Clinical pathways are care plans applicable to patient care procedures that present variations in practice and a predictable clinical course. They are designed not as a substitute for clinical judgement, but rather as a means to improve the effectiveness and efficiency of the procedures. This clinical pathway is the result of a collaborative work of the Sections of Endocrine Surgery and Quality Management of the Spanish Association of Surgeons. It attempts to provide a framework for standardising the performance of thyroidectomy, the most frequently performed operation in endocrine surgery. Along with the usual documents of clinical pathways (temporary matrix, variance tracking and information sheets, assessment indicators and a satisfaction questionnaire) it includes a review of the scientific evidence around different aspects of pre, intra and postoperative management. Among others, antibiotic and antithrombotic prophylaxis, preoperative preparation in hyperthyroidism, intraoperative neuromonitoring and systems for obtaining hemostasis are included, along with management of postoperative hypocalcemia.
Las vías clínicas son planes detallados de asistencia aplicables al tratamiento de pacientes con variaciones en la práctica y un curso clínico predecible. Sin pretender sustituir el juicio clínico de los profesionales, buscan una mejora en la efectividad y la eficiencia. La vía clínica que presentamos es el resultado del trabajo colaborativo de las Secciones de Cirugía Endocrina y Gestión de Calidad de la Asociación Española de Cirujanos, que intenta aportar un marco para normalizar la realización de la tiroidectomía. Junto con documentos habituales de toda vía clínica (matriz temporal, hoja de variaciones e información, indicadores de evaluación, encuesta de satisfacción), incluye una revisión de la evidencia científica en torno a diferentes aspectos del pre, intra y posoperatorio de esta intervención, la más frecuentemente realizada en cirugía endocrina. Entre otros, analiza la profilaxis antibiótica y antitrombótica, la preparación preoperatoria en hipertiroidismo, la neuromonitorización intraoperatoria, los sistemas para hemostasia intraoperatoria y el tratamiento de la hipocalcemia posoperatoria.
Any health care process requires a multidisciplinary and comprehensive approach. To that end, one of the tools available to health care professionals is clinical practice pathways and guidelines. Clinical pathways are health care plans applicable to patients with a specific disease that coordinate every dimension of the health care quality: those estimated by professionals (scientific–technical quality, interprofessional health care and coordination optimisation), by patients (information, participation and expectations adjustment) and by agents (efficiency and continuous assessment).1 These are tools that help to facilitate the multidisciplinary and systematised assistance to the patient but do not replace professional clinical judgement.2 The main objectives are the following: standardise professional performance in accordance with the best scientific evidence available, to reduce the unjustified variability of clinical practice and unnecessary costs associated to the procedure.
Thyroidectomy is the intervention most frequently performed in endocrine surgery, and has evolved in the last years, with a better knowledge of the pathophysiology of its complications and the incorporation of new assistance techniques in the pre-, intra- and postoperative scenarios. With the purpose of helping professionals incorporate the best practices and provide the best assistance to patients, the Endocrine Surgery and Quality Management sections of the Asociación Española de Cirujanos (Spanish Association of Surgeons) decided to create a clinical pathway for thyroidectomy (CPT). The boards of both sections assigned its performance to a joint and equal group of work. This clinical pathway is intended to become a useful tool in clinical decision-making, through a series of evidence-based guidelines with which the problems arising from the care of particular patients are solved.
Process Limits. Inclusion and Exclusion CriteriaThe clinical pathway starts when the surgeon confirms the surgical indication and advises the patient to have a thyroidectomy performed. Even though conceptually the exit limit is the hospital discharge, due to the existing variability in practice, we have incorporated a systematic review of certain innovative or controversial monitoring aspects, the follow-up and the eventual treatment of postoperative complications, once the patient has been discharged. Generally, the recommendations proposed in the CPT are applicable to all the patients subjected to thyroid resection. Exclusion criteria have been defined as: regional or general anaesthesia contraindication, urgent interventions and the performance of concomitant larger surgical procedures. The CPT has been divided into 2 basic documents: recommendations on key process points and CPT-related documents.
Recommendations on Key Process PointsGeneral ConsiderationsThey have been systematically prepared regarding high-variability aspects or aspects that required an update. The evidence-based medicine methodology has been followed, standardising the search and performing a critical assessment of the literature. Based on the level of evidence (LOE) determined, we have appraised several recommendations to minimise bias. We have based our work on original documents and clinical practice guidelines assessed in accordance with the guidelines from Appraisal of Guidelines for Research and Evaluation–AGREE–II (http://www.agreetrust.org).3
The LOE classification used is the one from the Oxford Centre for Evidence-Based Medicine in 2009 (http://www.cebm.net/?o=1025).4 It assesses diagnostic procedures, preventive and therapeutic interventions, risk and prognostic factors. To that end, a LOE is defined (Table 1) and a grade of recommendation (GR) is established in:
- -
Grade A: derived from level 1 consistent studies.
- -
Grade B: derived from level 2 to 3 consistent studies or extrapolations (use of data for clinical situations with potentially major differences) of level 1studies.
- -
Grade C: derived from level 4 studies or extrapolations of level 2–3 studies.
- -
Grade D: derived from level 5 evidence or from inconsistent or non-conclusive studies of any level.
Levels of Evidence and Grades of Recommendation From the Oxford Centre for Evidence-Based Medicine (March, 2009).
| Level | Aetiology/Treatment/Adverse effects/Prevention | Diagnosis |
|---|---|---|
| 1a | Homogeneous SRa of clinical trials | Homogeneous SRa of level 1 diagnostic studies or algorithms of decision or rating scales derived from 1b studies from different clinical centres |
| 1b | Clinical trial with narrow confidence interval | Diagnostic test validating cohort studiesb with good reference standards,c or algorithms of decision or rating scales tested in one clinical centre |
| 1c | “All or none” cases-seriesc | Highly specific and sensitive diagnostic test |
| 2a | Homogeneous SRa of cohort studies | Homogeneous SRa of level 2 diagnostic studies or higher |
| 2b | Individual cohort studies or poor-quality clinical trials (<80% follow-up) | Validating cohort studyb with good reference standards.c Studies derived from algorithms of decision or rating scales, or validated only upon divided samplesd or databases |
| 2c | Outcomes Research or ecological studies | |
| 3a | Homogeneous SRa of case–control studies | Homogeneous SRa of Level 3b studies or higher |
| 3b | Individual case–control studies | Non-consecutive patients study, or without a consistently applied reference standard |
| 4 | Case-series or poor-quality case–control studiese | Case–control studies with poor or non-independent reference standards |
| 5 | Expert opinion without explicit critical assessment, or based on physiology, ex vivo research or general principles | Expert opinion without explicit critical assessment, or based on physiology, ex vivo research or general principles |
SR: systematic reviews.
