Traumatic retroperitoneal injuries constitute a challenge for trauma surgeons. They usually occur in the context of a trauma patient with multiple associated injuries, in whom invasive procedures have an important role in the diagnosis of these injuries. The retroperitoneum is the anatomical region with the highest mortality rates, therefore early diagnosis and treatment of these lesions acquire special relevance. The aim of this study is to present current published scientific evidence regarding incidence, mechanism of injury, diagnostic methods and treatment through a review of the international literature from the last 70 years. In conclusion, this systematic review showed an increasing trend toward non-surgical management of retroperitoneal injuries.
Las lesiones traumáticas retroperitoneales constituyen un desafío para el cirujano de traumatología. Ocurren generalmente en el contexto de un paciente politraumatizado, con múltiples lesiones asociadas y en el que los procedimientos invasivos tienen un rol preponderante en el diagnóstico de estas lesiones. El retroperitoneo es la región anatómica que presenta mayores tasas de mortalidad, por lo que el diagnóstico precoz y tratamiento de estas lesiones adquiere especial relevancia. El objetivo de este trabajo es presentar la evidencia científica publicada hasta el momento en cuanto a su prevalencia, mecanismo lesional, métodos diagnósticos y tratamiento mediante una revisión de la literatura internacional de los últimos 70 años. Como conclusión, en esta revisión sistemática se pone de manifiesto una creciente tendencia al manejo no quirúrgico de las lesiones que afectan el retroperitoneo.
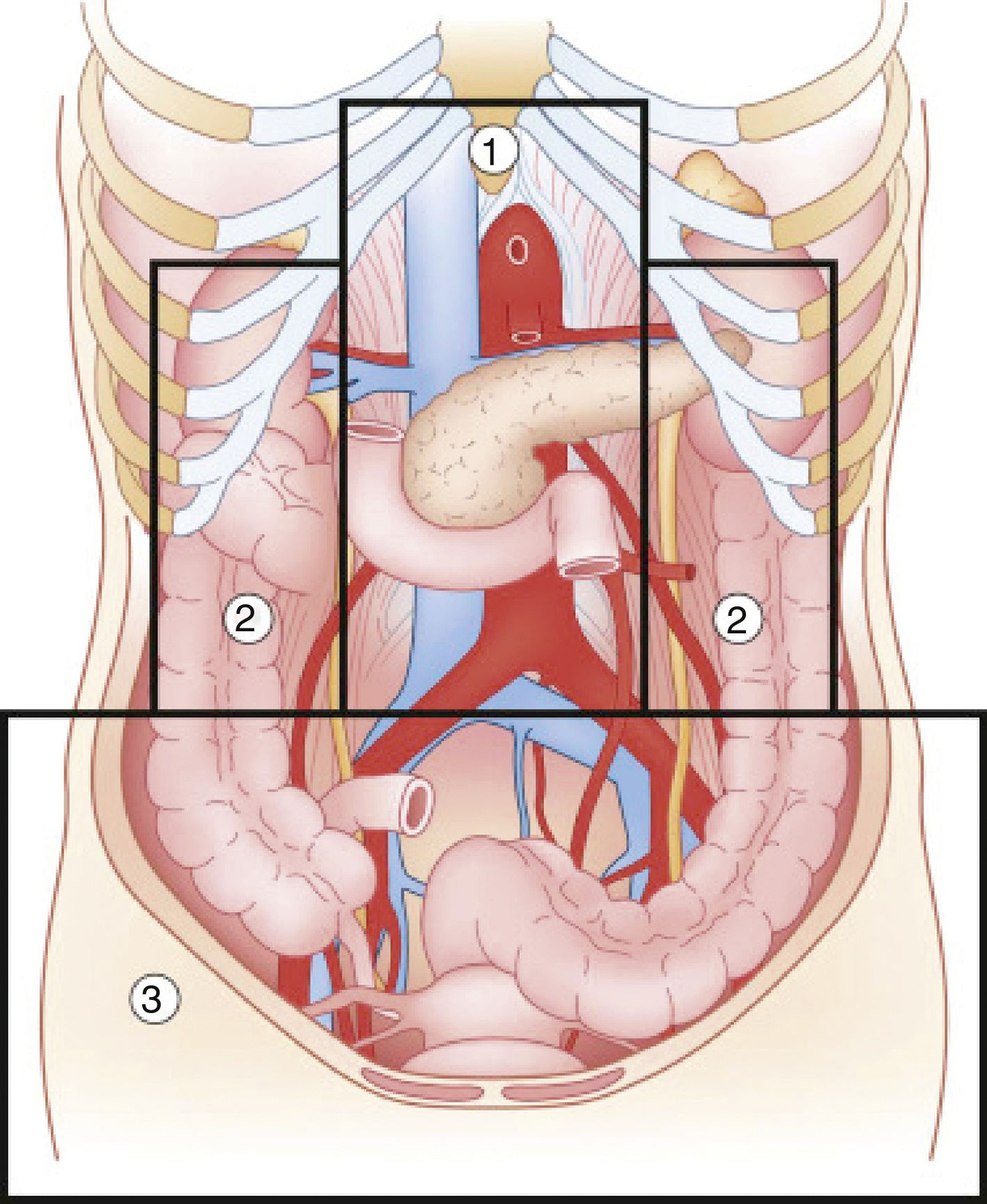
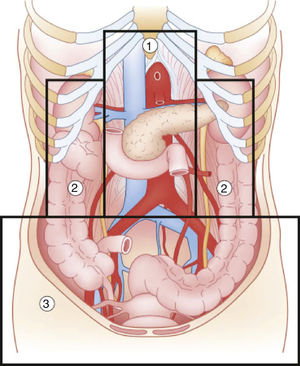
Abdominal trauma, both blunt and penetrating, occurs with a frequency of approximately 10% in torso trauma cases.1 The mechanism of injury varies depending on the country, socioeconomic status and culture. This trauma type is one of the main causes of morbidity and mortality in any age group. Trauma in the retroperitoneal compartment has the highest mortality rates.1 Considering its complex anatomy, the management of retroperitoneal injuries can vary widely (Fig. 1).2 The objective of this study is to carry out a review of the literature about retroperitoneal injuries, with an emphasis on their prevalence, diagnosis and management.
