Abdominal wall transplantation has been consolidated as an alternative to primary abdominal wall closure in intestinal and multiple organ transplant recipients. Given that it is feasible to obtain the visceral graft and the abdominal wall graft from the same donor, abdominal wall transplantation could offer satisfactory outcomes and be easily coordinated. Non-vascularized fascia is one of the alternatives for abdominal wall closure in transplantation. We report two cases of non-vascularized fascia transplantation in intestinal and multivisceral transplants, respectively. Both donors were young (23 and 18 years old). Both recipients had endured multiple previous surgeries, and no surgical alternatives for primary wall repair could be offered. In both cases, a complete abdominal wall flap was retrieved from the donor, however, due to the characteristics of the recipient's abdominal wall defect, only non-vascularized fascia was used after removing skin and subcutaneous cellular tissue from the graft. Abdominal wall transplantation is an option to consider for abdominal wall closure in patients with multiple previous surgeries and no alternatives for primary wall repair.
El cierre de la pared abdominal después del trasplante de órganos abdominales, especialmente en los casos de trasplante intestinal y multivisceral, sigue siendo un reto en muchos pacientes debido al importante número de intervenciones previas al trasplante, así como al edema de asas que dichos injertos presentan. Ante la imposibilidad de cierre primario y debido a las complicaciones en el uso de mallas, surge la opción del trasplante de la pared abdominal. Existen diversas opciones para dicho procedimiento, desde el empleo únicamente de la fascia no vascularizada hasta el trasplante de espesor completo de la pared abdominal. Revisamos la literatura en cuanto al uso de los injertos referidos y presentamos 2 casos de trasplante de fascia no vascularizada realizados en nuestra unidad de trasplante.
Primary abdominal wall closure after an isolated small intestine or multivisceral organ transplantation remains one of the most important challenges to resolve in this area. The great majority of these patients present wall closure complications, which can be attributed to the intestinal distension due to ischemia–reperfusion syndrome, associated intestinal edema and inelasticity of the abdominal cavity, which is generally reduced in volume after a history of multiple operations and associated infections, stoma placement and a high incidence of previous fistulae.1,2 These circumstances increase the risk of compartment syndrome, which can lead to ischemia or graft necrosis.3 As a result, some 20%–50% of recipients of this type of graft will require an alternative surgical technique to the conventional primary abdominal wall closure.4–6 In general, given the loss of wall structure in these recipients, they are considered poor candidates for reconstructive surgery, such as the separation of components or musculocutaneous flaps.
This can be resolved or treated by either reducing the size of the graft, or by expanding the capacity of the recipient. The general tendency to choose donors with lower weights, with a ratio between 1.1 and 0.757 or even to reduce the size of the grafts8,9 facilitates tension-free closure in many cases.1 Wall closure techniques using conventional mesh (absorbable or not) or biological mesh10 have presented disappointing results, probably due to a combination of tension in the closure and the effects of high doses of immunosuppressive drugs. The use of staged abdominal closure, advocated by the Birmingham group with 23 cases combining synthetic nylon prostheses (Silastic@) and negative pressure therapy, could be an alternative.11 Isolated skin closure is sometimes possible, despite the lower muscle layer not presenting as much elasticity. Interventions have even been proposed with a series of operations using expanders, which do not seem very recommendable due to the high complication rates (infection, hernia, fistula, seroma/hematoma, intestinal obstruction, mesh extrusion, etc.).12,13
The use of full or partial abdominal wall transplantation from the same donor as the intestinal or multivisceral graft, developed by Levi et al. since 2003,3 can be an interesting alternative in this context since they present obvious advantages in terms of obtaining a tension-free closure with a graft in normoposition that is well vascularized, avoiding the infectious complications of mesh that can lead to rejection (presentation as a maculopapular rash),14 all of which is achieved performed in a single surgery.2 The initial experiences of 15 and 17 patients have shown good results.2,15
Current State of Abdominal Wall Transplants in Organ TransplantationPartial Thickness TransplantNon-vascularized FasciaThere are two basic extraction techniques described in the literature:
- –
Miami technique3: this consists of the complete removal of the abdominal wall as if it were a complete graft, en bloc, to later separate the anterior lamina from the fascia of the rectus.