Information regarding family history of cancer or endocrine disease, irradiation or prior cervical surgery, node growth rate, presence of compressive symptoms (dyspnoea, dysphagia and dysphonia) and hyper or hypothyroidism has to be collected5 (LOE 2b, GR B). The absence of symptoms does not rule out malignity6 (LOE 3b, GR C). The physical examination has to include the thyroids and cervical lymph nodes (LOE 3b, GR B), and a description of their characteristics and location5–7 (LOE 3b, GR C).
Laboratory StudiesThe thyrotropic hormone serum level must be determined since it indicates the hormonal status6 (LOE 1b, GR A). Its descent indicates hyperthyroidism, excludes the risk of neoplasm and evidences the pertinence of a scintigram (LOE 2b, GR B). An increased TSH makes it advisable to determine anti-TPO antibodies. Antithyroglobulin antibodies must be determined if there is a suspicion of lymphocytic thyroiditis, with normal TPO concentrations (LOE 3b, GR C). The determination of thyroglobulin is not preoperatively justified.5,6
Besides the standard determinations, we would include liver function tests8 (LOE 5, GR D), calcium tests and phosphorus tests.9 The determination of parathyroid hormone (PTH) allows us to rule out hyperparathyroidism and count on the postoperative descent gradient as a hypocalcaemia predictor. Therefore, its systematic determination is recommended. Within the same context, the determination of vitamin D 25-OH is also useful and, therefore, its determination is also proposed for all patients.
There is no consensus regarding the routine determination of baseline calcitonin to rule out medullary carcinoma.10,11 This would be cost-effective in hereditary syndromes, single solid nodules, whenever there is family history of thyroid cancer or suspicion of malignancy or medullary carcinoma in the fine-needle puncture.5,7,12 Therefore, it is recommended restrictedly in these clinical scenarios (LOE 3b, GR B). Elevated figures must be confirmed with a calcium or pentagastrin (contraindicated in pregnant women) stimulation test.13
A blood cross match has to be anticipated in interventions for anaplastic cancers or advanced tumours requiring extensive cervical dissections, though, given the low LOE available on this matter, the implementation should always be approved by the Transfusion Commission or the appropriate authority for each specific centre (LOE 5, GR D).
Imaging TestsThe first exploration test to be performed is cervical ultrasound: it is harmless, cheap and it can be performed during the office visit (LOE 1c, GR A). It provides a lot of information on the glands size and echogenicity. It will describe the presence or absence of nodules, their number, size, location, shape, margins, content, echographic pattern, vascularisation and presence of calcifications5,6 (LOE 2b, GR C). It explores the presence of adenopathies, vascular abnormalities, cysts and other malformations.5,14,15 The elastography and the contrast-enhanced ultrasound may be useful to determine the benignity or malignancy of the thyroid nodule, but they are not currently a part of the study protocol5,6 (LOE 2b, GR C).
Neither magnetic resonance imaging nor computed tomography is routinely indicated since they are less cost-effective and informative than the ultrasound5 (LOE 5, GR D). They should be ordered in cases of compressive symptoms, suspicion of endothoracic extension or retrovisceral location. In case of malignity, they allow for the detection of adenopathies, local infiltration or distant metastasis. With this last purpose in mind, magnetic resonance imaging (or computed tomography without intravenous contrast) is mostly chosen because the iodinated contrast used for the computed tomography interferes with the possible postoperative use of radioiodine9 (LOE 5, GR D).
Positron emission tomography would mostly be useful in cases of suspicion of recurrence of thyroid cancer, undetected by conventional techniques. In the preoperative study, more than one third of the cases of an incidental focal thyroid uptake correspond to carcinomas. In nodules with follicular neoplasm cytology, it has shown discriminative capabilities due to its high sensitivity, although with low specificity.5,16 However, its systematic use cannot be recommended (LOE 2b, GR B).
The thyroid scintigram is not routinely indicated. It is mainly used for the study of hyperthyroidism5,6,9 (LOE 2b, GR B). In the solid nodule with undetermined cytology, hyperfunction makes it more unlikely to be carcinoma. It must never be used in pregnant women.
Fine-Needle Aspiration-PunctureCytological study is the test of choice for the diagnosis of non-hyperfunctioning thyroid nodules.5,6,10,17 The performance of the fine-needle aspiration-puncture (FNAP) under echographic control, the collection of sufficient sample and the examination by expert cytologists improve its performance (LOE 2b, GR B). FNAP is not indicated in infracentimetric nodules, except in the case of echographic findings indicative of malignancy. In the case of cystic-solid lesions, the solid component must be punctured and, in multinodular glands, the nodule most suspicious of malignancy, regardless of size. (LOE 5, GR D).17
Globally, 72% of FNAP end up being benign, 5% malignant, 17% undetermined and 6% are failed tests due to insufficient or inadequate sample.18 Currently, the Bethesda cytology classification is the one that is most widely used (Table 2 of Annex 1, supplementary material available on electronic issue).19 As we can see, it establishes 6 diagnostic categories, for which it constitutes a risk of malignancy (with percentages supported by a wide range of subsequent studies20,21) and a clinical recommendation (LOE 2b, GR B).
The FNAP allows for the diagnosis of anaplastic, medullary and papillary carcinoma, thyroid lymphoma and metastases, but not for the diagnosis of follicular carcinomas17,18 (LOE 5, GR D). A thick-needle biopsy may offer additional information on cervical masses and thyroids presenting non-conclusive results in the FNAP22 (LOE 2b, GR B).
LaryngoscopyThe preoperative verification of mobility of the vocal cords, either via fibro-laryngoscopy, or via indirect laryngoscopy, may help in deciding the surgical strategy7 (LOE 5, GR D). Even though all authors acknowledge its usefulness, they usually admit that they do not perform it routinely. Due to the low LOE available and the limited profitability of this technique in asymptomatic patients from the vocal point of view and without medical background that could be linked to a laryngeal motility disorder, we cannot recommend it routinely. It must always be requested under the following circumstances:
- 1.
In the case of history of cervical or thyroid surgery.
- 2.
If the patient presents dysphonia or changes in the voice tone.
- 3.
Whenever an intraoperative neuromonitoring is to be performed.
- 4.