Diagram of the retroperitoneal zones (source: Martin et al.2).
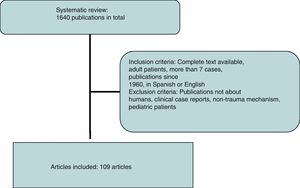
A systematic review of the literature was performed using the SCOPUS database under the criteria established by the reviewers, performing specific searches by organs using the following keywords: abdominal aorta, inferior vena cava, duodenum, pancreas, renal vessels, kidney, adrenal glands, ureters, and iliac vessels, associated with trauma. All publications in English and Spanish were included. Subsequently, a manual review was conducted from 1960 to date, excluding publications that did not refer to humans, clinical case reports or reviews of the literature, non-trauma mechanisms of injury and pediatric patients (Fig. 2).
PrevalenceThe incidence of retroperitoneal involvement in the literature is variable. In a study1 conducted on more than 6000 patients admitted to a specialized unit, 15% had abdominal involvement, 15% of which involved the retroperitoneum. Similarly, an incidence of 12%3 was reported in blunt abdominal trauma in hemodynamically stable patients by computerized axial tomography (CT). The kidney has been described as the most frequently affected retroperitoneal organ (18%), followed by the pancreas (3.7%) and the aorta (1%), with a predominance of blunt trauma over penetrating.1
While demonstrating a variable incidence, the abdominal organs most frequently affected by penetrating trauma are the liver and colon, followed by vascular injuries and the pancreas.4
Zone 1Zone 1, or the central zone, is delimited by the diaphragm above and reaches the aortic bifurcation below. It includes the aorta, the origin of the large vessels, the duodenum and the pancreas. Blunt trauma to this region affects the duodenum and the pancreas to a greater extent, with vascular lesions being less frequent. Most of the series analyzed report a duodenal injury rate that does not exceed 12%.4–9 Pancreatic injuries have an incidence that ranges between 1% and 12% of penetrating trauma, and 5% of blunt trauma.10 Mortality ranges from 10% to 46%, with ductal damage being an important predictor of morbidity and mortality.10 Regarding the mortality due to duodenal injury, some series report ranges from 15% to 47%, which increases to 67% with seven or more associated organs injured.4 The most frequent complication is duodenal fistula or dehiscence.