- –
Mount-Sinai/Favaloro technique16: a cross-incision is made in the subcutaneous tissue and skin, resecting the anterior lamina of the anterior rectus fascia en bloc through a bilateral subcostal incision with peritoneum.
In both cases, the graft becomes independent from the rest of the flap at the end of the extraction, after perfusion, which is not synchronous to the rest of the extracted organs. The graft is placed in preservation solution, and excess tissue and muscle are removed on the bench.
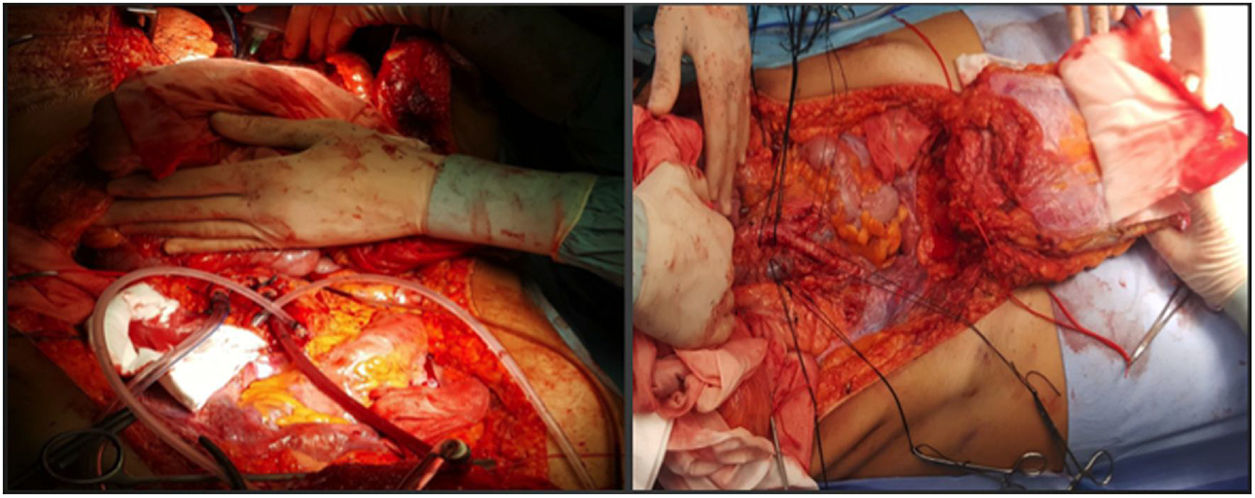
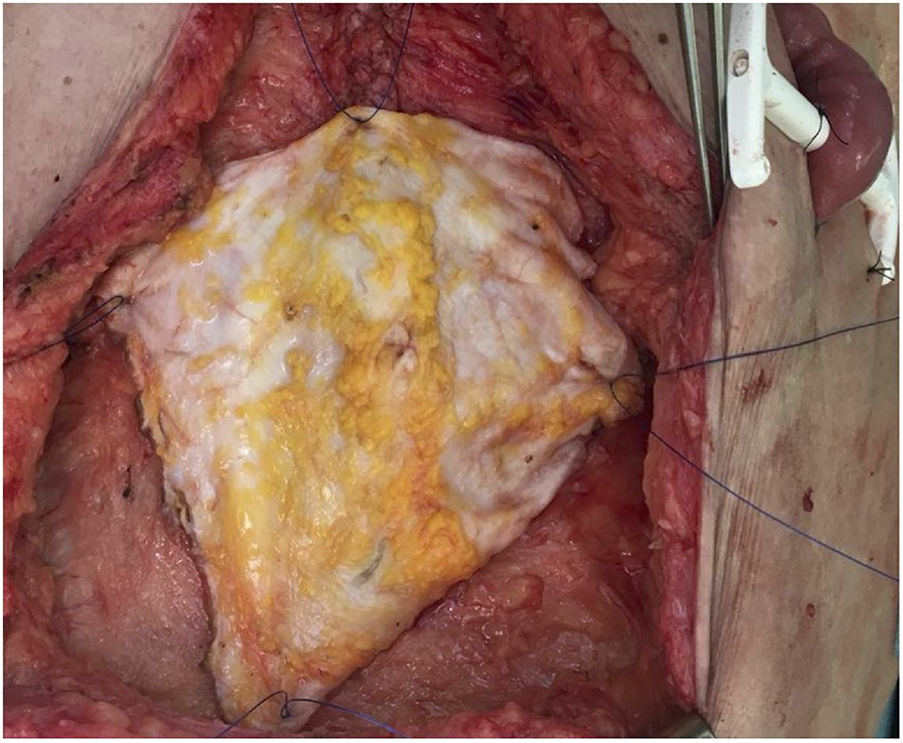
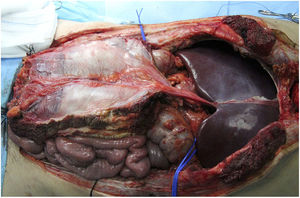
In our hospital, both external iliac arteries of the donor are cannulated, and the complete flap of the graft is perfused; subsequently, an extraction technique very similar to the Miami method is conducted. The objective is to obtain a complete graft from the donor (Fig. 1) to later decide the type