Neoadjuvant chemo-radiotherapy is the treatment of choice for rectal cancer in order to reduce local recurrence. Patients with a pathological complete response (PCR) have a better prognosis. The aim of this study was to determine the influence of PCR on the oncological outcomes in our patients.
MethodsAll patients with stage II/III rectal cancer treated with neoadjuvant chemo-radiotherapy and radical resection between 2007 and 2011 were identified from a prospective database, and grouped based on whether they achieved PCR or not (non-PCR). Clinical, histological and oncological outcome data were compared.
ResultsA total of 162 patients were included (62% men), with a mean age of 65 years. In terms of pre-operative TNM staging, 82 patients (50%) were T2, 75 (46%) were T3, and 5 (3%) were T4. Forty-two patients (25%) were N1, and 87 (53%) were N2. Low anterior resection and abdominoperineal resection were performed in 125 (77%) and 25 (15%) patients. Forty-three patients (26.5%) had postoperative morbidity. PCR was achieved in 19 patients (11.7%). After a median follow-up of 26 months, there are no recurrences in the PCR group, and in the non-PCR group, local recurrence was 1.4% (P=.78), and distant metastasis was 8.4% (P=.21). Overall survival (P=.39) and survival free of diseases (P=.23) were better in the PCR group, but the differences were not significant.
ConclusionPatients with pathological complete response have better oncological outcome.
La radioquimioterapia es el tratamiento de elección en el cáncer de recto para conseguir el control de la enfermedad. Los pacientes con respuesta patológica completa (RPC) presentan mejor pronóstico. El objetivo del trabajo es conocer nuestra incidencia de RPC y analizar los resultados oncológicos.
MétodosPacientes con neoplasia de recto estadios ii/iii, recogidos prospectivamente en el periodo comprendido entre 2007 y 2011. Los pacientes son sometidos a neoadyuvancia y a cirugía radical. Se dividen en 22 grupos según tengan o no RPC y se comparan las variables demográficas, clínicas e histológicas y su relación con la evolución oncológica.
ResultadosSe analizan 162 pacientes (62% varones) con una edad media de 65 a. La incidencia de RPC es del 11,7% (19 pacientes). El 50% de los pacientes son T2, el 46% son T3 y el 3% son T4, mientras que el 25% son N1 y el 53% son N2 antes de la neoadyuvancia. En 25 pacientes (15%) se ha practicado una amputación de recto y en 125 (77%) una resección anterior baja. La morbilidad global es del 26,5% (43 pacientes). Con una mediana de seguimiento de 26 meses, ningún paciente con RPC ha presentado recurrencia tumoral. En el grupo de NO-RPC la recidiva local es del 1,4% (p=0,78) y las metástasis del 8,4% (p=0,21), siendo la supervivencia global y la libre de enfermedad mayor en el grupo con RPC pero sin diferencias significativas (p=0,39, p=0,23).
ConclusiónLa presencia de RPC después de tratamiento neoadyuvante se relaciona con mejores resultados oncológicos.
Rectal cancer is one of the most frequent tumors in our society and is therefore the object of continuous analysis aimed at improving results.
The treatment of choice for stages II and III is radiochemotherapy followed by surgery.1 The objectives of this treatment are, first of all, to reduce tumor stage in order to reduce local recurrence rate and, secondly, to achieve tumor sterilization, meaning that there is no tumor found on clinical examination. This is known as complete clinical response (CCR).2
Although the response to neoadjuvant therapy varies greatly, between 10% and 30% of patients present absence of tumor cells in the surgical piece (ypT0N0), known as pathologic complete response (pCR).3 Although the therapeutic implications of pCR may be c ontroversial, it seems to be a factor for good prognosis.4
The objective of this study is to determine the incidence of pCR in our experience and to analyze its relation to oncological results.
Materials and MethodsThe patients studied were affected by rectal neoplasia located in the middle and inferior third of the rectum in stages II and III and had undergone neoadjuvant treatment between January 2007 and December 2011.
The patients were identified from a prospective database that included data for demographics, symptoms, diagnostic methods and biopsy with diagnosis of adenocarcinoma. The staging methods used were thoracoabdominal CT and endoanal ultrasound/rectal MRI for the local study. We excluded those patients who had undergone emergency surgery, patients with non-curative surgery (R1–R2 resections) and patients who died during the postoperative period as they logically did not have a follow-up.
