In recent years, with widespread laparoscopic cholecystectomy and liver transplantation, complications involving the biliary system are increasing. All current techniques have a high risk of recurrence or high morbidity.
Material and methodsA 3-dimensional collagen bile duct modified with agarose hydrogel was developed to substitute the affected extrahepatic bile duct. It was used in 40 guinea pigs and the histology and physiology were studied at 4 weeks, 3 months and 6 months after transplantation.
ConclusionsThe graft showed high potential in applications to treat hepatobiliary diseases which require surgery.
En la actualidad el tratamiento de la vía biliar extrahepática afectada por cáncer, estenosis, lesiones iatrogénicas y otras patologías consiste en la resección de las mismas, y una anastomosis en los casos má s graves de la placa hiliar biliar al intestino delgado.
Material y métodosNuestro grupo ha desarrollado un tubo tridimensional de colágeno y agarosa para reparar la vía biliar. Se emplearon 40 animales de experimentación, que fueron estudiados fisiológica e histológicamente a las 4 semanas, 3 y 6 meses.
ConclusionesNuestras pró tesis mostraron gran histocompatibilidad y mantenían una nor-mofisiología que hace pensar en las posibles aplicaciones clínicas que podrían tener en un futuro.
At present, the treatment of extrahepatic bile ducts affected by cancer, stenosis, iatrogenic injuries or other pathologies involves resection and anastomosis of the hilar plate to the small intestine in the most severe cases. In many cases, this technique is complicated by retrograde infections of the biliary tract caused by pathogens that ascend from the intestine, or due to complications such as anastomotic stricture that require another operation to redo the surgical technique. In a few cases, the stagnation of bile secretions and difficult bile flow require liver transplantation even in those cases where the only affected portion is extrahepatic. In those patients receiving liver grafts, stenosis of the biliary anastomosis can occur within a state of chronic rejection, thus requiring retransplantation.1
If there were an artificial tube that functioned morphologically and histologically like the native bile duct, it could be used as a substitute for the pathologic biliary tract. This would avoid complex surgical techniques that hinder the passage of bile, and ultimately avoid liver transplantation in those diseases in which the liver parenchyma is undamaged. Based on this idea and thanks to new biotechnology, our group is developing an artificial duct with the morphological, histological and functional properties of the native biliary tract.
In the current literature, there are few references to artificial ducts created to substitute pathological bile ducts, some with the help of biopolymers and others with histological structures of another type. Until now, these either did not have sufficient biocompatibility to allow for the flow of bile (biopolymers) or were not histologically integrated in the environment (intestine, blood vessels).2–4
As soon as we had developed the techniques for obtaining and holding collagen, our team modified a tubular three-dimensional structure of bovine collagen, covered by hydrogels, that was completely biocompatible. These new ducts were implanted into experimental animals (guinea pigs), substituting the native biliary tract; their histological and physiological functionalities were evaluated.
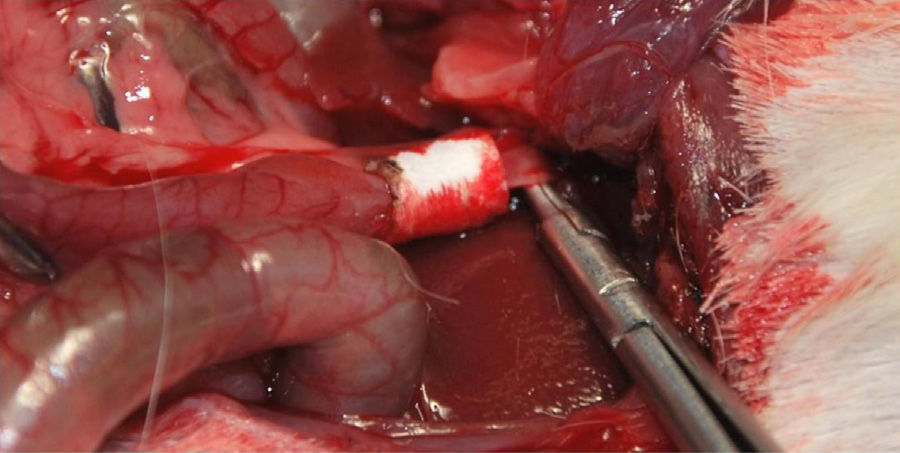
Material and MethodsBile Duct SubstituteThree-dimensional collagen tubes were constructed with a diameter of 4mm and a wall thickness of 1mm (Fig. 1). The size of the pores was 200nm. Subsequently, all the surfaces were coated with 2% agarose hydrogel. For storage, heaters were used at 37°C.