of graft and wall closure required by the recipient (depending on the preoperative imaging tests, especially volumetric computed tomography [CT], which must be confirmed by the tissue state at the time of implantation).
Although most series perform perfusion of the wall graft with Wisconsin preservation solution, at our hospital we feel that the use of the Celsior solution is superior due to its lower viscosity, although there is not enough evidence in the literature in this regard.17
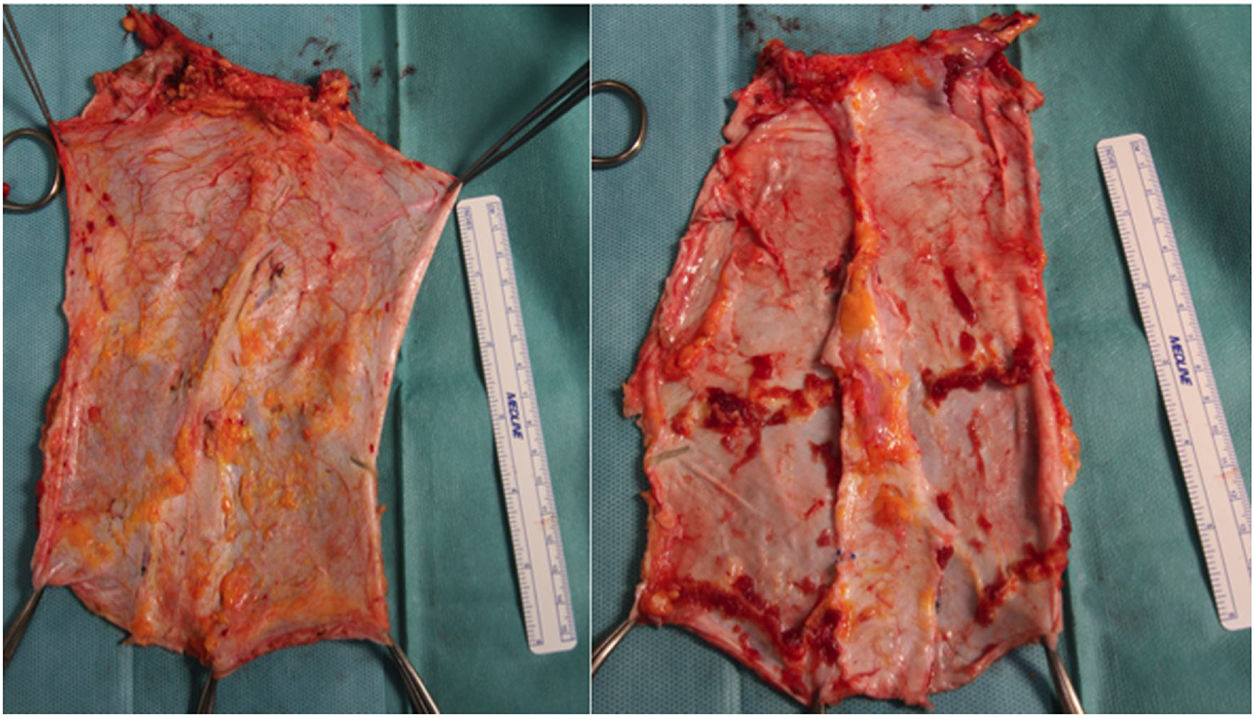
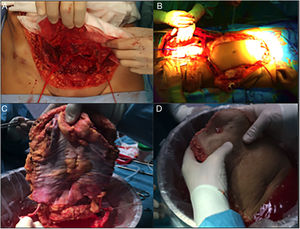
Subsequently, on the bench, the graft type is decided according to the needs of the recipient, and the unnecessary tissue is removed (Fig. 2). In the case of non-vascularized fascia, the excision of fat and muscle tissue is important because, without circulatory support, it would be very susceptible to developing necrosis and becoming a source of infection.