In cases of malignant or possibly malignant condition. In cases of advanced or anaplastic cancer, it should be supplemented with bronchoscopy and esophagoscopy.23
- 5.
In benign disease, whenever a greater risk of recurrence is foreseen, as in large endothoracic goitre with tracheal displacement or compression (LOE 4, GR C).
Patients must arrive euthyroid to the surgery, so antithyroid medication should not be preoperatively suspended24 (LOE 5, GR D). Occasionally, beta-blockers (propanolol) may be necessary for a better symptomatic control.25 The current antithyroid medication may have made unnecessary the preoperative use of Lugol's solution in Graves-Basedow disease26–29 (LOE 5, GR D).
Molecular Biology and Genetic StudyIt is performed on thyroid tissue samples. It may be useful in thyroid nodules with undetermined cytology. BRAF and RAS genes (N-RAS, H-RAS and K-RAS), and abnormal reorganisations type RET/PTC constitute the most studied somatic mutations associated with differentiated thyroid cancer. The gene expression for determination of messenger RNA or microRNA is also analysed.17,30 These are expensive determinations, with non-validated results, undefined usefulness and selective usage, although its medium-term incorporation to algorithms of decision is foreseen (LOE 2b GR B). On the other hand, mutational tests are essential upon suspicion of polyglandular syndromes (MEN2), in relation with the RET proto-oncogene.31
Intraoperative AspectsAntibiotic ProphylaxisIt is intended to reduce local bacterial load during the procedure.32 The surgical site infection incidence varies depending on the surgery type and location. If there is no prior tissue inflammation and the integrity of the aerodigestive tract is maintained, thyroidectomy should be considered a clean surgery that does not require antibiotic prophylaxis.33–38 The use of antibiotics in some patients with risk factors would be justified, when at least one of these is present: cancer, associated lymphadenectomy, airway opening, prolonged surgery or presence of at least one clinical risk factor: prior cervical radiation, recent chemotherapy, advanced age, malnutrition, diabetes mellitus, obesity, smoking, anaemia, peripheral vascular disease, inmunosuppression34 (LOE 3a, GR C). It will be administered in single dose before cutaneous incision and it must cover the most common pathogens in this area (Gram-positive cocci, including streptococcus species, negative coagulase staphylococcus and Staphylococcus aureus).39,40
Antithrombotic ProphylaxisThere are well-known risk factors for venous thromboembolism.41,42
Several models stratify such risk,43–45 such as Caprini (Supplementary material, Table 3 of Annex 1).45 This and the haemorrhage risk assessment46 are recommended to decide on prescribing thromboembolic prophylaxis. The guideline to antithrombotic therapy of the American College of Chest Physicians includes thyroidectomy in the same risk group as breast, urological and intestinal surgery and establishes some applicable therapeutic recommendations (Supplementary material, Table 4 of Annex 1).47
Most patients submitted to thyroidectomy are of at least moderate risk. On the other hand, pharmacological prophylaxis may increase the risk of haemorrhage in thyroid surgery up to 0.5%.48 Recent assessments of risk-benefit ratio recommend the preservation of pharmacological thromboembolic prophylaxis for high-risk patients, with c5 or more points as per Caprini's model of risk stratification for venous thromboembolism45,49 (LOE 2a, GR B).
Antinauseant ProphylaxisPostoperative vomiting and nausea appear in up to 71% of the cases after a thyroidectomy.50,51 They cause patient discomfort and an increase in venous pressure that compromises vascular ligatures or sealing areas, favouring haemorrhages. Preoperative dexamethasone reduces its incidence, the pain and the need for analgesics and it improves vocal function.52–54 The routine prophylactic use of antinauseant agents with a single preoperative dose of 8mg of dexamethasone is recommended55 (LOE 1a, GR A).
HaemostasisIt is mandatory to verify haemostasis after finishing the thyroid resection. Venous haemorrhage may be evidenced with Valsalva manoeuvres, applying positive expiratory pressure in the ventilation circuit. The Trendelenburg position at 30° assists in identifying additional bleeding points56 (LOE 2b, GR B). Cervical compression bandaging is not useful and makes it difficult to visualise a possible haematoma, so their use is not justified57 (LOE 1b, GR A).
Besides the classic haemostasis systems, we have new devices, such as the ultrasound energy system and the bipolar electrothermal vessel sealing system. In several meta-analyses, their use is advantageous over conventional systems regarding operating time, intra- and post-operative haemorrhage and hospital stay (LOE 1a, GR A).58–61 The current limited evidence does not allow for the extraction of recommendations regarding the advantages of using one of these devices over the other.62–68
The application of sealing and local haemostatic drugs (mainly fibrin-based) has been proposed. They may be useful to improve haemostasis and prevent postoperative seromas. In several studies, they reduce the debit measured in drainages,68 they prevent their use and reduce hospital stay.69–73 However, the products used are not comparable, the studies are limited and reduced on a case by case basis so their systematic application is not justified73 (LOE 2b, GR B).
Intraoperative BiopsyIt is not useful to rule out malignancy in lesions with cytology result of follicular neoplasm, since a detailed analysis is required to determine a vascular or capsular invasion.74,75 It would not be cost-effective in patients with a diagnostic FNAP of papillary carcinoma either.76–78 Some studies have raised awareness on the possible effect produced by frozen sections of the surgical specimen, that could alter the identification of vascular and capsular invasion, nuclear changes and the detection of microcarcinomas.74,75 Therefore, its systematic use is not recommended in thyroid nodule surgery. It should be reserved for cases of cytological suspicion of malignancy, unexpected intraoperative findings indicative of cancer or diagnostic confirmation of not very frequent lesions (LOE 2C, GR D).
Intraoperative NeuromonitoringSince the beginning of the xx century, the routine identification of the recurrent laryngeal nerve during thyroidectomy has been recommended to reduce its lesions.79 Moreover, the preservation of the external branch of the superior laryngeal nerve, anatomically related to the superior thyroid artery, is desirable.80
The introduction of intraoperative neuromonitoring in thyroid surgery is recent. In the most widely used method, an endotracheal tube with electrodes in the external part gathers the effect of the recurrent nerve stimulation through the contraction of the vocal cords. Its usage requires a preoperative and a postoperative laryngoscopy.81 Among other advantages, we can include:
- 1.
It may prevent bilateral recurrent lesion, if the surgeon does not act on the second side after verifying a loss of electromyographic signal in the former.82,83
- 2.
It may be specially useful in re-interventions, for surgeons with low volume of activity and from the medical-legal and teaching viewpoint.84,85
Some disadvantages have also been described:
- 1.