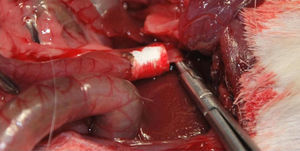
Implantation of the Artificial Ducts in Laboratory AnimalsThe artificial ducts were implanted in 40 Dunkin Hartley guinea pigs (Group 1: 13 guinea pigs; Group 2: 13 guinea pigs; Group 3: 14 guinea pigs) weighing approximately 1kg±200g. In all the animals, the extrahepatic bile duct was replaced in the portion between the cystic duct and the ampulla of Vater. For the intervention, a right subcostal incision was made to directly access the bile ducts, using an anesthetic induction of 20mg/kg of ketamine and maintaining a continuous infusion of propofol at 0.2mg/kg/min. Having identified the common hepatic duct in the hepaticojejunal ligament, it was divided circumferentially below the junction of the cystic duct. Using the surgical microscope, the collagen prosthesis was implanted with the aid of 10/0 sutures made of slow-absorption polylactic acid (Fig. 2). The experimental animals were housed in accordance with current regulations. During the first 7 postoperative days, analgesia and antibiotics (azithromycin) were administered subcutaneously.
Morphologic and Functional StudyThe animals were sacrificed at 4 weeks, 3 months and 6 months after bile duct substitution. At each instance, histological studies were done using hematoxylin–eosin stains. Similarly, the specimens were exposed to epithelial membrane antigens and low molecular weight keratin. All these were compared with specimens obtained from the native bile duct of 10 control guinea pigs. They were all similarly studied.
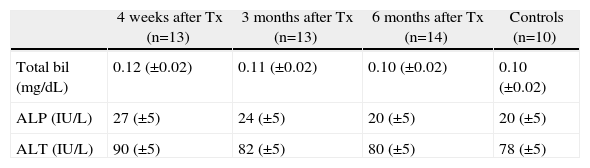
ResultsAll the experimental animals survived until they were sacrificed (after 4 weeks in Group 1, 3 months in Group 2 and 6 months in Group 3) after implantation of the artificial duct. They developed physiologically in a similar way to the control animals in terms of weight gain and growth (1.9kg±200g). In all groups, blood levels of bilirubin and liver enzymes were measured after sacrifice, and normal levels were observed in the 40 operated animals (Table 1).
Changes in the Biliary and Hepatic Enzymes in the Blood Tests of the Transplanted Guinea Pigs Throughout the Study Period Compared With the Normal Levels of the Control Group.
| 4 weeks after Tx (n=13) | 3 months after Tx (n=13) | 6 months after Tx (n=14) | Controls (n=10) | |
| Total bil (mg/dL) | 0.12 (±0.02) | 0.11 (±0.02) | 0.10 (±0.02) | 0.10 (±0.02) |
| ALP (IU/L) | 27 (±5) | 24 (±5) | 20 (±5) | 20 (±5) |
| ALT (IU/L) | 90 (±5) | 82 (±5) | 80 (±5) | 78 (±5) |
The results are presented as group mean±standard deviation.
Macroscopically, all implanted artificial ducts seemed to have completely biodegraded after 4 weeks, although adhesions and slight swelling persisted in the surrounding tissues. The specimens extracted after 3 months (3 and 6 months) had been completely resorbed, and the appearance was comparable to that of the native bile duct, with the persistence of mild adhesions of the surrounding tissues. The macroscopic appearance of the liver was completely normal, with no observed signs of cirrhosis.