Among the vascular lesions, inferior vena cava injury stands apart, representing 30%–40% of abdominal vascular injuries. Their overall mortality rate varies from 34% to 70%, and factors for morbidity and mortality include both the level of the injury and the existence of active bleeding or other associated lesions.11 Mortality due to suprahepatic and retrohepatic lesions varies between 78% and 100%, while adrenal injury mortality ranges from 33% to 66% versus 25% mortality in infrarenal vein cava involvement.11,12 Prehospital mortality rates are reported to range from 30% to 50%, and these figures are maintained after hospital admission.12 Abdominal aorta injuries are around 0.2%,13 and its high immediate mortality rate is the second most common cause of death in blunt trauma injuries. It is estimated that 80% of patients die before hospital care, and between 50% and 78% do so after.14,15
Zones 2Zones 2, or the lateral zones, are the areas between the diaphragm and the aortic bifurcation, delimited medially by the renal vessels and laterally with Toldt's fascia, so they therefore encompass the adrenal glands, kidneys, renal vessels, ureters, and a portion of ascending and descending colon.
The incidence of renal injuries stands out in this anatomical region at approximately 1.2%.16 Few urological lesions are life threatening, although they represent some of the most frequent complications. However, adrenal injuries have a lower incidence, situated at around 0.4%.17 Renal vascular damage, on the other hand, occurs in less than 5% of blunt traumas. Mortality associated with renal trauma is between 5%18 and 11%,16 while the mortality rate secondary to arterial injuries reaches 20% in some series.19 Mortality associated with the adrenal gland is very variable, ranging from 8%20 to 32%. It is noteworthy that the mortality rate associated with unilateral injury is 0% in certain studies.17,21
Zone 3Zone 3, or the pelvic zone, is delimited by the aortic bifurcation above. It includes the iliac vessels, distal ureters, distal sigmoid colon and the rectum.
The incidence of iliac vessel injuries is less than 1%. Specifically, they represent between 2% and 6.5% of vascular lesions.22 The associated mortality ranges between 25% and 42%, although in some series this figure can reach 62%, presenting higher mortality rates in penetrating trauma injuries. Within this group, mortality increases in combined arterial and venous injuries (43%–62%) versus isolated venous injuries (6%–45%).22,23
Mechanism of InjuryZone 1According to the data obtained from the review of the literature, penetrating trauma is most frequently associated with aortic injuries. The most frequently associated vascular lesions affect the inferior vena cava, and only 2% of the cases present isolated vascular injury.15 The most common aortic injury is intimal disruption, which may be partial or involve the entire aortic circumference, and may associate thrombosis and/or dissection. If the complete thickness is affected, this may lead to the formation of an aneurysm or rupture and subsequent death by exsanguination. The infrarenal aorta represents 50% of cases, however, this region has the lowest mortality rates.15 In the same way, vena cava injuries occur in more than 80% of cases with penetrating trauma, and gunshot wounds are the main mechanism.12 The adrenal region is the most frequently affected, while in blunt trauma there is an increase of retrohepatic lesions.11