The neoadjuvant regimes were 5-FU both in continuous perfusion as well as intravenous bolus or orally (capecitabine). The protocol used most often was continuous infusion for 6 weeks. Preoperative radiotherapy was performed with 3–4 fields with a mean dose of 50.4Gy.
Four weeks after the end of the treatment, rectal MRI was repeated to re-evaluate the tumor stage, and 6 weeks after neoadjuvant therapy radical surgery was carried out using the total mesorectal excision technique.
The pathologic stage of the tumor was reported in accordance with the American Joint Committee on Cancer5 and the degree of tumor regression was determined with the Dworak classification.6 The studies were always done by expert pathologists.
pCR was defined by the absence of adenocarcinoma cells both in the rectal wall as well as in the mesenteric lymph nodes of the surgical specimen (ypT0N0). The patients were divided into 2 groups according to presence of pCR or no-pCR and we compared the clinical and histologic variables as well as their relationship with oncologic outcome: tumor recurrence, overall survival and disease-free survival.
The statistical analysis was done with the SPSS 15.0 program (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to verify the normalcy of the sample. The data are presented as mean±standard deviation and in the follow-up as medians (range). The differences between qualitative variables were analyzed with Pearson's χ2 test or Fisher's exact test, and the quantitative variables with Student's t test. A P<.05 was considered statistically significant. The survival functions were estimated by the Kaplan–Meier method and the log rank test was used to compare the samples.
ResultsThe study group included 162 patients (62% males), with a mean age of 65, 19 of whom presented pCR (11.7%).
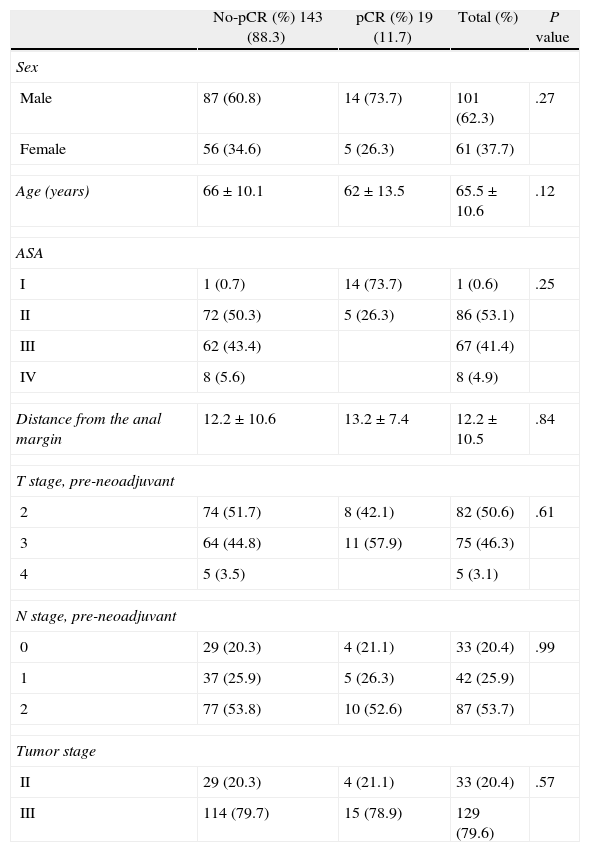
There were no demographic differences in the clinical stage of the tumor between the pCR and no-pCR patients (Table 1), with a pre-neoadjuvant therapy T2 stage of 50% and T3 of 46% of the total series with lymph node infiltration of 79.6%.
