In view of the current pandemic by SARS-CoV-2 it deems essential to understand the key concepts about the infection: its epidemiological origin, presentation, clinical course, diagnosis and treatment (still experimental in many cases). The knowledge about the virus is still limited, but as the pandemic progresses and the physiopathology of the disease is understood, new evidence is being massively published. Surgical specialists are facing an unprecedented situation: they must collaborate in the ER or medical wards attending these patients, while still needing to make decisions about surgical patients with probable COVID-19. The present narrative review aims to summarize the most relevant aspects and synthetize concepts on COVID-19 for surgeons.
Ante la pandemia por SARS-CoV-2 resulta fundamental conocer los aspectos claves de la infección: su origen epidemiológico, presentación, curso clínico, diagnóstico y los tratamientos empleados (aún experimentales en muchos casos). El conocimiento sobre el virus es limitado, pero a medida que progresa la pandemia y se conoce más su fisiopatología, se está publicando nueva evidencia de forma masiva. Los especialistas quirúrgicos se enfrentan a una situación sin precedentes: deben colaborar en plantas médicas o urgencias atendiendo a estos pacientes y además tomar decisiones sobre pacientes quirúrgicos con posible COVID-19. Esta revisión narrativa pretende resumir los aspectos más relevantes y sintetizar los conceptos básicos sobre COVID-19 para los cirujanos.
The current pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) is an unprecedented situation for healthcare systems. The high incidence of cases has saturated these systems, leading to the reorganization of hospitals and the availability of all resources for the treatment of patients with COVID-19. This has had a significant impact in the field of surgery, as many elective surgical procedures have been suspended, operating rooms closed for the use of the ventilators in intensive care units, and surgical staff redistributed to other medical services. Both the Spanish Ministry of Health and numerous scientific societies have published guidelines and recommendations for medical professionals. The Surgical Infection Division of the Spanish Association of Surgeons (Asociación Española de Cirujanos, or AEC) and the specific Surgery-AEC-COVID-19 workgroup have reviewed the scientific evidence and available guidelines, synthesizing key concepts about COVID-19 in order to facilitate the transfer of this knowledge to surgeons.
MethodsA review of the literature has been conducted using PubMed and platforms with specific COVID-19 resources: LitCovid,1 Cochrane Library,2 Lancet Resource Center,3 SpringerNature,4 BioMed central5 and JAMA network.6 Likewise, for other specific aspects, we have consulted updated information and documents from the Spanish Ministry of Health, the World Health Organization (WHO), Centers for Disease Control (CDC), as well as the recommendations of surgical societies including: AEC, American College of Surgeons, Society of American Gastrointestinal and Endoscopic Surgeons, European Cancer Organization and the Association of Surgeons of Great Britain and Ireland.
The co-authors conducted the bibliographic search, reviewed the selected articles, adapted the content for synthesis. The most relevant topics were grouped in an orderly manner to facilitate accessibility, consultation of this document. The authors have provided a narrative review of the literature available for certain key aspects of COVID-19, epidemiology, clinical presentation, diagnosis, treatment, which are of special interest to the readers of the journal. Specific concepts related to surgery have also been included, based on available evidence.
ResultsEpidemiology and TimelineThe new coronavirus responsible for severe acute respiratory syndrome (SARS-CoV-2) was first detected in December 2019 in the city of Wuhan (Hubei Province, China). Several cases of atypical pneumonia of unclear origin were detected. As the first infected patients had been to the Huanan market (Wuhan), this site was the suspected ground zero of the epidemic and was closed on January 1, 2020.7 The disease is considered a zoonosis, although it still has not been determined in which animal the disease originated. Based on the genetic sequencing of the virus, either bats or the pangolin would be the most probable animal of origin.8 The virus was sequenced in early January 2020, which identified it as a new type of coronavirus.9
On January 12, 2020, China openly shared the gene sequencing of the new coronavirus on the gisaid.org platform. On January 13, the first case outside China was detected in Thailand, which was an individual who had traveled to Wuhan. In the following days, cases were detected in Japan, Korea and other Asian countries. On January 20, the USA reported the first positive case, a traveler who arrived in Washington state that had also come from Wuhan. The first case in Europe was reported by the French Ministry of Health on January 24th.