In the review of 13 published series, the most frequent mechanism of duodenal injury was penetrating, with 1550 cases (90.7%) out of a total of 1709 patients.4–9,24–30 The data reveal the higher incidence of firearm injuries over knife wounds. In blunt duodenal trauma, there is an increased association with pancreatic lesions, with fewer hepatic and colic injuries.25 Within this group, there is a predominance of traffic accidents over falls. Underlying these lesions is the shear force mechanism between fixed and mobile portions, as well as compressive forces against the spinal column.31 Penetrating pancreas trauma occurs in an isolated manner in 11% of cases. The second duodenal portion is the most frequently affected, with a variable incidence of 35%–62.5%, followed by the 3rd and 4th portion (12%–28%), with the first duodenal portion being the least affected.5–8,25,28,30,31 The most common pancreatic injury affects the body and tail versus the head and neck of the gland. The American Association for the Surgery of Trauma proposes aclassification according to 5 degrees of duodenal and pancreatic injury.32
Zones 2In the United States, between 80% and 95% of renal trauma occur due to traffic accidents, falls or assaults.18 In all the analyzed series, the rate of penetrating injuries is lower, with the proportion of injuries by gunshot versus stab wounds being variable. The most frequent is the presence of contusions, followed by lacerations, renal ruptures and vascular pedicle injuries. In adrenal lesions, theoretically there is an increase in the inferior vena cava pressure, which would explain the predominance of right-sided injuries.17,20,33–36 Other mechanisms described are deceleration, affecting the adrenal arterioles, and crushing between the spinal column and neighboring organs, which is associated with a greater probability of associated ipsilateral lesions.20,37 Ureteral injuries are uncommon, accounting for only 17% of penetrating urological trauma, and the blunt mechanism is a rare cause of injury.38
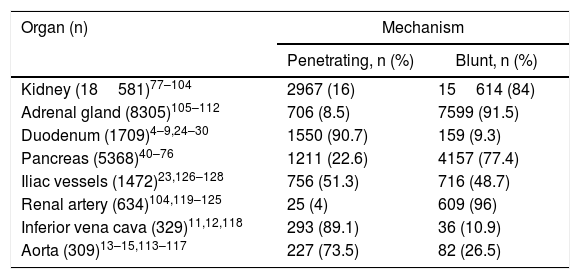
Zone 3Penetrating trauma is the main cause of injury, with isolated venous injury being the most common (41%),22 followed by isolated arterial injury (33%) versus the combination of both (25.5%). In 73% of blunt trauma, there is an association with pelvic fractures, which increases the probability of genitourinary and intestinal injuries. The reported incidence of vascular injury is 3.5% in complex pelvic fractures, which increases to 7% in severe fractures.39Table 1 shows the most frequently affected organs according to the mechanisms of injury and age ranges.
Organs Affected According to the Mechanism of Injury.
| Organ (n) | Mechanism | |
|---|---|---|
| Penetrating, n (%) | Blunt, n (%) | |
| Kidney (18581)77–104 | 2967 (16) | 15614 (84) |
| Adrenal gland (8305)105–112 | 706 (8.5) | 7599 (91.5) |
| Duodenum (1709)4–9,24–30 | 1550 (90.7) | 159 (9.3) |
| Pancreas (5368)40–76 | 1211 (22.6) | 4157 (77.4) |
| Iliac vessels (1472)23,126–128 | 756 (51.3) | 716 (48.7) |
| Renal artery (634)104,119–125 | 25 (4) | 609 (96) |
| Inferior vena cava (329)11,12,118 | 293 (89.1) | 36 (10.9) |
| Aorta (309)13–15,113–117 | 227 (73.5) | 82 (26.5) |
The retroperitoneal location helps unmask the early clinical signs found in the diagnosis of the usual injuries.31 Some of the most frequent symptoms are pain and abdominal distension, although up to 8% of patients remain asymptomatic.130 Both peritoneal aspiration and focused abdominal ultrasound (Focused Abdominal Sonography for Trauma) can be positive, although they may give both false negatives and false positives.25,26 In hemodynamically stable patients, the use of CT with oral and intravenous contrast stands out, with high sensitivity and specificity, although in 40% of cases of early pancreatic trauma this test may be normal.