Demographic and Clinical Variables.
| No-pCR (%) 143 (88.3) | pCR (%) 19 (11.7) | Total (%) | P value | |
| Sex | ||||
| Male | 87 (60.8) | 14 (73.7) | 101 (62.3) | .27 |
| Female | 56 (34.6) | 5 (26.3) | 61 (37.7) | |
| Age (years) | 66±10.1 | 62±13.5 | 65.5±10.6 | .12 |
| ASA | ||||
| I | 1 (0.7) | 14 (73.7) | 1 (0.6) | .25 |
| II | 72 (50.3) | 5 (26.3) | 86 (53.1) | |
| III | 62 (43.4) | 67 (41.4) | ||
| IV | 8 (5.6) | 8 (4.9) | ||
| Distance from the anal margin | 12.2±10.6 | 13.2±7.4 | 12.2±10.5 | .84 |
| T stage, pre-neoadjuvant | ||||
| 2 | 74 (51.7) | 8 (42.1) | 82 (50.6) | .61 |
| 3 | 64 (44.8) | 11 (57.9) | 75 (46.3) | |
| 4 | 5 (3.5) | 5 (3.1) | ||
| N stage, pre-neoadjuvant | ||||
| 0 | 29 (20.3) | 4 (21.1) | 33 (20.4) | .99 |
| 1 | 37 (25.9) | 5 (26.3) | 42 (25.9) | |
| 2 | 77 (53.8) | 10 (52.6) | 87 (53.7) | |
| Tumor stage | ||||
| II | 29 (20.3) | 4 (21.1) | 33 (20.4) | .57 |
| III | 114 (79.7) | 15 (78.9) | 129 (79.6) | |
There was also no significant difference in the height of the tumor from the anal margin between the two groups (7.1 vs 6.9cm).
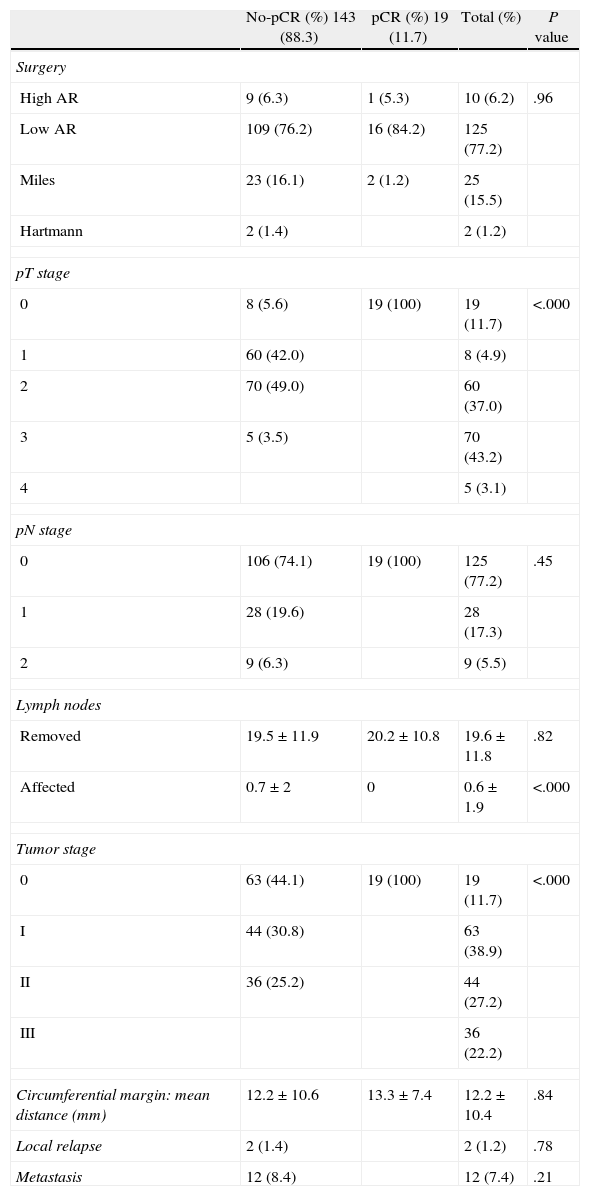
In the overall series, high anterior resection was performed in 10 cases (6.2%) and low anterior resection in 125 cases (77.2%), abdomino-perineal amputation of the rectum in 25 (15.5%) and the Hartmann technique in 4 (1.2%), with no significant differences between the two groups (pCR vs no-pCR) with regard to surgical techniques. An end colostomy was constructed in 27 patients (16.7%) and protective ileostomy in 77.2%. In 6.2% (10 cases), no stoma was performed.
In the radiological staging, compared before and after neoadjuvant therapy, reductions were seen in tumor stage, wall invasion and lymph node infiltration. There was logically a significant difference between the two groups, with a decrease in tumor stages (Tables 1 and 2).