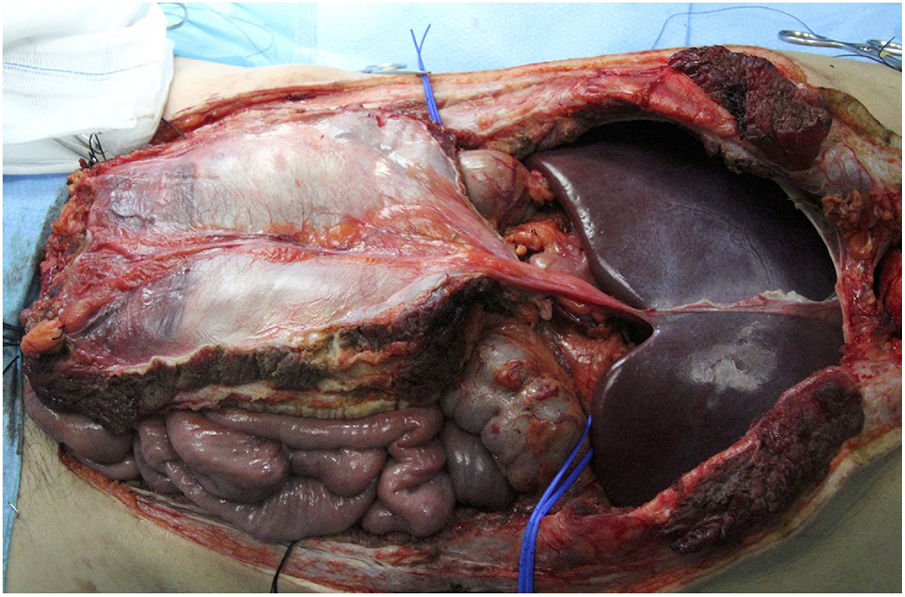
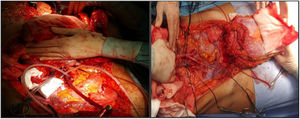
Vascularized FasciaThis is indicated in cases of isolated liver implants and those combined with intestinal transplants. Liver extraction would be performed in association with the falciform ligament and the posterior lamina of the rectus sheath, which would maintain a certain degree of flow from the artery of the falciform ligament (usually from the left liver) and drainage through the umbilical vein, with partial vascularization of the peritoneum and posterior lamina of the rectus sheath (Fig. 3). The artery of the falciform ligament appears in 67% of autopsies, although only in 2%–24% of angiographies.18 It seems a very interesting option in pediatric donors and recipients, since in adults it is obliterated. Apparently, this graft has a greater resistance to infection than non-vascularized fascia and greater integration with the surrounding tissue. The Chicago group19 needed to access the cavity of a recipient up to 3 times, observing good viability of the lamina (despite this, the patient died 51 days later due to a fungal infection). In the cases of Duke University,20 it was used as a sort of reinforcement mesh for closure, with no apparent complications in both cases (Table 1).