On January 30, 2020, the WHO declared a Public Health Emergency of International Concern (PHEIC), and the number of cases continued to rise in several countries, including Iran, Italy and Spain. On March 11, 2020, the WHO declared COVID-19 had progressed to pandemic status.10
Situation in SpainTable 1 shows the chronology of the most relevant events in Spain in terms of the start and evolution of the epidemic.
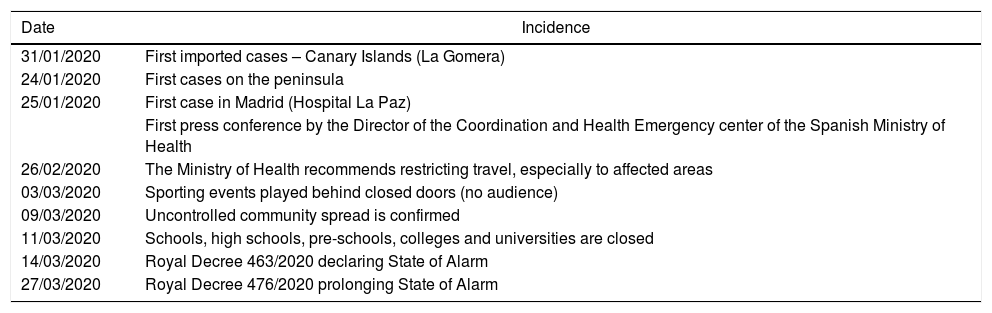
Timeline of the Most Relevant Events in Spain Regarding the Onset and Evolution of the Epidemic.
| Date | Incidence |
|---|---|
| 31/01/2020 | First imported cases – Canary Islands (La Gomera) |
| 24/01/2020 | First cases on the peninsula |
| 25/01/2020 | First case in Madrid (Hospital La Paz) |
| First press conference by the Director of the Coordination and Health Emergency center of the Spanish Ministry of Health | |
| 26/02/2020 | The Ministry of Health recommends restricting travel, especially to affected areas |
| 03/03/2020 | Sporting events played behind closed doors (no audience) |
| 09/03/2020 | Uncontrolled community spread is confirmed |
| 11/03/2020 | Schools, high schools, pre-schools, colleges and universities are closed |
| 14/03/2020 | Royal Decree 463/2020 declaring State of Alarm |
| 27/03/2020 | Royal Decree 476/2020 prolonging State of Alarm |
By April 17, 2020, a total of 188 068 cases had been registered in Spain, with 19 478 deaths and 72 963 cured patients (currently, these numbers are still rising). However, the estimates of undiagnosed cases are much higher. The Community of Madrid has been one of the most affected, with the highest number of confirmed cases (> 51 993 on April 17, 2020).11
Characteristics of the VirusCoronaviruses are a broad family of viruses first described in the 1960s. They are spherical, encapsulated viruses that contain single-stranded RNA surrounded by a protein coat. Protein S ‘spikes’ cause the characteristic structures giving it its crown-like appearance, and they are what determine the tropism of the virus and its fusion with host cells. There are several known coronaviruses circulating in animals and humans. An interspecies ‘jump’ can sometimes occur, as in the case of SARS in 2002 (which originated in civets) or MERS in 2012 (originating in dromedaries), causing serious novel respiratory diseases in humans. The main difference between these and the current SARS-CoV-2 is the transmissibility between humans that the latter has shown.
