Metamizole magnesium is one of the most frequently used analgesics in the treatment of perioperative pain.1 This drug came on the market in 1921 and is currently authorized in South America and in 10 countries of the European Union (EU), including Spain. In October 2018, the Spanish Agency for Medicines and Health Products (AEMPS) published a communication on the risk of agranulocytosis and the growing use of metamizole in Spain, as its consumption has doubled in the last 10 years.2 We are concerned about the dosage of metamizole, due to its well-known serious adverse effects. A recently published review has analyzed a total of 1448 cases reported from 1985 to 2017,3 with a mortality rate of 16%. No lethal dose has been established, but doses greater than 10 g or in a short period of time can cause nausea, vomiting, abdominal pain, renal function deterioration, central nervous system symptoms, and even shock.4 The most common adverse effects are hypotension (frequency ≥ 1/100), followed by dermatological reactions (≥1/1000), leukopenia, anaphylactic reaction, asthma, maculopapular rash (≥1/10 000), agranulocytosis (including case fatalities), thrombocytopenia, toxic epidermal necrolysis, Stevens-Johnson syndrome, shock and phlebitis (<1/10 000).5
In clinical practice, metamizole is used at a parenteral dosage of 2 g every 8 h, which is changed to an oral dose of 575 mg every 8 h when the clinical situation improves. As a result of this difference and the AEMPS communication on metamizole and its adverse effects, a review was conducted of the pharmacokinetics, expert consensus and technical specifications of the different presentations marketed in Spain in order to determine the most appropriate dosage of metamizole.
At the pharmacokinetic level,5,6 when administered orally, metamizole undergoes non-enzymatic hydrolysis in the stomach acid, transforming into its main active metabolite, 4-methyl-amino-antipyrine (4-MAA). Once absorbed, it is metabolized in the liver by oxidation, demethylation and acetylation. Intravenous administration
is the fastest to reach maximum levels, followed by intramuscular and oral routes, the latter having almost 100% absorption. Therefore, pharmacokinetics do not justify a four-fold higher dosage parenterally versus orally. The difference in the dosage of both formulations seems to be related to the indications, meaning that it may be associated with greater clinical benefit in patients whose pain is more intense, a faster effect is required, and the patient is also usually intolerant to oral administration. However, this reasoning does not determine the most appropriate pattern.
On December 13, 2018, after a review of medicines containing metamizole and given that their adverse effects may be dose-related, the European Medicines Agency (EMA) made a consensus recommendation of the maximum daily dose in the European Union (EU)7 and addressed inconsistencies in product information marketed in many EU member states. The recommendations included a maximum single oral dose of 1 g up to 4 times a day in patients over the age of 15, and a maximum daily dosage of 5 g when the formulation is parenteral. These recommendations were sent to the European Commission (EC), which issued a final legally binding decision on March 20, 2019 that is valid throughout the EU.
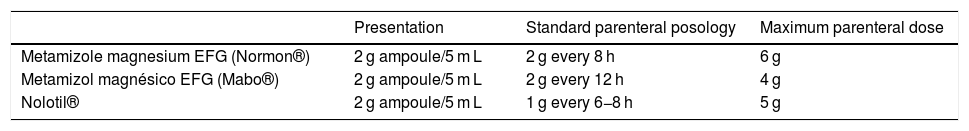
Table 1 shows the presentations marketed in Spain and the dosage according to the drug data sheets. The biggest differences are in the Normon® metamizole magnesium data sheet,6 which recommends a 2 g vial/8 h (exceeding EU recommendations), versus the Mabo® date sheet,8 which recommends 2 g/12 h, or the Nolotil® data sheet,5 which recommends 1 g/6−8 h and a possible maximum daily dose of up to 5 g. Regardless of the presentations, the dose of 2 g/8 h frequently continues to be used in hospitals. This exceeds the maximum daily dose of 5 g and the usual pattern of 1 g every 6−8 h or 2 g/12 h. In our opinion, with these dosages, the equivalence to the oral dose of 1–2 capsules every 8 h makes more sense.
Marketed presentations in Spain for parenteral dosage and posology according to the drug data sheet.
| Presentation | Standard parenteral posology | Maximum parenteral dose | |
|---|---|---|---|
| Metamizole magnesium EFG (Normon®) | 2 g ampoule/5 m L | 2 g every 8 h | 6 g |
| Metamizol magnésico EFG (Mabo®) | 2 g ampoule/5 m L | 2 g every 12 h | 4 g |
| Nolotil® | 2 g ampoule/5 m L | 1 g every 6−8 h | 5 g |
We conclude that we are overdosing parenteral metamizole in routine daily practice. After a multidisciplinary consensus was reached at our hospital, we recommend a dosage of 1 g/6−8 h, thus guaranteeing adherence to the binding decision of the EC. It is true that the current presentations of 2 g ampoules do not facilitate its applicability, and we hope that pharmaceutical companies will revise their drug data sheets in order to unify criteria, adjusted to the recommended dosage and to promote the safe use of metamizole. Lastly, this information should be transmitted to all medical professionals in order to correct this extended clinical practice, because, in this instance, common practice is not always the best practice.
Please cite this article as: Melgarejo-Ortuño A, Ribed-Sánchez A, Giménez-Manzorro Á, Zorrila-Ortúzar J, Sanjurjo-Saez M. Estamos sobredosificando el metamizol por vía parenteral? Cir Esp. 2021;99:68–70.