Its usage does not prevent recurrent paralysis since it only predicts it when a lesion has already occurred.85,86 Vagal nerve continuous stimulation could detect reversible electromyographic changes, but the results are not tested in a reliable manner.87
- 2.
It has a low positive predictive value.88 Upon loss of signal, the possibilities of paralysis are 30%–75%. Some of the causes of false positives are endotracheal tube displacement, equipment problems, a blood-filled surgical field and persistence of neuromuscular blockade.
- 3.
Its usage may delay total thyroidectomy to a second surgery. In up to 90% of the patients without nervous section, acting on the second side would not add risk due to intraoperative recovery of the nerve function.89
- 4.
It is doubtfully cost-effective. It does not reduce operating time and adds direct costs and operating room time.88
Two meta-analyses analyse its usefulness.84,85 One did not show nervous lesion rates reduction after its use.84 In the other one85,86,90–94 it only reduced significantly the risk of transient lesion of the external branch of the superior laryngeal nerve. Taking this information into account, we cannot recommend its routine usage (LOE 5, GR D).
Parathyroid Autogenous TransplantationThe parathyroid autogenous transplantation in the sternocleidomastoid muscle is a widely spread manoeuvre, although there are doubts regarding its actual degree of usefulness.95 In principle, it is only indicated when any gland has been totally devascularised or has been inadvertently removed. The best prevention is to maintain the glands in situ and vascularise them with a thorough technique, since the permanent hypoparathyroidism rate significantly increases after autogenous transplantation of more than 2 glands95 (LOE 4, GR C).
Use of DrainagesIt may be avoided practically in 90% of thyroidectomies,96 since:
- -
They do not prevent haemorrhage or make the possible re-intervention any faster.
- -
Its limited debit does not rule out haematoma, since they may be obstructed with clots.
- -
They do not prevent postoperative seromas and collections.
- -
They may increase the surgical wound infection rate.
- -
They cause patient discomfort and prolong hospital stay.
In summary, as gathered in the review of the Cochrane Collaboration (valid for patients without thyroid endothoracic extension, coagulopathy or lymph node dissections), they do not offer benefits and are unnecessary.97–99 Therefore, their use is selectively recommended (LOE 1a, GR A).
Postoperative AspectsRecovery Room StayThe minimum period of stay recommended is 6h100,101 (LOE 5, GR D). Possible complications, such as nausea and vomiting, pain, alterations in the respiratory function (breathing difficulty, laryngeal stridor) and cardiovascular alterations, haemorrhage causing asphyctic haematoma and other complications can be treated.101,102 In the absence of complications, an oral intake 4–6h after surgery may be initiated, preferably on demand103–106 (LOE 2b, GR B).
Monitoring of the Parathyroid Function and Treatment of HypocalcaemiaHypocalcaemia is the most frequent complication after a bilateral thyroidectomy. It occurs transiently in 30% of the patients and stays permanently (after surgery) in 2%. Its symptoms may start up to 72h after the thyroidectomy. Test procedures are required to rule it out prematurely (LOE 2a, GR B).107–110 Due to its minimum incidence, they would not be cost-effective after hemithyroidectomy.111
The tendency to perform ambulatory surgery112,113 has encouraged the development of several modalities for its premature detection.114–117 The isolated determination of calcaemia would have a maximum reliability at 72h, increasing hospital stay. Total corrected calcium may be measured with total protein and albumin (more affordable and extended) or the ionised.117,118 The measurement of PTH figures, taken during the first 24h after thyroidectomy, is useful to predict hypocalcaemia119 (LOE 2a, GR B). Associated to calcaemia, it provides maximum reliability.119–125 Since its reference values and measurement units vary,125 its relative level of descent is more easily generalised as of the preoperative to postoperative values,126 with the most predictive gradient located at 40%–75%.110,119,120,126–128
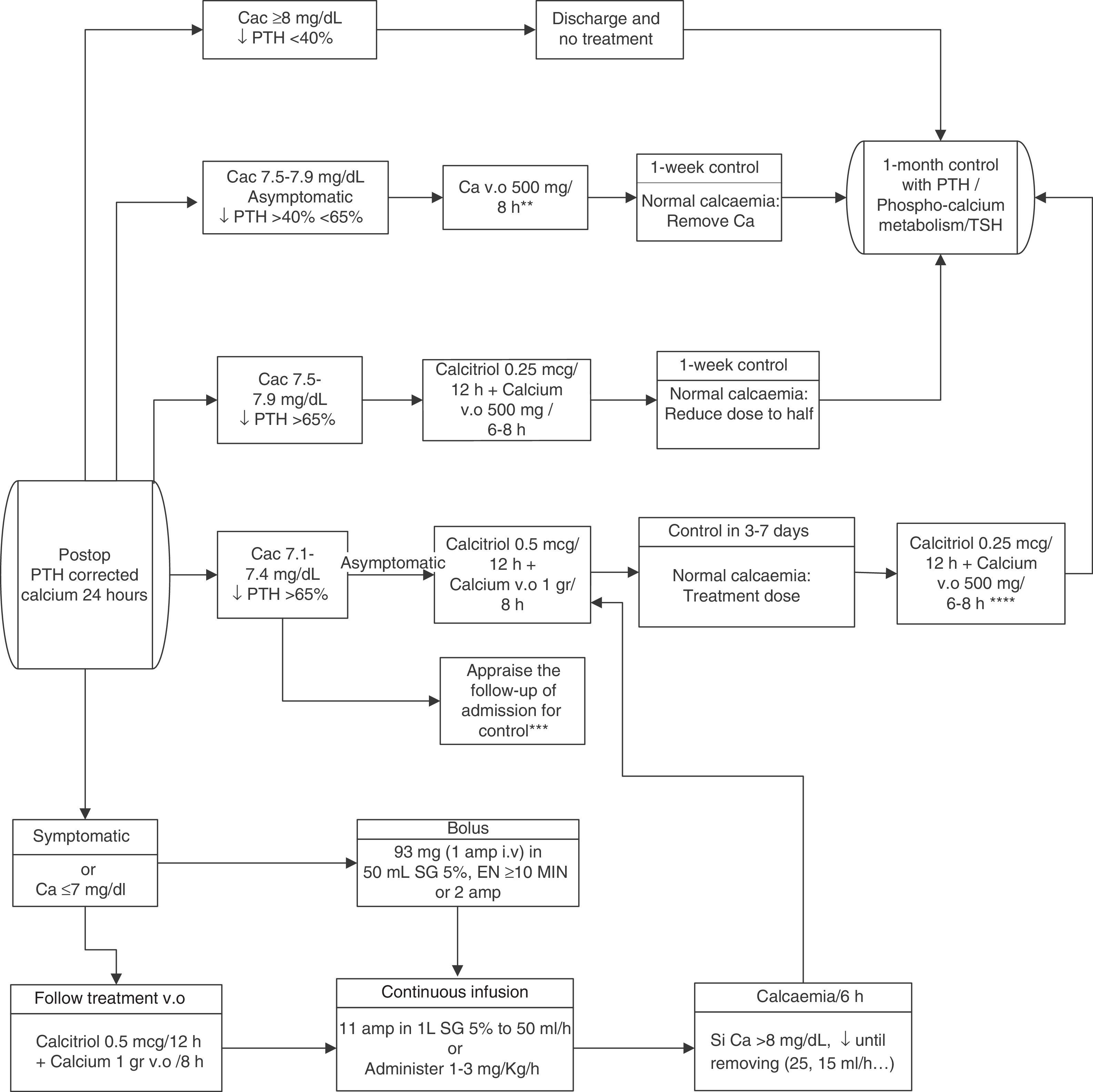
If we lack PTH figures, we may establish a cut-off point of 15pg/mL. Patients with higher values will not need calcium if they present calcaemias higher than or equal to 8mg/dL and they will be treated with low doses of calcium if they present calcaemias below 8mg/dL.