On February 11, 2020, the official nomenclature was established for the virus as SARS-CoV-2, and the disease it causes as COVID-19.12 It is a highly transmissible virus. The main form of transmission is droplets (large particles > 5 μm, moving 1–2 m) that are produced when an infected person sneezes or coughs. Transmission by contact with surfaces or fomites contaminated by these droplets is also relevant, with subsequent entry of the infection by touching the nose, eyes or mouth. Transmission by aerosols (small particles < 5 μm, moving more than 1 m) is also possible and has stirred up controversy. This factor is especially relevant in the healthcare setting. In the operating room, the possibility of aerosolization when performing intubation or any airway-related maneuver is of concern. Aerosolization also seems possible when using electrocoagulation or laparoscopy (if the patient is infected) when performing these techniques on infected tissue, mainly the respiratory tree.13
In vitro studies have shown that the virus can survive on multiple types of surfaces (metal, plastic or glass), where it can remain for up to 9 days.14 Disinfection of surfaces is therefore essential to minimize the contagion risk. Thorough hand washing (with soap and water) is recommended as a preventive measure, using either soap (due to the ability of the soap to disintegrate the viral lipid capsule) or hydroalcoholic gels. Likewise, solutions of 62%-71% alcohol (ethanol), 0.5% hydrogen peroxide and 0.1% sodium hypochlorite are highly virucidal (time of around one minute), which should be used in surface disinfection. Other biocides such as 0.02% chlorhexidine gluconate or 0.05%-2% benzalkonium chloride are less effective.15,16
The WHO has made available formulations to for individual/institutional/local production of alcoholic solutions in case of shortages or distribution problems.17
Prevention of Transmission and InfectionAs the specific mechanism of transmission of the virus is known, it is essential to establish preventive measures. In the case of transmission by droplets, the basic measures are:18
- •
Cover mouth/nose when sneezing, or use a disposable tissue and throw this away immediately.
- •
Symptomatic patients should cover their nose and mouth with a mask, one without a valve to avoid the dispersion of droplets.
- •
Wash hands with soap and water or hydroalcoholic solution.
- •
Maintain interpersonal distance (some studies even recommend 1.8-2 meters).
- •
Avoid touching your face (eyes, nose and mouth).
- •
Follow pertinent recommendations made by health authorities (local/national/international).
In the situation of an epidemic, it is essential to adopt a series of public health measures for the population, which will depend on the evolution of the epidemic and the local context:
- •
Vigilance: rapid detection of cases in order to establish pertinent isolation measures as early as possible during the containment phase.
- •
Screening at entry points: detection of imported cases and adequate information to persons coming from affected areas.
- •
Contact studies of confirmed cases to quickly identify infected individuals, and isolation (at home) of positive patients to avoid new contacts for 2 weeks (in the containment phase).
- •
Social distancing measures: these may include avoiding crowds or large gatherings or public events, and even mandatory quarantine of the population (mitigation phase).
- •
Healthcare authorities should inform the public about the measures implemented, number of cases and status of the epidemic; health education measures are also necessary.
The clinical presentation is highly variable, and different conditions have been observed, from mild (catarrhal symptoms) to very severe (adult respiratory distress syndrome). There are asymptomatic patients who pose an epidemiological problem due to their ability to transmit the virus unnoticed. The average incubation period is about 5 days (ranging from 0 to 14 days), and 97.5% of patients develop the disease in the first 12.5 days of incubation.19
The most frequently described clinical symptoms have been fever, cough (with/without expectoration) and general malaise. Other symptoms have been described with varying frequencies, including dyspnea, headache, asthenia, myalgia, odynophagia, nasal congestion/discharge, anosmia, ageusia, syncope, confusion, neurological symptoms, ophthalmological symptoms (conjunctivitis and dry eye) and cutaneous (rash skin eruptions).20
A percentage of patients report the presence of diarrhea, vomiting and abdominal pain as relevant symptoms. SARS-CoV-2 RNA has been identified in stool samples from infected patients, and the viral receptor ACE2 has been found with high expression in gastrointestinal epithelial cells, indicating that the virus could easily infect and replicate in the gastrointestinal tract. It has also been observed that the virus continues to be eliminated in the feces after the initial symptoms have passed.21 However, fecal-oral transmission has not been confirmed at this time.