Surgical Technique and Histologic Variables.
| No-pCR (%) 143 (88.3) | pCR (%) 19 (11.7) | Total (%) | P value | |
| Surgery | ||||
| High AR | 9 (6.3) | 1 (5.3) | 10 (6.2) | .96 |
| Low AR | 109 (76.2) | 16 (84.2) | 125 (77.2) | |
| Miles | 23 (16.1) | 2 (1.2) | 25 (15.5) | |
| Hartmann | 2 (1.4) | 2 (1.2) | ||
| pT stage | ||||
| 0 | 8 (5.6) | 19 (100) | 19 (11.7) | <.000 |
| 1 | 60 (42.0) | 8 (4.9) | ||
| 2 | 70 (49.0) | 60 (37.0) | ||
| 3 | 5 (3.5) | 70 (43.2) | ||
| 4 | 5 (3.1) | |||
| pN stage | ||||
| 0 | 106 (74.1) | 19 (100) | 125 (77.2) | .45 |
| 1 | 28 (19.6) | 28 (17.3) | ||
| 2 | 9 (6.3) | 9 (5.5) | ||
| Lymph nodes | ||||
| Removed | 19.5±11.9 | 20.2±10.8 | 19.6±11.8 | .82 |
| Affected | 0.7±2 | 0 | 0.6±1.9 | <.000 |
| Tumor stage | ||||
| 0 | 63 (44.1) | 19 (100) | 19 (11.7) | <.000 |
| I | 44 (30.8) | 63 (38.9) | ||
| II | 36 (25.2) | 44 (27.2) | ||
| III | 36 (22.2) | |||
| Circumferential margin: mean distance (mm) | 12.2±10.6 | 13.3±7.4 | 12.2±10.4 | .84 |
| Local relapse | 2 (1.4) | 2 (1.2) | .78 | |
| Metastasis | 12 (8.4) | 12 (7.4) | .21 | |
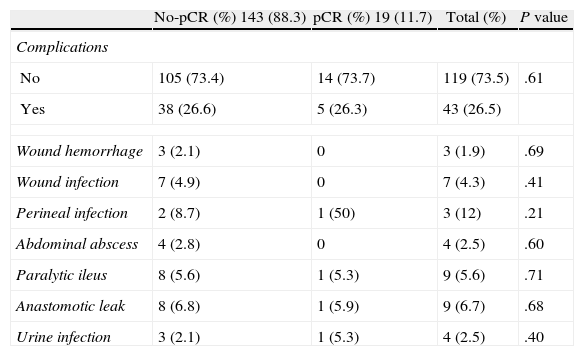
Complications were seen in 26.5% of the patients, with no differences between the 2 groups regarding the percentage and type of complications. Table 3 reports the most important: overall incidence of anastomotic leak was 6.7%, and infection of the perineum was 12%.
Mortality.
| No-pCR (%) 143 (88.3) | pCR (%) 19 (11.7) | Total (%) | P value | |
| Complications | ||||
| No | 105 (73.4) | 14 (73.7) | 119 (73.5) | .61 |
| Yes | 38 (26.6) | 5 (26.3) | 43 (26.5) | |
| Wound hemorrhage | 3 (2.1) | 0 | 3 (1.9) | .69 |
| Wound infection | 7 (4.9) | 0 | 7 (4.3) | .41 |
| Perineal infection | 2 (8.7) | 1 (50) | 3 (12) | .21 |
| Abdominal abscess | 4 (2.8) | 0 | 4 (2.5) | .60 |
| Paralytic ileus | 8 (5.6) | 1 (5.3) | 9 (5.6) | .71 |
| Anastomotic leak | 8 (6.8) | 1 (5.9) | 9 (6.7) | .68 |
| Urine infection | 3 (2.1) | 1 (5.3) | 4 (2.5) | .40 |
There has been no case of tumor recurrence in the pCR group while 14 patients (9.8%) of the no-pCR group did present recurrence. These were 2 local relapses (1.4%) and 12 distant metastases (8.4%).