Reposition should be more aggressive, including calcitriol, in the case of PTH figures below 15pg/mL.
Therapeutic strategies in the cases of hypocalcaemia include the selective or routine reposition (depending on targeted needs) of oral calcium, associated to calcitriol or not, the active form of vitamin D. This association is more effective but requires a closer monitoring to prevent hypercalcaemias129–132 (LOE 2b, GR B). Intravenous calcium is reserved for very symptomatic patients or patients with calcium below 7–7.5mg/dL.133–136 It must be associated to oral treatment to regularise calcaemia more rapidly (LOE 2b, GR B). For oral replacement, the more recommended compounds are carbonate or calcium citrate. For the intravenous treatment (in slow perfusion), calcium gluconate, in 10ml ampoules at 10% containing 93mg of elemental calcium is preferred. Another option is calcium chloride in 10ml ampoules at 10% containing 270mg of elemental calcium, although it has more adverse effects.
In the cases of untreatable or serious hypocalcaemias, a concomitant hypomagnesemia (levels of Mg <0.7mEq/L or 1.4mg/dL) should be ruled out. For a rapid reposition, an intravenous treatment is required,137–139 though oral reposition is always preferred. The urgent treatment (intravenous) is performed by immediately administering 6–12mmol/L of magnesium sulfate (Sulmetin®), and 40mmol in the following 5h. A phial contains 150mg, 12mEq or 6mmol of Mg2+. 1–2 phials should be administered in 10–20min (never one phial in less than 10min). Orally, 15mmol/day should be administered (around 400mg of magnesium oxide). Supplementation must be maintained until oral intake improves and magnesemia is higher than 2mg/dL. There is more hypocalcaemia in patients with preoperative vitamin D deficiency so it would be advisable to maintain their preoperative levels within adequate ranges.135,136,140
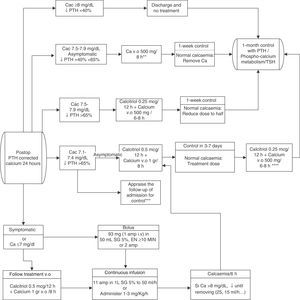
In conclusion, to minimise the risk of hypocalcaemia and favour one-day postoperative stays, we propose the joint determination of calcaemia (the day after the intervention) and postoperative PTH (extracted 4–24h after the end of the surgery, based on the centres availability). For this last one, a gradient descent of 65% of its preoperative value shall be considered to indicate substitution treatment.127 If there was a higher probability of hypocalcaemia due to intraoperative incidences, an oral “prophylactic” treatment may be prematurely established. The recommended reposition guidelines are presented in Fig. 1.
Therapeutic algorithm for calcium reposition. Amp: ampoules; Ca: calcium; Cac: corrected calcium. i.v.: intravenous; DS: dextrose solution; p.o.: per os (oral administration); PTH: parathyroid hormone; TSH: thyrotropic hormone.
*Calcaemia can be measured with total corrected calcium (with proteins or albumin) or with ionic calcium. The values proposed herein pertain to corrected calcium.
**The doses reflected pertain to elemental calcium.
***Appraise admission in very symptomatic patients or in patients with severe signs of hypocalcaemia, distance or difficult access to health care centre or prediction of difficulty in ambulatory handling.
****Appraise calcaemia figures and weight.
The current standard is at least one-night postoperative hospitalisation (LOE 5, GR D), and the minimum period of hospital observation is 6–8h. Ambulatory surgery is possible for selected patients.112,140–145 The American Thyroid Association has proposed relative contraindications (Supplementary material, Table 5 of Annex 1)146 and some conditions the patient must meet before ambulatory hospital discharge are:
- 1.
Ability to drink fluids and take oral medication.
- 2.
Adequate pain control with oral analgesics.
- 3.
Complete recovery of preoperative movement capacity and performance of basic daily activities.
- 4.
Adequate clinical condition after wound review, ruling out inflammation or cervical haematoma, dysphonia, dysphagia and dyspnoea.
- 5.
Adequate oxygenation, vital signs and blood pressure.
- 6.
Adequate social support and understanding of instructions.
The recommendations for supplementation with thyroid hormone vary depending on the disease treated. After total thyroidectomy in a non-hyperfunctioning benign disease, daily doses of 1.6mcg/Kg of levothyroxine are recommended for the first week. For patients over 65 years of age or cardiac patients, a lower initial dose is recommended. The objective is to maintain normal TSH figures at 4–6 weeks. Long-term hormone supplementation should be implemented by the endocrinologist and, once the dose has been adjusted, one annual determination of TSH would be sufficient.9,147 After a lobectomy, it is not necessary to start treatment, assessing the need for supplementation through TSH at 4–6 weeks. Due to the non-negligible percentage of patients with recurrence of nodular disease after hemithyroidectomy, an echographic and clinical control by a surgeon or endocrinologist would be advisable every 2 or 3 years.148
During the hyperthyroidism postoperative period, antithyroid drugs will be suspended. Beta-blockers must be progressively reduced throughout one week. Substitution with levothyroxine may be started a week later at a dose of 1.7mcg/kg.24,149
In malignant conditions (differentiated thyroid cancer), the dose will depend on the disease stage, the intention to administer radioiodine and the way in which the TSH is intended to be stimulated. If ablation is not scheduled or if it is performed using recombinant TSH, substitution with levothyroxine at 1.6–2mcg/kg will be started to achieve TSH inhibition (<0.1mUI/L). In the cases of high risk of recurrence, suppressive doses of TSH (<0.01mUI/L) will be required5,7,150,151 (LOE 2b, GR B).