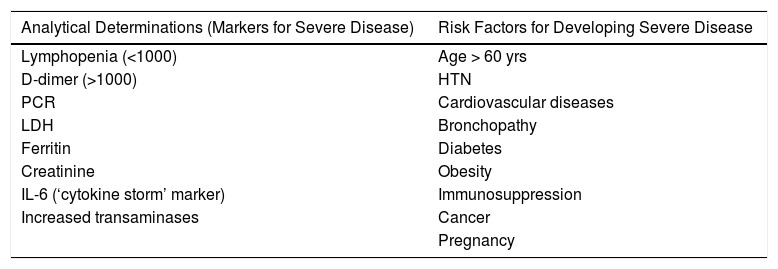
Preliminary data obtained mainly from studies carried out in the Chinese population seem to indicate that elderly patients with associated comorbidities (HTN, cardiopathy, diabetes, cancer, obesity, etc.) are at increased risk of serious presentations.22 It is estimated that more than 80% of total cases are mild/moderate, but 14% are severe and 5% critical.23 Patients with the highest mortality in the Chinese retrospective studies were males with a mean age 17 years older than survivors (68 vs. 51 years) who had some type of underlying pathological process (HTN, diabetes, cardiovascular disease or COPD). Furthermore, the presence of various associated comorbidities also increased the risk of poor progress.24Table 2 shows some serious risk factors and laboratory abnormalities that have been associated with poor clinical outcomes.
Lab Work Alterations and Risk Factors for Severe Disease Associated With Poor Clinical Outcomes.
| Analytical Determinations (Markers for Severe Disease) | Risk Factors for Developing Severe Disease |
|---|---|
| Lymphopenia (<1000) | Age > 60 yrs |
| D-dimer (>1000) | HTN |
| PCR | Cardiovascular diseases |
| LDH | Bronchopathy |
| Ferritin | Diabetes |
| Creatinine | Obesity |
| IL-6 (‘cytokine storm’ marker) | Immunosuppression |
| Increased transaminases | Cancer |
| Pregnancy |
It has been observed that cases with poor present sudden worsening (usually in the second week of illness), with the appearance of dyspnea and respiratory failure that usually lead to the need for oxygen therapy and occasionally intubation and ICU care around days 10 to 12.25,26 The usual cause of death is uncontrolled sepsis and respiratory failure. This poor evolution seems to correlate with the development of a ‘cytokine storm’ on the 7th day, which occurs as a result of the virus’s interaction with the patient’s immune system.27
DiagnosisMicrobiologyThe molecular diagnosis is based on RT-PCR techniques that study specific sequences of the virus genome. When the SARS-CoV-2 genome was openly shared, several groups began working to develop diagnostic RT-PCR tests based on genomic regions. In general, it is recommended to detect a less specific area for screening (the envelope gene or E gene) and another more specific one for confirmation (RNA-dependent RNA polymerase, or RdRP).28 There are different combinations of sequences according to the protocol developed by several laboratories. Despite having high sensitivity and specificity, these tests can present false negatives.29 Generally, these are due to the sample being insufficient or unrepresentative, taken too early or too late in the course of the disease, or degraded during transport or handling. In negative cases where suspicion or symptoms persist, it is recommended to repeat the RT-PCR in a few days.
Although nasopharyngeal or oropharyngeal samples are the easiest to obtain, bronchial samples (for example, bronchoalveolar lavage) are more effective for diagnosis yet more complex to obtain and require a higher level of biosecurity for personnel during extraction. The virus has also been detected in other biological samples (blood, feces, and saliva).30
Serological techniques are able to detect IgM and IgG antibodies. There is evidence that these techniques begin to detect antibodies from day 5 after the onset of symptoms, and they can be useful for population-based seroprevalence studies. There are already commercially available serological tests (immunochromatography) performed with whole blood that may be useful in diagnosis.
Cell culture is used for research. It takes several days, so it is not useful for clinical diagnosis, but it enables us to study the cytopathic effect of the virus on cell lines and to obtain virions. In this instance, biosecurity facilities (minimum BSL III) are necessary.
RadiologyIn the initial stages of the disease, changes may not be observed on plain radiography, or even on CT scan if performed during the first 2 days of symptoms. However, as the condition progresses, the sensitivity of CT increases, especially after the 6th day, when almost all patients with COVID-19 will present some type of alteration.