Since most duodenal injuries occur in the context of penetrating trauma, their diagnosis is made during exploratory laparotomy. The findings that suggest duodenal injury are the presence of bile, crepitations in the duodenal fascia and the presence of retroperitoneal or perirenal hematoma31 as well as necrosis of peritoneal fat and peripancreatic edema.30,130 These latter findings are also related to ductal pancreatic lesions together with the presence of transparent fluid in the bed. Additionally, in the diagnosis of vascular injuries, there may be the presence of a pulsatile mass, reduction in or absence of femoral pulses, coldness, cyanosis or weakness of the lower extremities.13 Neurological symptoms are described in around 70% of patients presenting aortic injury, and shock rates of up to 82% in the largest published series.15
The presence of both macro- and microscopic hematuria varies between 66%39 and 96% of patients according to some series, although other indirect signs may be noted, such as pain in the affected flank or signs of hemodynamic instability. When renal injury is suspected, the most widely used diagnostic method in hemodynamically stable patients is CT with intravenous contrast, which provides for classification of the lesions and rules out contrast extravasation.129 This not only demonstrates active hemorrhage but is also related to greater kinetic energy and therefore is an indicator of the severity of the associated injuries.20 In adrenal injuries, the most frequent finding is an oval hematoma measuring 2–3cm,37 and they present more than 90% of associated injuries. In addition to the use of CT and angiography, the use of urethrography is recommended in the presence of perineal hematomas or pelvic ring disruptions.
TreatmentThe initial assessment and management of patients presenting with abdominal trauma are based on the indications established by the Advanced Trauma Life Support (ATLS®) guidelines of the American College of Surgeons. The initial objective of surgical management is based on 3 points: controlling the site of the hemorrhage, controlling gastrointestinal contamination and reviewing the abdominal cavity for diagnostic purposes.
Isolated vascular injuries with no indication for urgent laparotomy may benefit from non-operative management, including intravascular therapy, while surgery is indicated in cases of bleeding or arterial thrombosis. In the series by Lopez-Viego et al.,15 the procedure most commonly used is arteriorrhaphy (53%). For the approach of the supramesocolic region, the Mattox maneuver is used, which involves the medial mobilization of the kidney and left colon along with the spleen and tail of the pancreas. As an alternative, the Kocher maneuver is used, although its evaluation through the smaller omentum is possible. In contrast, the approach of the inframesocolic region requires cephalic and medial mobilization of the small intestine and right colon, known as the Cattle–Braasch maneuver, which facilitates the evaluation of the portal vein and inferior cava.
Proximal and distal vascular control of the injured vessel is imperative, including proximal control at the supraceliac level of the aorta at the esophageal hiatus. In unsuccessful cases or when this is not possible, left thoracotomy is performed with thoracic aortic clamping or placement of an intravascular occlusion balloon (Resuscitative Endovascular Balloon Occlusion of the Aorta).130 Recent studies suggest that this device is an effective method for the treatment of hemorrhages in which direct compression is not possible, providing temporary aortic occlusion and temporary control of bleeding with an increase in mean blood pressure. In venous injuries, lateral venorrhaphy is recommended regardless of its location and when possible, including injuries of the mesenteric veins and inferior vena cava, provided that doing so does not endanger the patient's life, although ligature of the inferior cava in zone 1 has been described as a last resort.131
The most commonly used technique in the repair of iliac artery injuries is primary arteriography, followed by bypass. Meanwhile, in venous lesions, the use of ligation prevails over venorrhaphy.23 The largest published series has demonstrated a greater use of intravascular techniques in blunt trauma. Embolization of the internal iliac artery is not associated with higher rates of short-term complications, although in the long term an increase in the incidence of paresthesia is observed.128 As for the duodenum, when the hemodynamic situation and absence of associated injuries allow, conservative treatment can be proposed, especially patients with maintained duodenal wall integrity.9,129 For correct exploration of the 4 duodenal portions and its anterior and posterior sides, it is necessary to perform the Kocher maneuver, the Cattell–Braasch maneuver, or both, and division of the ligament of Treitz may be necessary.6,31