International Experience With Abdominal Wall Transplantation.
| Hospital | Type of Vascular Anastomosis | N of Cases | Complications |
|---|---|---|---|
| Tx entire wall thickness | |||
| Miami4 (2003) | Macrovascular iliac-iliac | 12 (6 IT; 4 MOT; 2 MMOT) | Thrombosis (2)Secondary closure (2)Infections (7) |
| Oxford2 (2008–2014) | Microvascular epigastric-iliac | 17 cases (12 IT; 5 MMOT) | Infections (6)GVHD (2)Acute rejection (5) |
| Bolonia21 (2005) | Microvascular epigastric-iliac | 3 cases (3 IT) | Lymphoproliferative syndrome (1) |
| Chennai (Vayda et al. Unpublished results) (2015) | Microvascular epigastric-iliac | 1 case (1 IT) | |
| Indiana (Viana et al. Unpublished results) (2013) | Macrovascular iliac-iliac | 1 case (1 IT) | |
| Groningen23 (2016) | Microvascular epigastric-iliac | 1 case (1 IT) | |
| Tx vascularized fascia | |||
| Oxford2 (2007) | 1 case (1 IT) | ||
| Chicago19 (2010) | 5 cases (1 LKT, 4 TH) | Sepsis (1)Secondary closure (2) | |
| Universidad Duke20 (2012) | 2 cases (2 MOT) | ||
| Tx non-vascularized fascia | |||
| Miami24 (2009) | 13 cases (6 MOT, 4 TH, 2 MMOT, 1 LIT) | Infections (7)Withdrawal (2) | |
| Oxford2 (2007) | 1 case (1 IT) | ||
| Argentina16 (2007) | 19 cases (13 IT, 4 MOT, 2 LIT) | Graft losses (3)Infection (7/17) | |
| Mount Sinai24 (2009) | 1 case (IT) | Sepsis (1) | |
| Berlín25 (2012) | 5 cases (5 MOT) associated VAC | ||
| Hospital 12 de Octubre (2018) | 2 cases (1 IT, 1 MOT) | Sepsis (1) | |
GVHD: graft-versus-host disease; LT: liver transplant; LIT: liver and intestinal transplant; LKT: liver and kidney transplant; IT: intestinal transplant; MOT: multiple-organ transplant; MMOT: modified multiple-organ transplant; Tx: transplant.
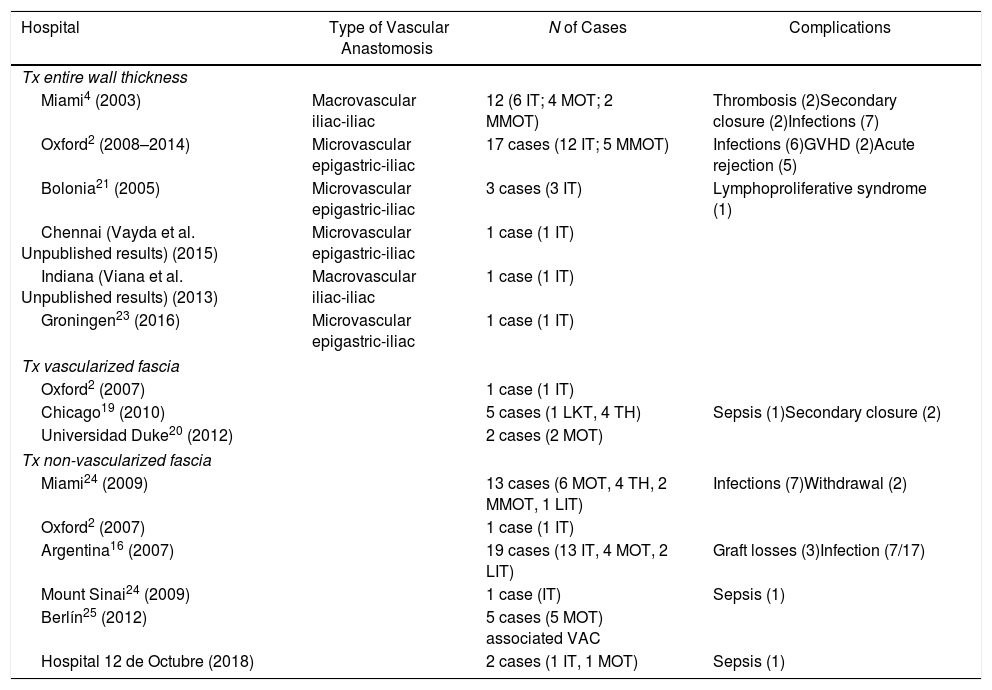
This seems the most physiological alternative from an anatomical perspective. Historically, it is the first type of wall transplantation that was carried out, which is surprising, given that it is the most complex technique, both for extraction and for implantation. The extraction is done in 2 steps, at the beginning and at the end of the multiple-organ extraction. From the beginning of the extraction, a full-thickness flap incision is made “at home”, leaving the musculocutaneous flap mobilized and connected to the donor by the inferior epigastric vessels. Once the multiple-organ extraction has been carried out, the aorta is cannulated and the wall graft is perfused prior to extraction and placement in ice with preservation solution (Fig. 4). In our hospital, selective cannulation of both external iliac arteries is preferred (ligating distal to the inguinal canal—femoral artery—and in the origin of the external iliac artery) and independent of the perfusion process of the rest of the organs to be extracted.