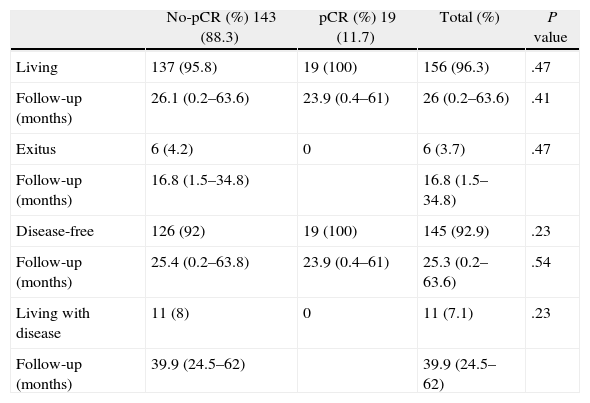
Table 4 shows the oncological results. The mean total follow-up of the 156 (96.3%) living patients was 26 (0.2–63.6) months, and for the 6 patients who had died (3.7%), mean follow-up was 16.8 (1.5–34.8) months; 5 (3%) died because of the tumor itself and 1 (0.7%) due to causes unrelated with the tumor. No significant differences were observed between the two groups. Mean follow-up of the 145 disease-free patients was 25.3 (0.2–63.6) months, and 11 patients had metastatic disease.
Oncological Results.
| No-pCR (%) 143 (88.3) | pCR (%) 19 (11.7) | Total (%) | P value | |
| Living | 137 (95.8) | 19 (100) | 156 (96.3) | .47 |
| Follow-up (months) | 26.1 (0.2–63.6) | 23.9 (0.4–61) | 26 (0.2–63.6) | .41 |
| Exitus | 6 (4.2) | 0 | 6 (3.7) | .47 |
| Follow-up (months) | 16.8 (1.5–34.8) | 16.8 (1.5–34.8) | ||
| Disease-free | 126 (92) | 19 (100) | 145 (92.9) | .23 |
| Follow-up (months) | 25.4 (0.2–63.8) | 23.9 (0.4–61) | 25.3 (0.2–63.6) | .54 |
| Living with disease | 11 (8) | 0 | 11 (7.1) | .23 |
| Follow-up (months) | 39.9 (24.5–62) | 39.9 (24.5–62) |
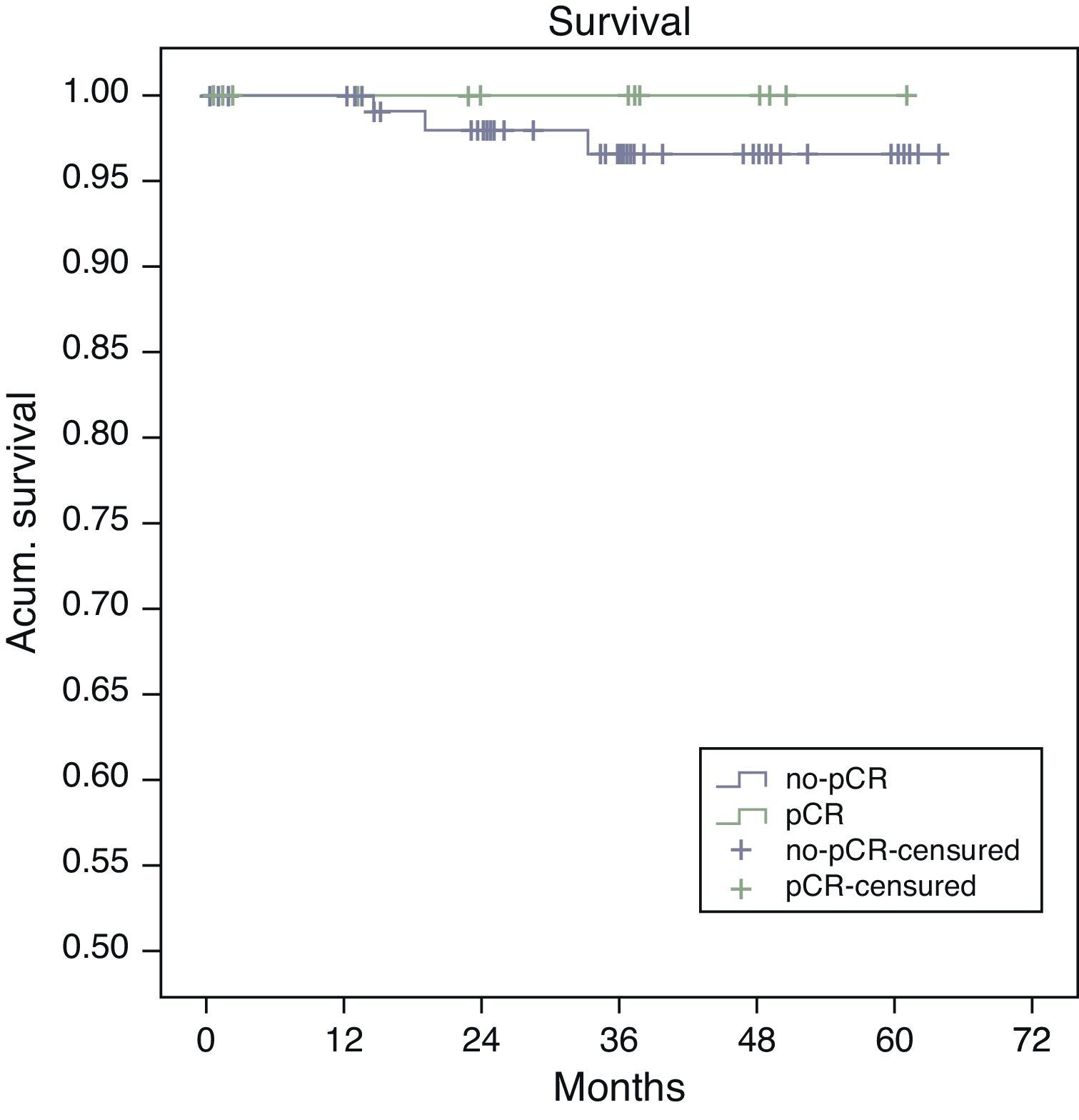
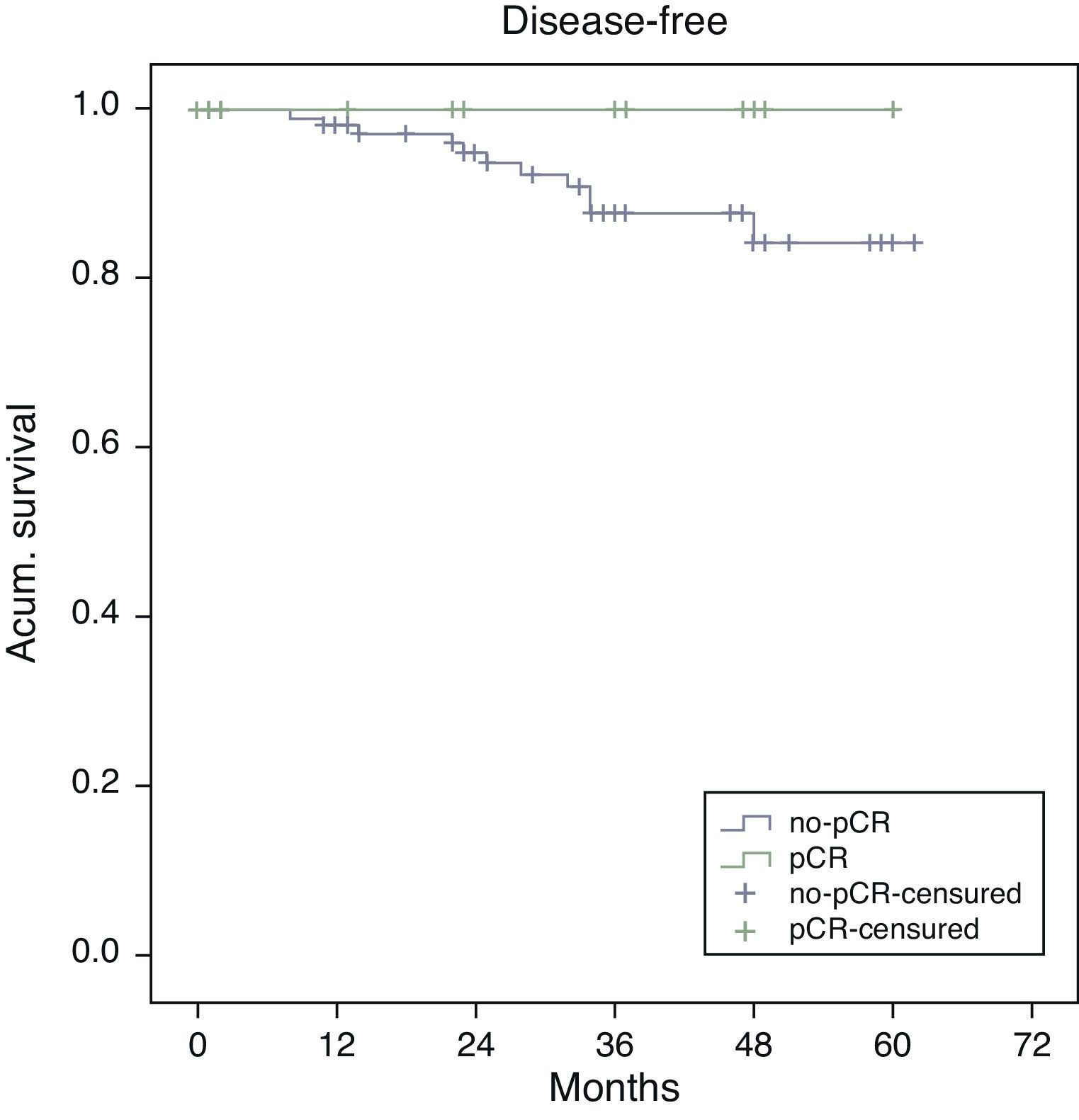
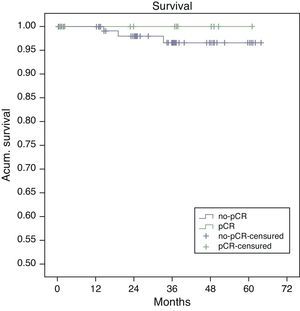
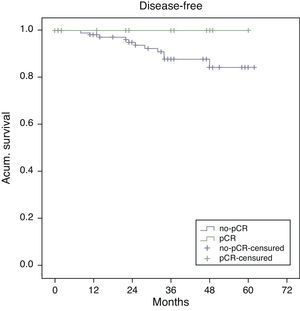