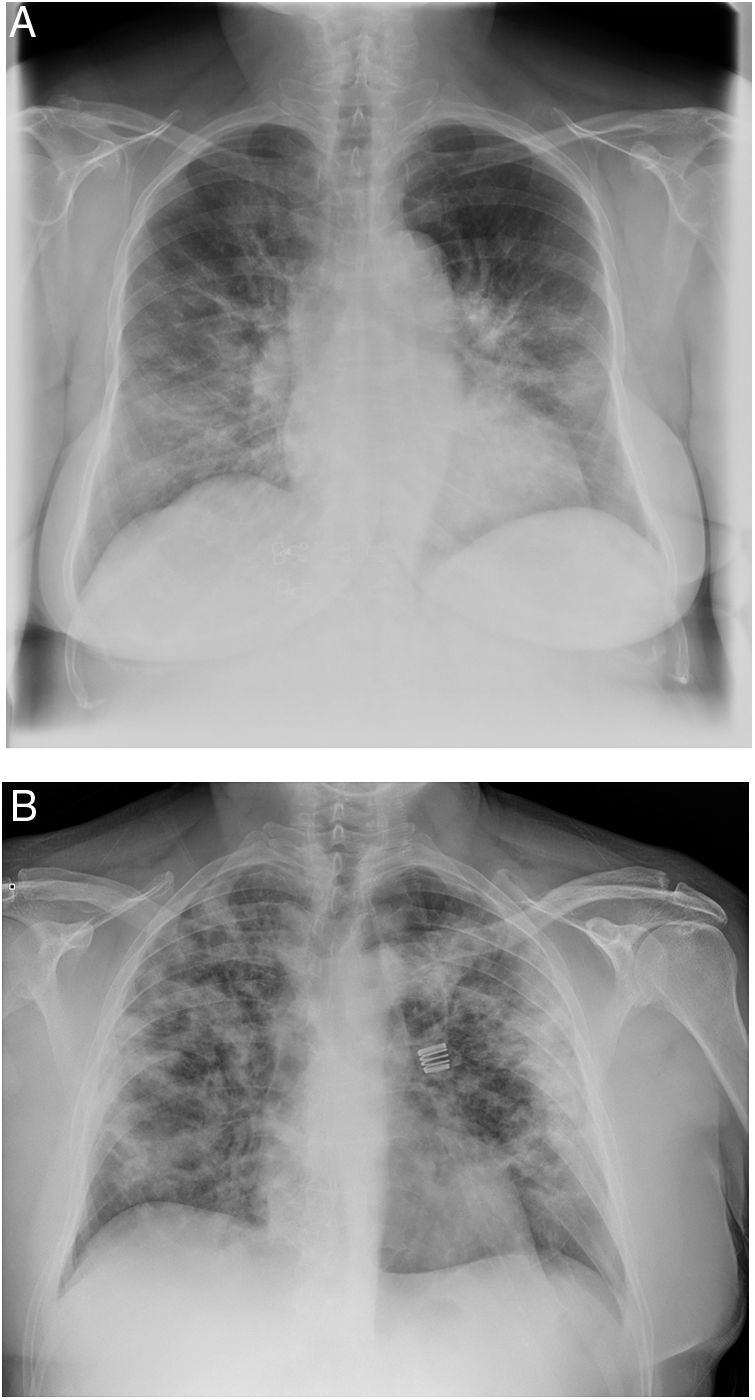
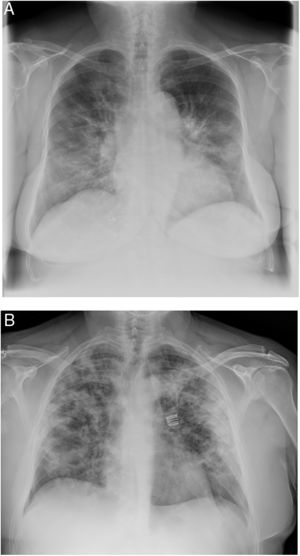
When the radiological image is abnormal, certain common patterns are seen.31 Asymmetrical areas of alveolar or interstitial opacity (patches or diffuse) are identified on simple chest radiograph. The most common pattern is bilateral pneumonia, with subpleural ground glass opacities, poorly defined margins and a slight predilection for the right lower lobe (Fig. 1).
Examples of simple chest radiology of patients with pneumonia in the context of COVID-19.