Ideally, it is the solution for patients lacking the abdominal wall and even skin for closure. It would therefore avoid the need to reduce the graft or to limit the pool of donors in terms of the graft-to-recipient weight ratio of 0.75. Given the weights of adult recipients in our environment, competition for pediatric donors is common, so the ability to expand the weight range guarantees a greater breadth and quality of available organs.
Reconstruction of the wall graft is performed in different ways, depending on the vascular and anatomical structure of the recipient wall. An anastomosis is usually used between a patch of the external iliac artery and the inferior epigastric artery of the donor with the bifurcation of the iliac arteries of the recipient because of its greater diameter. The Bolonia group22 uses a direct anastomosis between the inferior epigastric vessels of the donor and recipient, except in one of the 3 cases in which the superficial iliac circumflex artery was used due to an injury to the recipient's inferior epigastric artery.
The possible detection of intestinal or multivisceral organ rejection through wall biopsies has been proposed, although these may not be as sensitive or specific as intestinal biopsies (no rejection was detected in the case of Bolonia, even though it existed in the intestine); however, they could have great research value.22
Our ExperienceWe present the cases of 2 patients in whom it was impossible to perform primary closure of the abdominal wall or repair surgery due to several surgical procedures prior to the transplantation.
Case 1The patient is a 50-year-old woman who had been referred to our hospital due to short bowel syndrome secondary to multiple resections (6 interventions) for mesenteric ischemia. After 3 years as a candidate for intestinal transplantation and due to parenteral nutrition, she developed chronic liver disease and declining kidney function, making her a candidate for multivisceral transplant. The patient presented a very significant abdominal wall defect measuring 10cm in transversal diameter×15cm in length, with the consequent decrease in abdominal size.
A multiple-organ transplantation was performed following the standard technique. At the time of wall closure, the abdominal wall defect was insurmountable, associated with significant redundant skin, which provided for closure with the non-vascularized anterior lamina of the fascia of the rectus from the donor, without requiring the complete abdominal graft. The patient presented multiple infectious complications that led to her death (pneumonia and abdominal collections), with no apparent relationship with the abdominal wall on the imaging tests performed or the autopsy. No abdominal reoperation was required after the transplantation.