In regards to the control of parathyroid function, if the patient has required a substitute treatment, a premature analytical control is recommended. Maximum doses of calcium and vitamin D require analytical control after 3 days or the reduction of the intake of calcitriol and/or oral calcium after 3 days and analytical control a week later. Lower doses allow for control after a week. A laboratory test with PTH is recommended a month after surgery to assess the recovery of the parathyroid function7,24,152–155 (LOE 2b, GR B).
The vocal function must be appraised. Even though there is some controversy and insufficient evidence, and it depends on availability in each centre, a postoperative laryngoscopy is advisable in all cases, especially if one has been preoperatively performed, as quality control of the units9,156 (LOE 5, GR D). It is essential in patients with preoperative motility alteration of the vocal cords, and in those with postoperative development of dysphonia, phonoasthenia, bitonal voice or swallowing disorder or if an intraoperative neuromonitoring has been performed.
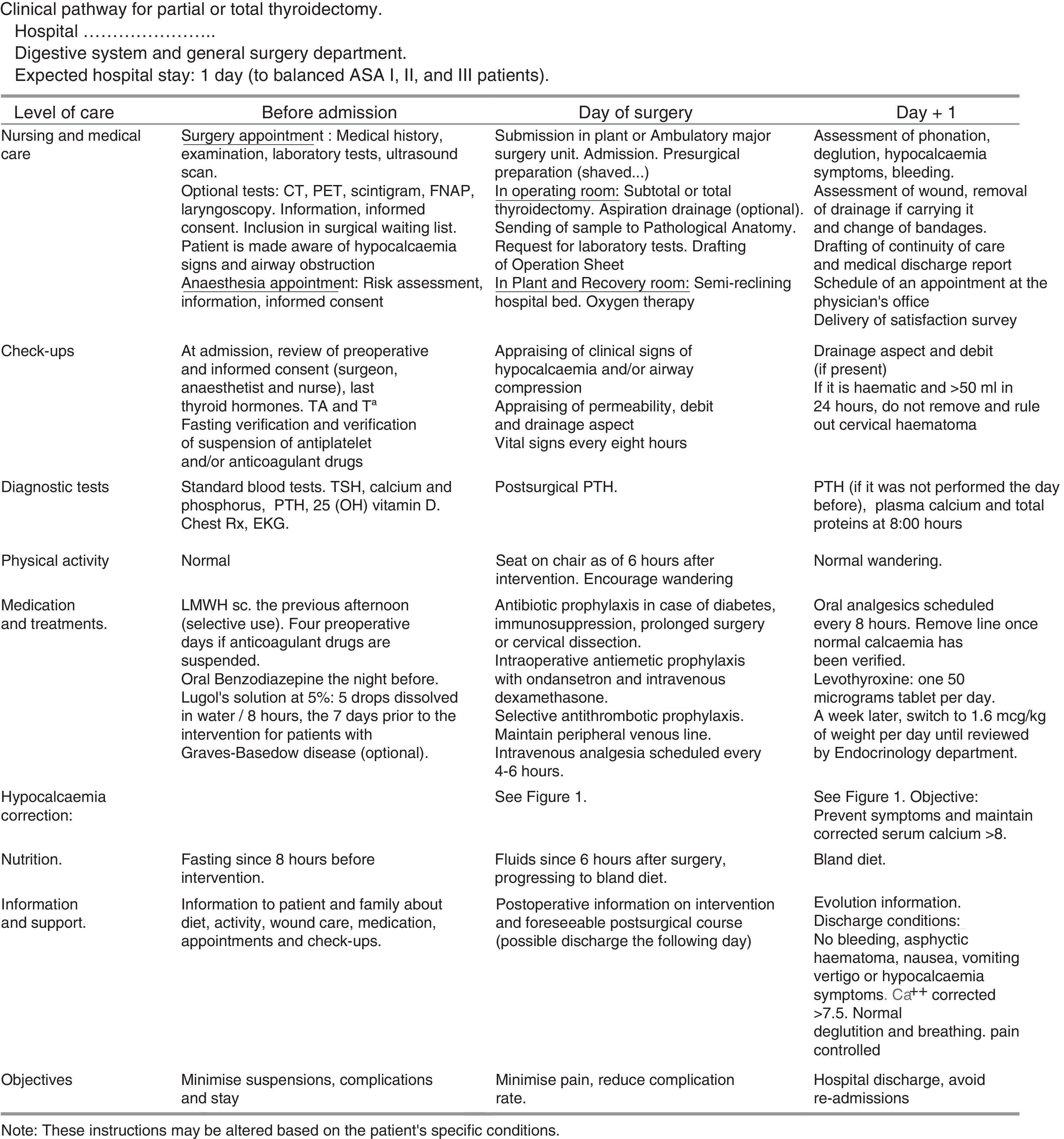
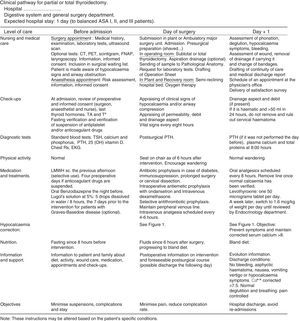
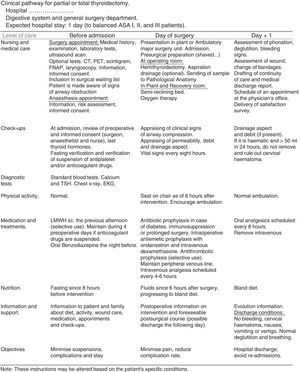
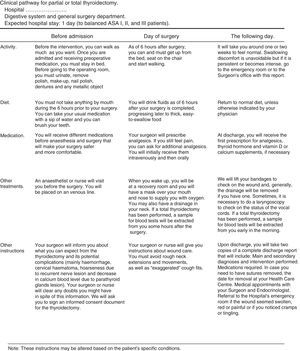
Documents Related to the Clinical Pathway for ThyroidectomyTime matrix. Chart that relates time (in divisions by days or hours) with actions and interventions performed on the patient: assessments and assistance, laboratory test or determinations, medical treatments, nursing care, medication, activity, diet, information, admission or discharge criteria. It is attached in Fig. 2.