Estimated 5-year survival, represented by Kaplan–Meier curves (Fig. 1), was 100% in the pCR group and 93% in the no-pCR group, with no significant differences (P=.39; log rank). The estimated calculation of 5-year disease-free survival was 100% in the pCR group while in the no-pCR group it was 84%, with no significant differences (P=.23; log rank) (Fig. 2).
The mean time elapsed before the appearance of metastasis was 26.5 (8–40) months and the mean time before the appearance of local recurrence was 23 (14–32) months.
DiscussionOne of the most important advances in the treatment of rectal cancer is the capability to predict or identify patients who present clinical-radiological evidence of CCR after preoperative radiochemotherapy. The regression of the tumor is considered a factor of good prognosis7 and has led to the appearance of new therapeutic concepts with a watch-and-wait attitude and intensive follow-up to avoid aggressive surgery. This is the “wait and see” approach proposed by Habr-Gama et al.8,9
Although there is a reduction in tumor size and tumor stage in up to one-third of cases,10 this approach is not free of risk since CCR does not always correlate with pCR, because only in some patients with CCR (between 25% and 50%) is the presence of pCR confirmed (after radical surgery). Furthermore, lymph node infiltration is not predicted and it is found in up to 25% of T0 patients.11 Therefore, it is necessary to demonstrate its role as a factor for good prognosis although this may actually mean a change in attitude in selected patients.12
The demographic variables of our series are similar to those described in the literature12 with a mean age in the sixth decade of life and a predominance of males. With regard to tumor stage, our series presents a lower incidence of stage ii compared with results from a recently published meta-analysis (20% vs 34%) and a greater incidence of stage III (79% vs 64%), with a similar mean distance between the tumor and the anal margin12 (5.3 vs 7.1cm).
Our incidence of pCR of 11.7%, within the described limits (10%–30%), is lower than other rates reported.8,9 Brazilian studies2,8,9 have demonstrated that when there is no evidence of tumor (CCR) detected by clinical-endoscopic-radiological examination, a wait-and-see attitude provides the same survival as radical surgery, later confirming the existence of pCR in the surgical specimen. But these same authors10 indicate that this approach can fail in one-fifth of cases. Therefore, the main problem is to identify those patients who may benefit from this change in attitude. With the aim of re-staging our patients, we ordered post-radiochemotherapy MRI. In no case did we find a radiologic CCR that was able to modify our approach, although this change in attitude should still be taken in a controlled clinical trial.