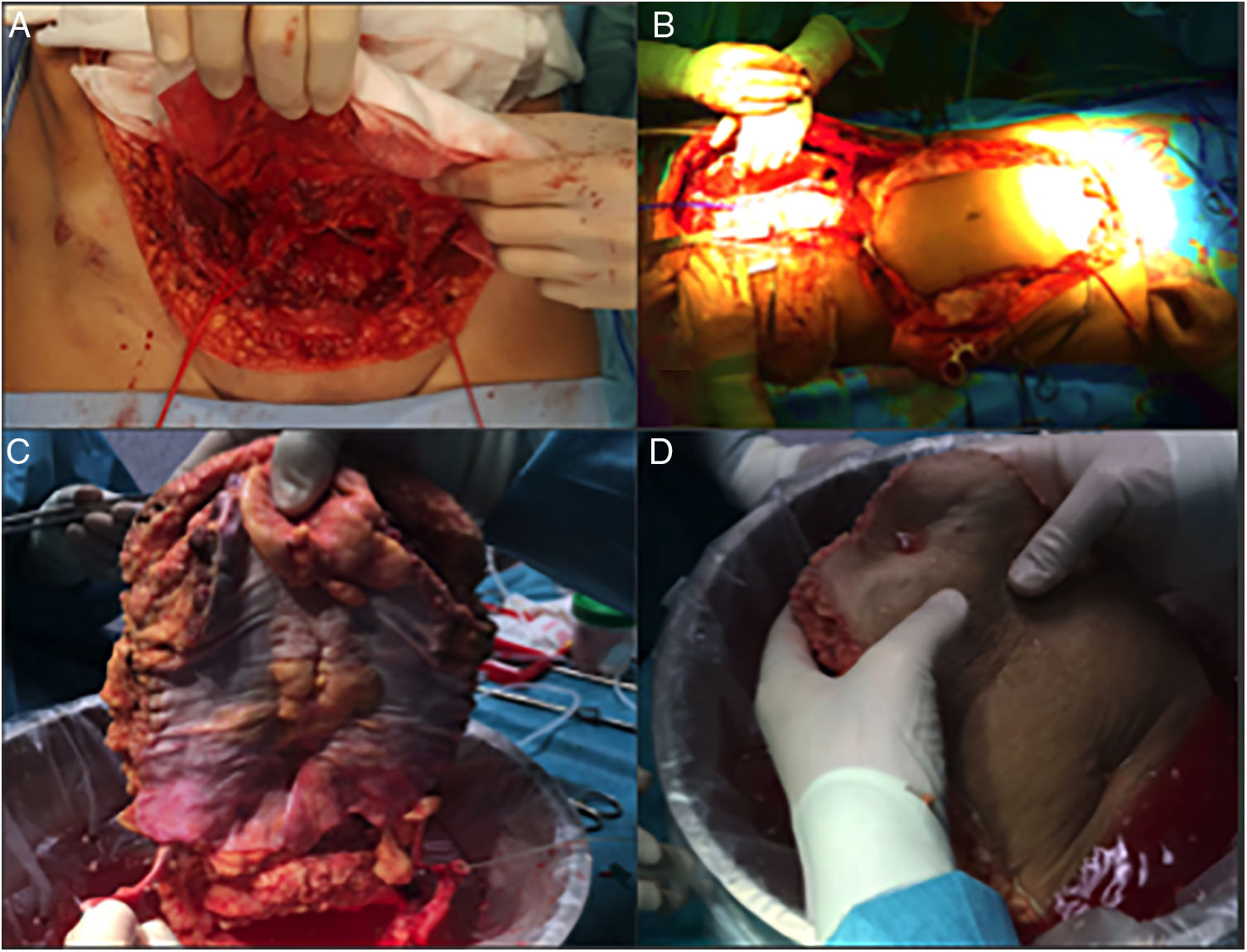
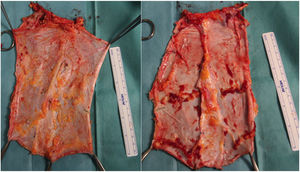
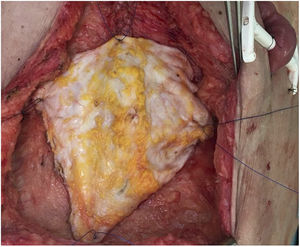
Case 2A 60-year-old woman was referred to our hospital due to a desmoid tumor with infiltration of the abdominal wall. She had undergone 2 previous laparotomies that confirmed the unresectability due to extensive involvement of the superior mesenteric artery. Two years after being added to the waiting list, an isolated intestine transplant was performed following the standard technique. At the time of closure, and due to the post-reperfusion syndrome of the graft, the abdominal wall defect was impossible to close (defect of 16cm in length×20cm in width). A non-vascularized transplant of the anterior lamina of the rectus sheath was conducted, which provided for primary skin closure (Fig. 5). The postoperative period was uneventful, except for an episode of mild rejection, which required the addition of everolimus to the immunosuppressive regimen with corticosteroids and tacrolimus. On the follow-up CT scan, excellent integration of the fascia flap was observed (normal CT scan, with no observed wall defect or bulging) with no collections or signs of infection.