Multiple surgical techniques have been employed, ranging from primary closure to the Whipple technique. All authors agree on the recommendation of primary closure as the initial choice technique, which is successful in up to 85% of patients and should preferably be transversal, with no tension. More complex procedures are reserved for the severest injuries.5–9,25 If this is not possible, resection can be considered with primary end-to-end anastomosis or anastomosis of a jejunal loop over the defect itself. In grade I or II pancreatic injuries, it is recommended to initially opt for conservative treatment, even when the finding is intraoperative. In grade III or IV lesions, the surgical approach is recommended, for which several options have been proposed, from simple suture to segmental resection; the decision should be individualized for each case, taking into account both the extent of the injury and the surgeon's experience. The initial maneuvers involve hemostasis and debridement of the necrotic tissue, which may require pancreatojejunostomy or pancreaticogastrostomy to avoid pancreatic insufficiency. The Whipple technique is reserved for unreconstructable injuries, large tissue losses, severe duodenal or head of the pancreas injuries, or in cases with transection of the proximal Wirsung duct in the neck of the pancreas, averting resection of the spleen and tail of the pancreas to preserve its endocrine and exocrine function.9,25,26,31,132 On the other hand, the risk of anastomotic leakage and morbidity is significant, as is the learning curve of the technique, which is more complex than resection.133
In renal trauma, conservative treatment is advocated in hemodynamically stable patients with no other surgical indication,82 with a success rate surpassing 90% for grades I to IV, and up to 35% for grade V.88 There is an increase in the use of embolization, with failure rates of less than 15%. Delays in surgical indication have not been shown to be associated with a higher incidence of complications in the most current series.86 Conservative treatment of a damaged but perfused kidney in selected patients results in less morbidity than nephrectomy. The non-surgical treatment of renal lacerations due to blunt trauma associated with extravasation is associated with fewer complications, which can generally be treated by endourological or percutaneous methods. In lacerations due to penetrating trauma, non-surgical treatment may be considered in stable patients, with no associated lesions and correctly staged, with a high index of suspicion to avoid ureteral injuries. The success of non-surgical treatment can be improved with the use of angioembolization.18,82,83
Surgical treatment ranges from the use of nephrorrhaphy to nephrectomy, either partial or total, with variable rates between series (13%–38%).94,96 Cases have been described requiring delayed nephrectomy due to post-traumatic complications. There is an association between the degree of renal injury of the American Association for the Surgery of Trauma and the significant decrease in post-traumatic renal function from grade III on.92 In the management of avulsions and renal lacerations, embolization is proposed as a safe alternative to surgical treatment, citing success rates higher than 94%.19,121 Another consideration would be to perform angioembolization to avoid nephrectomy and the loss of renal tissue after other surgical procedures. In renal vascular lesions, there is a growing tendency toward conservative management; the percentage of nephrectomies is variable, surpassing 30% in some series compared to percentages of around 18% in the most recent publications. In the series by Sanghtong et al.,120 only 27% of the total patients underwent surgical exploration of the renal artery, 67% of which underwent nephrectomy versus 32% vascular reconstructions. The use of vascular reconstruction is controversial, with percentages of late nephrectomies of up to 35% being cited, while currently it is not used in the presence of a functioning contralateral kidney.19
Most publications about ureteral injuries are composed of case reports and small series, so there is no consensus for their management. It seems clear that the presence of shock upon admission or severe colon injury requiring colectomy precludes ureteral repair, so diversion or primary nephrectomy are proposed in these circumstances.
Regarding the management of adrenal trauma, this will depend on the extent of the damage, state of the contralateral gland, hemodynamic status and associated injuries.33,35,37 Conservative treatment is the most widely accepted, relegating surgical treatment to the presence of associated injuries or hemodynamic instability.20,33,36,37 With active extravasation of contrast during angiography and patient stability, embolization has been proposed as a safe alternative comparable to surgery.33 The complex adrenal vascularization allows for selective embolization to lead to a localized infarction that does not lead to glandular insufficiency. In the largest series to date, it is estimated that around 1% of adrenal lesions require adrenalectomy.17 Other than excision, procedures to achieve hemostasis are suture of the affected gland or the application of hemostatic agents and packing.
