et al.,68 | 2019 | RS | Miscellaneous | 25 | Soft tissue | 9.6 months | 8.9 months |
| Koizumi et al.,69 | 2019 | CR | SCC | 1 | Pancreas | 11 years | 24 months DF |
| Mahmodlou et al.,70 | 2018 | CR | SCC | 1 | Eye | 94 months | NR |
| Saito et al.,71 | 2017 | CR | SCC | 1 | LNSkeletal muscle | 6 months | 13 years DF |
| Hart et al.,72 | 2017 | CR | Adeno | 1 | Gallbladder | 3 months | NR |
| Datta et al.,73 | 2016 | Case series | Adeno | 1 | Scalp | 36 months | NR |
| Triantafyllou et al.,74 | 2015 | Case series | Adeno | 2 | Skin | 11 months, 4 months | 37 months DF, 16 months death |
| Sun et al.,75 | 2014 | CR | SCC | 1 | Renal | 9 months | 12 months |
| Sanyal et al.,76 | 2005 | CR | SCC | 1 | Spleen | 15 months | 7 months DF |
| Lim et al.,77 | 2004 | CR | SCC | 1 | Renal | 11 months | NR |
RS: retrospective study, CR: case report, LN: lymph nodes, NR: not reported, DFI: Disease-free interval, DF: Disease-free, OS: overall survival, SCC: squamous cell carcinoma, Adeno: adenocarcinoma.
Although recurrence usually means widespread metastatic disease, a subset of patients only develop oligometastatic recurrent disease, which is defined as a state of limited metastatic burden, usually including from 1 to 5 lesions in various fields.1,40 In 1995 Hellmann, reported evidence of improved disease control and even cure when local therapy is implemented.1,60 Several locoregional therapies have been implemented for the management of metastatic lesions of other malignancies, such as colorectal cancer. Due to the latest improvements in perioperative care and surgical technique, the surgical resection of recurrent oligometastatic EC may be a reasonable, safe, and feasible approach.
Depyrene et al.,11 analyzed 766 consecutive patients with recurrent disease after curative esophageal resection for EC. Among patients with solitary organ metastasis, surgical resection (n=20) was associated with superior survival when compared to no surgery group (n=63), with a median survival rate after initial diagnosis of recurrence of 54.8 months versus 11.6 months, respectively (P=0.0004).11 Although no statistically significant difference was reached, patients who were operated on for isolated locoregional recurrence survived for an average of 75 months compared with the non-surgically treated group who had a median survival of about 17 months.11
Several studies sought to define prognostic factors in an attempt to determine the patient population who would benefit from surgical treatment of oligometastatic recurrence. More specifically, Procopio et al.,30 found that a disease-free interval longer than one year may be used as a criterion to select patients who could show favorable outcomes after surgical treatment of metachronous liver lesions. Several other studies have also demonstrated the prognostic value of longer time to recurrence in determining survival after surgical treatment of recurrence.1,4,42,43,56,61 Primary tumor TNM stage has also been suggested as another factor that may be associated with survival after surgery for EC recurrence.4,21,61,62 In patients with disease recurrence in the liver and lung, increased metastatic tumor size was also associated with worse outcomes after surgical therapy.4,37 On the other hand, good response to chemotherapy for the management of hepatic recurrence was identified as a predictor of biologically favorable type of disease and therefore, a potential selection criterion for curative liver surgery.34 Kaya et al.,63 reported that patients with distal gastroesophageal carcinomas and those receiving chemotherapy for ≥3 months might benefit from surgical management of metachronous metastatic disease. The primary location of the tumor has also been identified as a prognostic factor in patients with recurrent disease,10 while it has also been associated with the location of metastasis.39 In a registry analysis of gastroesophageal carcinomas treated surgically for metastatic disease, the presence of a HER2- positive primary malignancy treated with trastuzumab was the only factor predictive of superior survival.64
Limitations of the studies included in this review are their retrospective nature, the limited number of analyzed patients, leading to non-statistically significant and robust results, the associated selection bias and the heterogeneity of the baseline characteristics of the included study populations. Despite the fact that there are no strict guidelines regarding the therapeutic approach in each recurrence site, our study indicates that surgical management may be feasible in well-selected cases. Although recurrent disease after esophagectomy is common, a recommended treatment strategy and consensus algorithm is yet to be established. Patients with oligometastatic disease being free of disease longer than 12 months, with isolated one-field lymph node metastasis or solitary lesions in organs (i.e., liver, lung) might benefit the most from chemotherapy and surgical management of metachronous recurrence.1 However, the need for prospective studies to further elucidate the role of this combination, patient selection criteria, and consistent classification of EC recurrence to reach more meaningful conclusions is apparent. The multicenter FLOT3 study with metastatic gastroesophageal tumors showed that a subset of carefully selected patients might benefit from surgery following chemotherapy at the stage of oligometastatic disease.4 The FLOT5 study is now enrolling patients and aims to assess the effect of chemotherapy alone versus chemotherapy plus surgical resection on overall survival and quality of life in patients with oligometastatic adenocarcinoma of the stomach or esophagogastric junction.4,65
ConclusionPatients with oligometastatic recurrence after esophagectomy for EC have traditionally been managed with chemotherapy and/or radiation. However, emerging data suggest that carefully selected patients may exhibit more favorable survival outcomes after chemotherapy and surgical treatment. Future prospective studies will pave the way toward that direction and will allow us to draw more robust conclusions.66–77
Authors contributionsDS, MV, MS, IK, AK made substantial contributions to the conception or design of the work, IAZ, KSM, and TD revised it critically for important intellectual content, TD supervised this research project
Funding statementThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors report no conflict of interest.