DiscussionAbdominal wall transplantation has been consolidated as a valid alternative for wall closure in patients receiving another abdominal organ transplant. In up to 40% of cases, closure of the abdominal wall will complicate multivisceral or intestinal transplantation, so this issue needs to be resolved.26 The long-term results, especially for vascularized grafts, predict a good future, to the point of having demonstrated piloerection and a certain degree of autonomic muscle contraction in some grafts.27
The choice of one graft type or another is based on the need to repair the abdominal wall of the recipient. Depending on the size of the defect, the use of the graft that least complicates implantation is prioritized. In many cases, the defect is musculoaponeurotic and does not need the additional full thickness required by adding a double vascular anastomosis. The vascularized fascia alternative is limited to pediatric patients who require at least liver transplantation.
Although non-vascularized grafts present a higher incidence of complications, especially infectious, they seem a reasonable alternative, especially in older patients with a higher degree of atherosclerosis that hinders adequate vascular support or entails added risk when creating anastomoses.24 This atherosclerotic load should be measured in adult recipients by CT scan with arterial contrast when calculating the risk–benefit ratio.
In pediatric recipients, vascularized alternatives probably gain in importance. Benefits such as a potential diagnosis of rejection, or at least less invasive sampling with fewer potential complications, make it a very attractive alternative for abdominal wall closure in patients with multiple reinterventions28 and, at the outset, better vascular status.
The resistance and integration in the tissues of the different abdominal wall transplant types are striking, showing that these techniques are very solid wall closure alternatives, especially in the case of reoperations. In published series,22 they demonstrate significant strength, with a remarkable lack of adhesions of the intestines to the wall graft.16 In our series with non-vascularized grafts, the presence of macroscopic fibrosis in the necropsy of one patient and on imaging tests of the other demonstrated good integration without the need for vascular support.
New classifications such as that proposed by the Light et al. group29 could be useful in the preoperative planning of graft needs, as well as to be able to compare the different grafts beyond their vascular support.
The fact that multivisceral and intestinal transplants represent a very low percentage of the total influences the lower use of abdominal wall transplantation and has probably greatly limited its diffusion. However, the possibility to use this type of graft in liver transplant recipients with hostile abdominal walls could extend the indication. This is especially true given the incidence of complications with polypropylene mesh (and the fact that mesh cannot come into contact with the viscera) and the poor results with expanded polytetrafluoroethylene mesh in terms of reoperation and infection. The alternatives with biological mesh used at our hospital30 only presented acceptable results in pediatric recipients with a small wall defects, and at a very high economic cost, which is why they are no longer used in adult recipients.
The use of such a transplant without associated transplants of other viscera seems at least controversial given the needs for immunosuppression, and the alternatives with mesh and compounds are more viable in non-immunosuppressed patients.
ConclusionAbdominal wall transplants are a valid alternative in patients undergoing transplantation with previous abdominal wall damage (impeding the repair due to the loss of muscle layers) or mismatched donor/graft sizes with the need for graft reduction.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Justo I, Manrique A, Calvo J, Marcacuzco A, Caso Ó, García-Sesma Á, et al. Utilidad del trasplante de la pared abdominal en el trasplante de órganos. Nuestra experiencia inicial. Cir Esp. 2019;97:247–253.