Our results are similar to other reports13,14 and there is no difference between the clinical-histologic stages or in the treatment depending on the presence of pCR or not.
Several factors influence pCR. First of all, there is the state of the tumor itself (the smaller the wall invasion, the greater the response15), and then there are the different neoadjuvant therapy regimes.16 Without a doubt, one of the most important factors is the interval between neoadjuvant therapy and surgery. The effect of radiochemotherapy is variable in extension and in duration. As it is a continuous process, the optimal interval between the end of the treatment and the date for surgery is controversial.17
After the Lyon trial, standard treatment included surgery after 6 weeks, with a pCR rate of 26%.18 However, some tumors may have pCR after 8 weeks, finding 16% pCR before 8 weeks and 31% pCR after 8 weeks.19 It has even been suggested that the radiosensitizing effect may last for up to 12 weeks,20 and that the patients would benefit from additional cycles of chemotherapy.21
Our protocol was based on surgery 6 weeks after the end of neoadjuvant therapy, but, given the results obtained, we have changed the surgery to 8 weeks afterwards with a new tumor re-staging MRI 6 weeks after the end of radiochemotherapy.
The results regarding surgical technique used and morbidity are within the ranges of Spanish studies.22 The clinical results are also similar to those described, with no differences between the patients with and those without pCR.23
The histologic results and morbidity are similar to those reported in another national series with a similar mean follow-up,24 where a reduction was observed in the final histologic stages in both lymph node as well as wall invasion. The histologic examination after neoadjuvant therapy and surgery showed a significant decrease in the overall tumor stage of the series, with 22% in stage III, 27% in stage II and 38% in stage I.
The optimization of the total mesorectal excision surgical technique has caused a decrease in the local recurrence rate, which has decreased from values of 30%–40% to 4%, with improved results resulting from surgical teaching programs. The impact of pCR on local recurrence is also important since it causes a greater decrease in its percentages, with mean rates of 0.7%, overall survival above 90% and disease-free survival at 87%.12
Our rate of local recurrence of 1.4% and metastasis of 8.4% in the no-pCR group and the absence of tumor recurrence in the pCR group are similar to published data.12 These data reinforce the impact of pCR on oncological results, with no relapses in this group after a mean follow-up of 23.9 months (range: 0.4–61). These results concur with other reports25 of 0.9% recurrences and 8.9% metastases, but with a longer follow-up (46 months), although the mean detection of pelvic recurrence was 26 months, and in our series it was 23 months.
The incidence of local recurrence in patients with pCR is very low. Nevertheless, the rate of metastasis has not decreased as much and indicates the need for adjuvant chemotherapy. The current challenge is to be able to identify those patients who could benefit from more doses of chemotherapy.21
Considering that 55%–80% of local recurrences of rectal cancer occur within the first 2 years of follow-up,12,26 and despite the fact that our series has an average follow-up time of 26 months, we can affirm that patients with pCR present better oncological results. Nevertheless, it is also important to state that, at present, local recurrence has appeared in up to one-third of the patients more than 5 years after neoadjuvant therapy.2 Meanwhile, in other studies, mean local recurrence time is 26 months,25 which may cause controversy when developing follow-up protocols.
In our series, all the patients whose death was tumor-related were from the no-pCR group. There were longer overall (100% vs 93%; P=.39) and disease-free survivals (100% vs 84%; P=.23) in the pCR group compared with the no-pCR group.
In conclusion, the presence of pCR after radiochemotherapy implies better oncological results and is a positive prognostic factor that indicates less recurrence and longer survival. In our experience, however, due to the small number of patients and the short follow-up period, there are no significant differences.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Codina Cazador A, Farres Coll R, Olivet Pujol F, Martin Grillo A, Pujadas de Palol M, Gómez Romeu N, et al. Resultados clínico-oncológicos de la respuesta patológica completa en el cáncer de recto después de tratamiento neoadyuvante. Cir Esp. 2013;91:417–423.