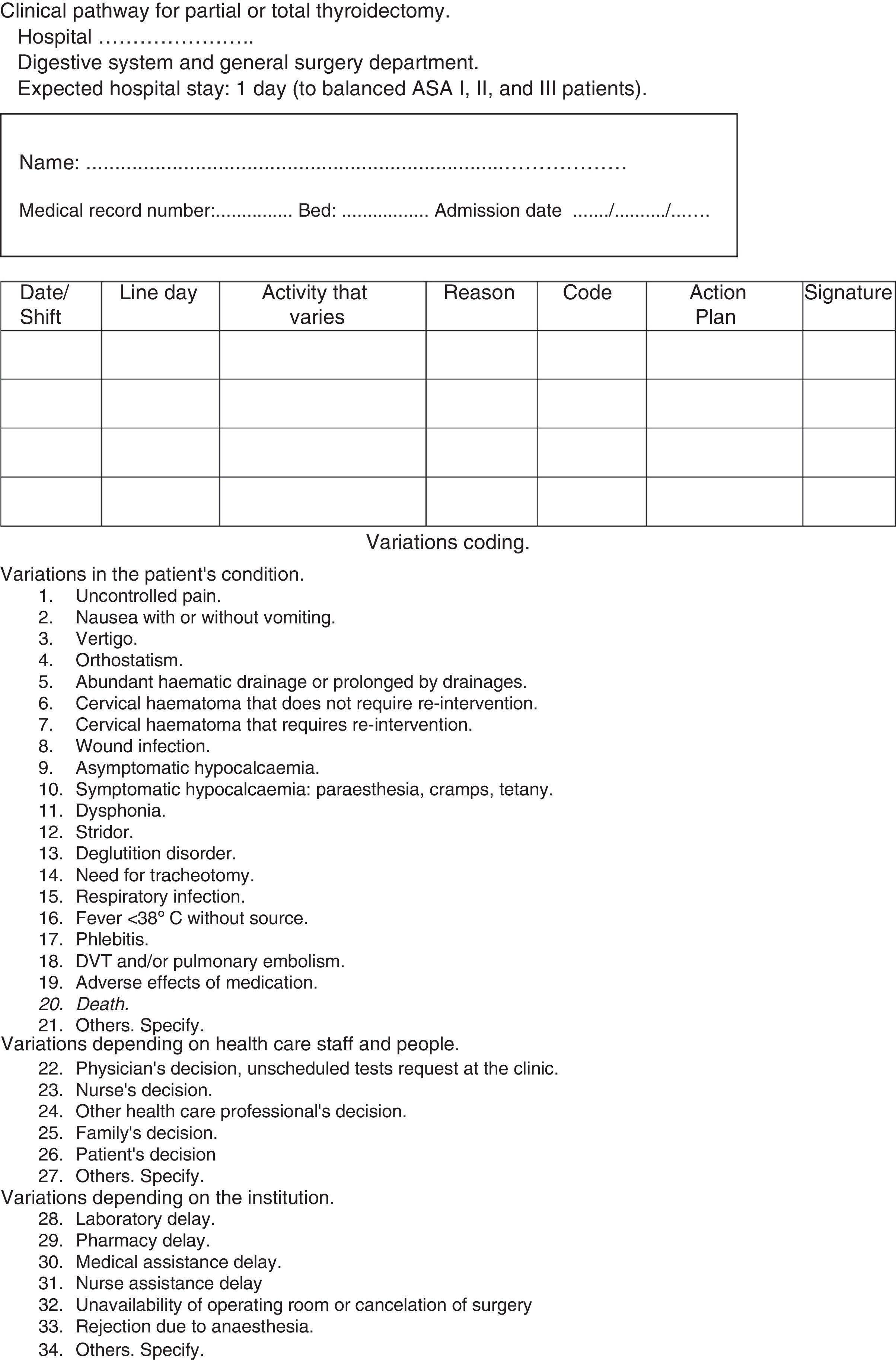
Variations sheet. It gathers the variations that occurred from the original plan and the solution adopted. It assigns codes to the most relevant variations. It is gathered in Fig. 3.
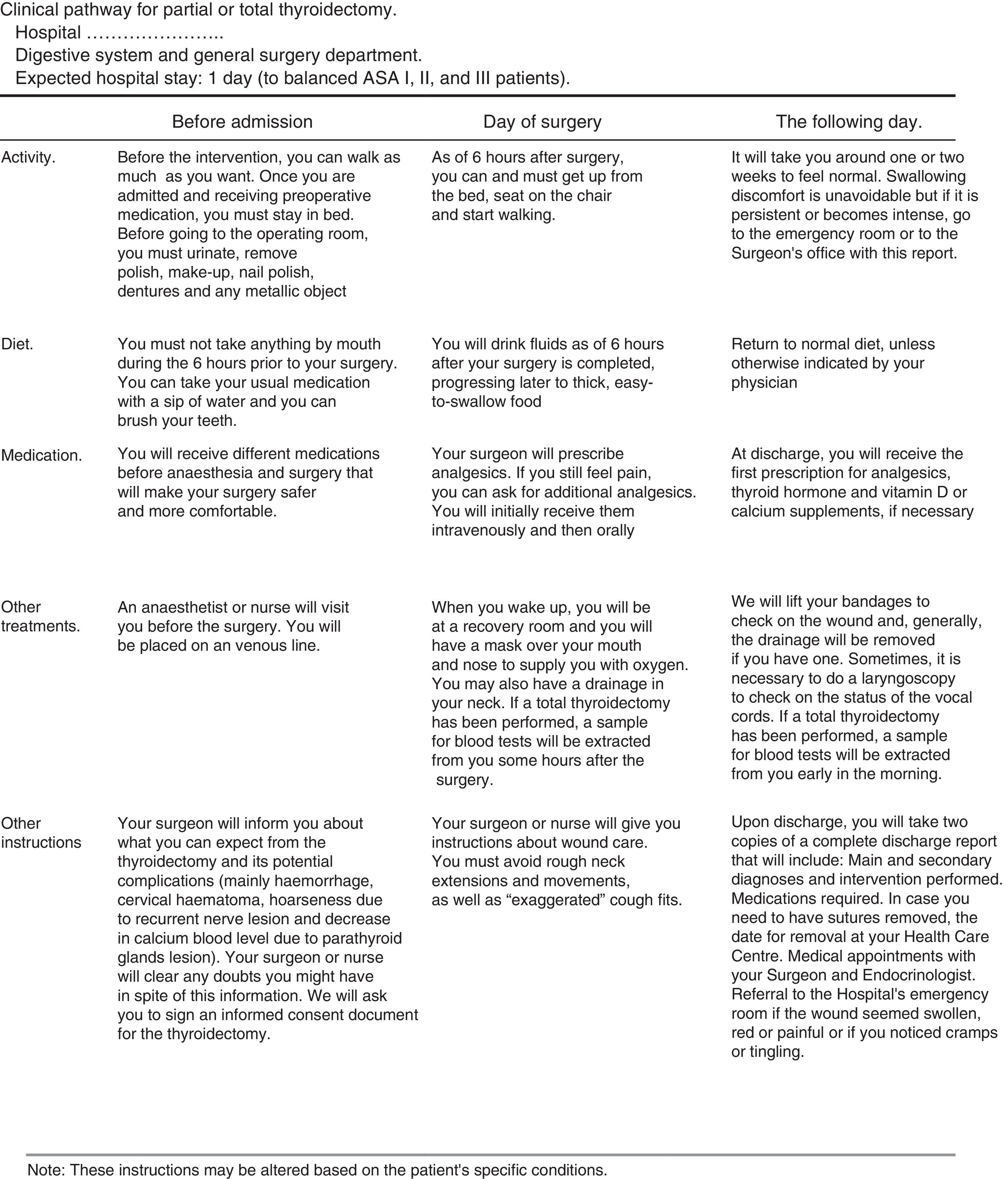
Patient's information sheet. It provides information on the activities to be performed during the process. Its awareness increases collaboration and reduces the anxiety induced by the intervention (Fig. 4).