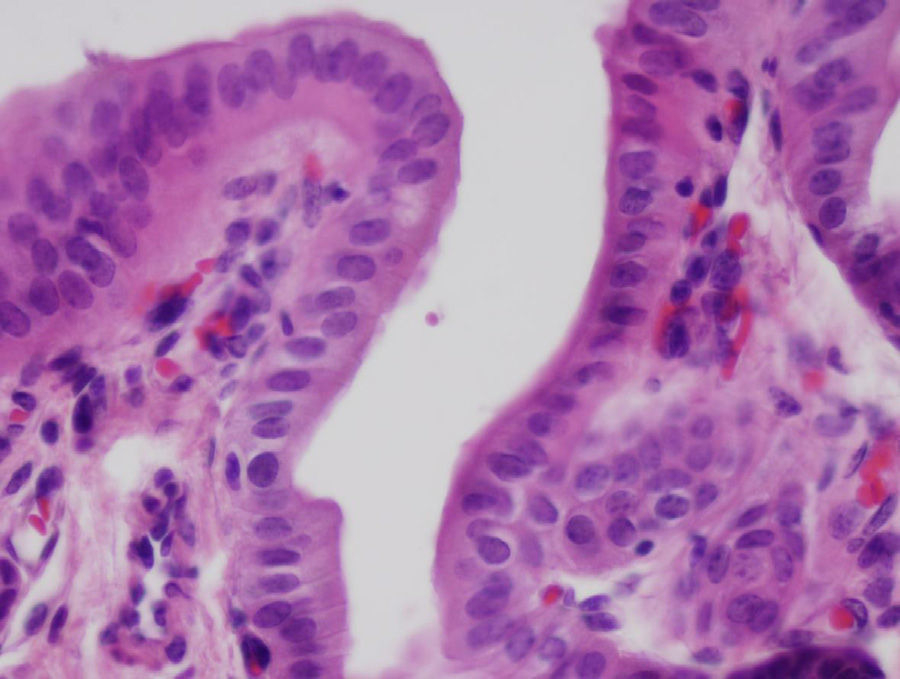
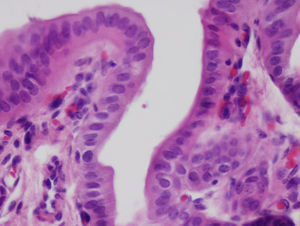
After 4 weeks, the artificial duct was partially reabsorbed and some granulomas were observed at the distal ends where polylactic acid sutures had been used. We observed some areas with the presence of glandular tissue under the biliary epithelium. The entire length of the collagen duct had been re-epithelialized with the same thickness in its entirety, although with some irregular areas (Fig. 3). In the study of the duct at 3 and 6 months, the artificial material was undetectable as it was covered by simple columnar epithelium. The collagen that formed the artificial tube had been replaced by dense connective tissue and was covered on its intraabdominal side by an epithelium with characteristics similar to that of the mesothelium of other organs. There was hardly any presence of glandular tissue beneath the surface of the biliary epithelium. In all cases, the liver tissue suffered no alterations, without presence of cirrhosis or bile stasis, as demonstrated with the histological sections obtained from the liver parenchyma. We saw no trace of the sutures that had been used. The transition between the ends of the collagen duct and native bile duct could not be located due to the absence of tissue differences between the different portions.
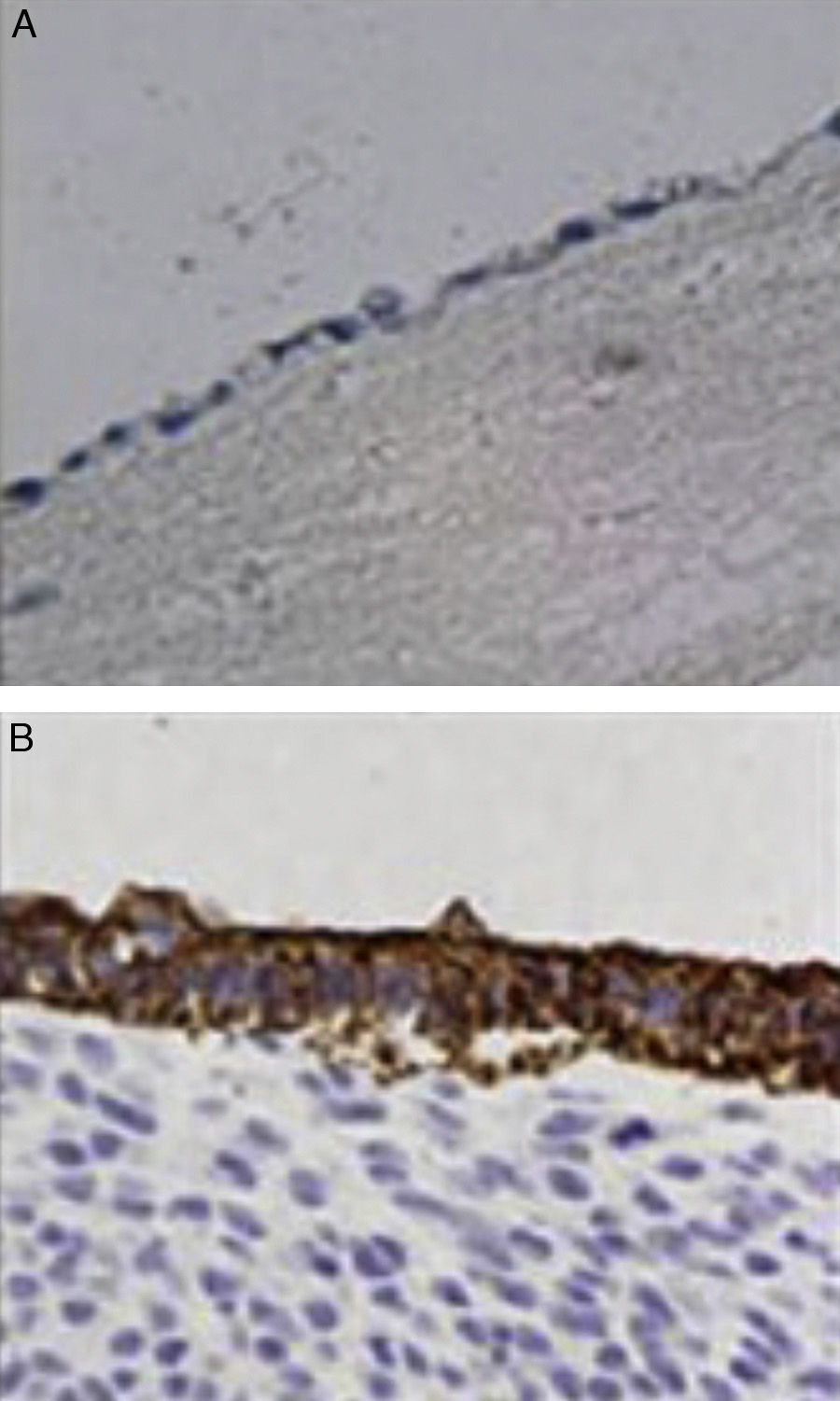
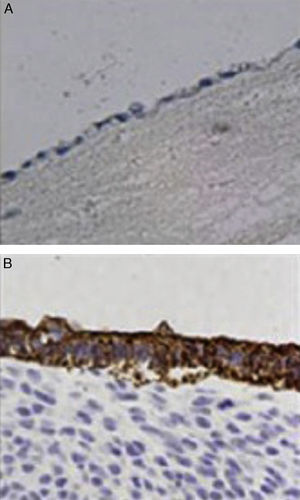
ImmunochemistryAll specimens were exposed to antibodies against epithelial membrane and low molecular weight keratin (CAM 5.2 antibody). All cells lining the duct lumen were positive for the antibody against epithelial membrane and showed organization similar to the epithelial cells of the bile duct. Furthermore, positivity for antibodies shown by low molecular weight keratin was lower than that observed in controls (Fig. 4).
DiscussionThe three-dimensional implanted duct becomes morphologically and physiologically integrated within the biliary tract, while maintaining the functions of bile transport and avoiding techniques with high morbidity, mortality and healthcare costs. The tissue integration that occurs after week 4 suggests that the structure serves as a scaffold for regeneration from the ends of the native bile tissue. We could construe multiple applications that could be developed for the use of these materials in current clinical practice. In the presence of stenosis or cancer of the extrahepatic bile duct, these segments can be removed and replaced with an artificial duct using end-to-end anastomosis. Thus, we are able to preserve the papilla of Vater and maintain the biological functions of this valve while preventing retrograde infections of the biliodigestive anastomoses. If our project becomes feasible, the constant availability of substitute bile ducts can avoid high-risk bypasses and these can be used in a multitude of techniques that require the removal of bile duct segments. This would avoid complications and, ultimately, the need for transplantation.
We consider our prostheses correctly integrated when there are no complications after implantation. There should be no signs or symptoms of bile flow obstruction caused by stenosis of the duct, nor should there be any signs indicating intraabdominal bile leakage due to dehiscence or leakage points in the anastomoses. None of these complications was present in our cases. The positive function that we observed in the artificial ducts created in the study periods suggests that these grafts could be applied in clinical practice.