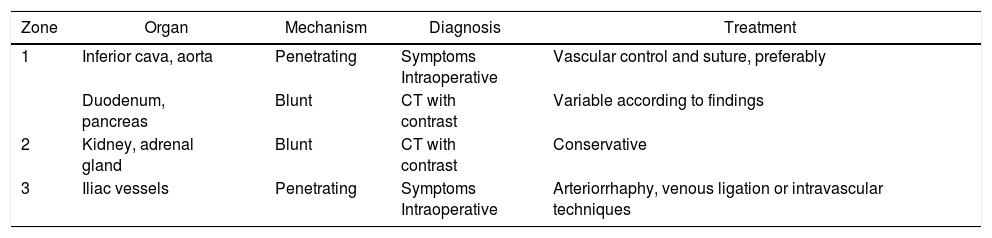
Finally, in the management of bone injuries, the main objective is to achieve hemostasis as well as to restore the integrity and stability to the pelvic ring before definitive stabilization.134 In minor pelvic lesions with mechanical and hemodynamic stability, non-surgical management can be considered. In contrast, in moderate lesions (with hemodynamic stability and mechanical instability) after radiological diagnosis, the possibility of embolization can be considered individually when faced with surgical treatment or conservative management. Severe pelvic lesions are characterized by hemodynamic instability, regardless of the mechanism of injury according to the classification of the World Society of Emergency Surgery.134 In these cases, the possibility of temporary stabilization measures (pelvic compression belt), preperitoneal packing, placement of an intravascular occlusion balloon and angioembolization should be considered in addition to the surgical approach.134Table 2 describes the most frequently affected organs, classified by zones, mechanism of injury, diagnostic method and treatment.
Most Frequently Affected Organs and Their Corresponding Approach.
| Zone | Organ | Mechanism | Diagnosis | Treatment |
|---|---|---|---|---|
| 1 | Inferior cava, aorta | Penetrating | Symptoms Intraoperative | Vascular control and suture, preferably |
| Duodenum, pancreas | Blunt | CT with contrast | Variable according to findings | |
| 2 | Kidney, adrenal gland | Blunt | CT with contrast | Conservative |
| 3 | Iliac vessels | Penetrating | Symptoms Intraoperative | Arteriorrhaphy, venous ligation or intravascular techniques |
- -
The highest mortality rates are related to large vessels, so the diagnostic suspicion is essential.
- -
The most frequent aortic injuries are infrarenal, while vena cava injuries are predominantly adrenal.
- -
The therapeutic objectives are based on the control of hemostasis/contamination and the assessment of injuries.
- -
In the presence of hemodynamic stability and absence of contraindication, conservative management, including angioembolization, should initially be considered. Otherwise, the use of sutures is preferable to more complex maneuvers.
- -
Conservative treatment is the most widely accepted, and surgery is defined by the presence of associated injuries or instability. Within the surgical approach, conservative maneuvers are more frequent than nephrectomy or adrenalectomy.
- -
A high index of suspicion of ureteral injuries is necessary.
- -
Vascular reconstruction is reserved for patients with anomalies of the contralateral kidney.
- -
Iliac vessel injuries prevail in importance due to their associated high mortality.
- -
Arteriorrhaphy and venous ligation stand out, together with an increase in the use of intravascular techniques.
- -
The management of bone injury is based on the multidisciplinary approach and individualized for each case.
The authors have no conflict of interests to declare.
Please cite this article as: Petrone P, Magadán Álvarez C, Joseph D, Cartagena L, Ali F, Brathwaite CEM. Abordaje y manejo de las lesiones retroperitoneales traumáticas. Cir Esp. 2018;96:250–259.