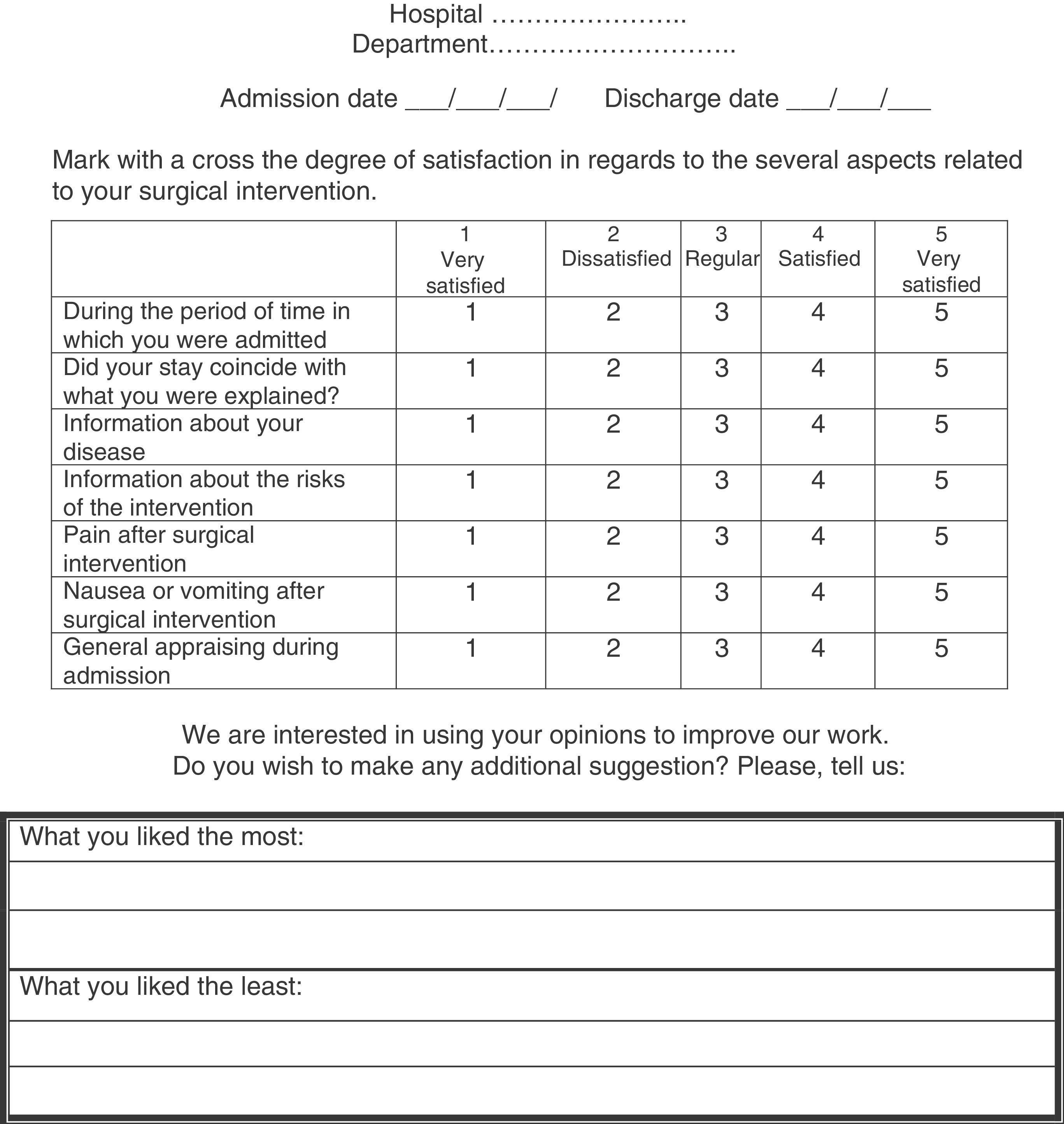
Satisfaction survey. It uses indicators of perception, assessment and improvement. Increasing patient satisfaction is not always more expensive. It is included in Fig. 5.
Assessment indicators. A group of relevant indicators has been selected, defining their formula, type, justification, origin, exclusions, necessary clarifications and relevant bibliography. They are gathered in the supplementary material (Table 6 of Annex 1).
FundingSupport received for the performance of this study in the way of grants: none.
Conflict of InterestsNone of the authors has received any funding related to the performance of this study or declares any conflict of interests.
Please cite this article as: Villar del Moral JM, Soria Aledo V, Colina Alonso A, Flores Pastor B, Gutiérrez Rodríguez MT, Ortega Serrano J, et al. Vía clínica de la tiroidectomía. Cir Esp. 2015;93:283–299.
Some information for this Clinical Pathway for Thyroidectomy was publicly announced on October 25, 2013, during the XIX National Meeting of Surgery held in Burgos.