Some authors have recently published successful results in the formation of artificial organs, such as blood vessels5,6 and the small intestine7,8 using cell cultures. This principle has been used by authors such as Rosen et al. to facilitate the reconstruction of the circumference of the bile duct using intestinal submucosa, leading to re-epithelialization.4 In our case, re-epithelialization occurred from the ends of the artificial duct and extended along its entire length by cell migration through the three-dimensional pore structure of the collagen. The rate of re-epithelialization was similar at both ends. This characteristic would allow these collagen ducts to be used in other clinical situations, while making surgical techniques easier. Roughly speaking, we could say that the biliary pluripotent cells are viable and that they differentiate into epithelial cells uniformly, migrating through the implanted duct. More targeted histological studies are necessary in order to support this statement.
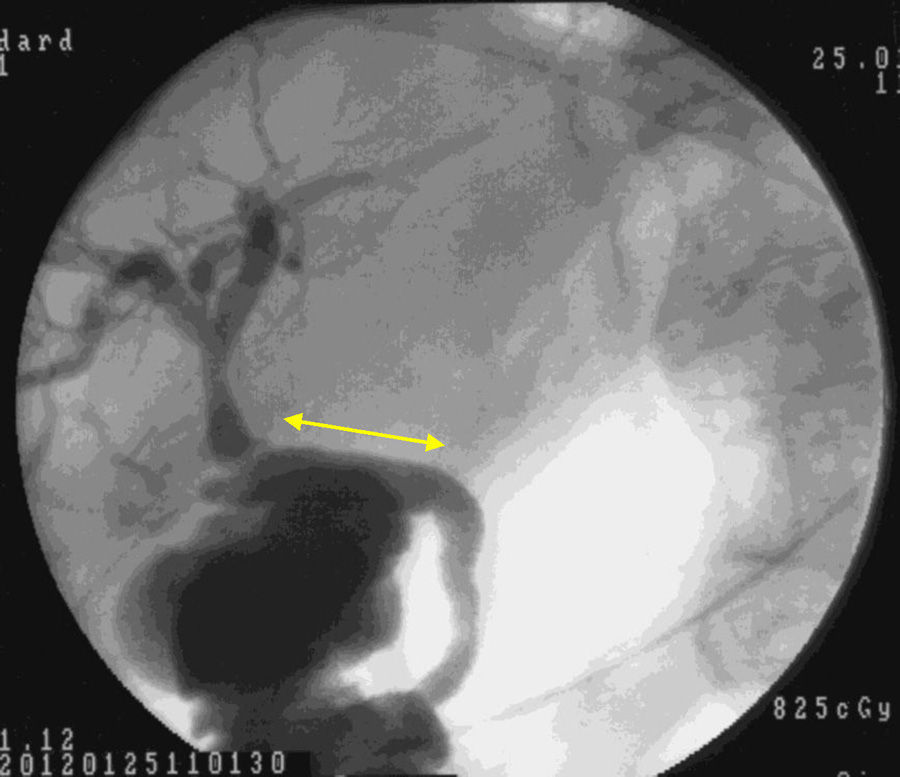
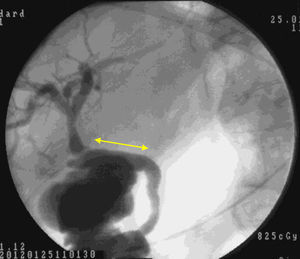
Macroscopic examination showed that 4 weeks after implantation our biocompatible duct was completely similar to the native bile duct morphologically. Histology studies and immunohistochemistry demonstrated that histologically the tissue disposition was similar with analogous antigen expression. In the new environment created, the bile flowed through the duct into the duodenum (Fig. 5). Through our research, we have been unable to determine which factors stimulate cell differentiation and re-epithelialization, either through bile components or chemotactic stimuli.
Our three-dimensional collagen tube implanted in experimental animals acquired a function and form similar to those of the native bile duct 4 weeks after grafting. The creation of an artificial bile duct opens new therapeutic avenues for the treatment of diseases of the extrahepatic biliary tract, thus avoiding complex surgical techniques with high socio-economic costs, morbidity and mortality. If future studies confirm the long-term viability of these prostheses, our approach will certainly have a great many clinical applications.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Pérez Alonso AJ, Del Olmo Rivas C, Machado Romero I, Pérez Cabrera B, Cañizares Garcia FJ, Torne Poyatos P. Reconstrucción del conducto biliar mediante tubos tridimensionales de colágeno. Cir Esp. 2013;91:590–594.