A) Moderate pneumonia with reticular-alveolar consolidations in the bilateral lower and middle lungs; B) Severe pneumonia showing extensive bilateral involvement with alveolar opacities and a tendency towards consolidation in the periphery of both lungs.
The most characteristic findings on thoracic CT are multiple ground glass opacities with peripheral lung consolidations.32 If these atypical findings are identified (even in asymptomatic patients), the diagnosis of COVID-19 should be considered. CT stratification has been used to assess severity and is the most sensitive diagnostic test, detecting lesions that are sometimes not identified on plain chest radiography. This may be especially relevant in the early identification of cases, for example preoperatively in patients with suspected disease requiring surgery.
LaboratoryRegarding lab results, increased C-reactive protein (CRP) levels with normal procalcitonin and lymphopenia (<1100 cells/μl) are characteristic. It should be noted that severe cases (compared to moderate ones) more frequently presented intense lymphopenia, hypoalbuminemia and higher levels of ALT, LDH, PCR, ferritin and d-dimer, as well as IL-2R, IL-6, IL-10 and TNF-α.33 Current evidence suggests that the exaggerated immune response mediated by the cytokine storm leads to acute lung injury and acute respiratory distress, which, without treatment and support, lead to death in a large proportion of patients.
In severe cases, it is recommended to rule out coinfections with other respiratory pathogens (Influenza A and B, Pneumococcus and Legionella) and to extract blood for cultures. In the first series published in China, 50% of the deceased presented a second infection during hospitalization.20
TreatmentTo date, there is no known effective treatment for COVID-19. However, several molecules have demonstrated in vitro activity against SARS-CoV2 and they are being investigated and used off-label.
There are currently various clinical trials underway from different initiatives, public and private, national and international, to try to establish which treatment may be effective for COVID-19.
The WHO has proposed an international clinical trial (SOLIDARITY trial) to evaluate the efficacy of the different therapeutic modalities proposed in affected countries, which have been used empirically or based on limited evidence.34 It is an adaptive, open, randomized trial that allows the study branches to be modified as more data become available. Currently, the molecules under evaluation are:
- 1)
Remdesivir (initially developed and previously evaluated for the Ebola virus).
- 2)
Lopinavir/ritonavir (enhanced antiretroviral therapy used for HIV).
- 3)
Lopinavir/ritonavir in combination with interferon β.
- 4)
Chloroquine or hydroxychloroquine (CQ/HCQ) (antimalarial drugs that have been used successfully in China).35
- 5)
All of them will be compared with standard support treatment without any molecule with potential antiSARS-CoV2 action.
The Spanish Ministry of Health frequently updates the drugs proposed for treatment as data and new therapeutic options are registered. Currently some of the drugs being considered in the protocol are: CQ/HCQ, lopinavir/ritonavir, remdesivir, tocilizumab, sarilumab, interferon beta-1B and interferon alfa-2B.36
CQ/HCQ has demonstrated an antiviral effect in vitro, and it therefore has been considered a possible candidate for treatment and has been widely used empirically. However, other in vivo experiments with a murine model and SARS-CoV-2 have not shown that CQ/HCQ was able to inhibit viral replication. Following a recent French observational study, with no control arm or randomization and a very limited number of patients, it was suggested that combined treatment with CQ/HCQ and azithromycin could more rapidly reduce viral load.37 In contrast, another trial (with 11 patients) found no benefit (clinical or antiviral) of this combined therapy in patients with severe COVID-19.38 The real usefulness of this guideline has not yet been clearly established, although there are other multiple clinical trials under development (mostly in China).39
When administering any of these off-label treatments, it is essential to evaluate the pharmacological interactions with the patient’s current medication and potential toxicity. Serious interactions can occur (such as QT prolongation with CQ/HCQ)40 and occasionally there are relevant contraindications (in cardiac or HIV-positive patients, etc.). Web portals such as www.hiv-druginteractions.org provide for quick consultation of interactions.
The utility of plasma from convalescent patients is being tested for the treatment of critically ill patients with COVID-19,41 and several laboratories around the world are investigating the development of a possible vaccine.
Implications for SurgeonsThe AEC, as well as other national and international scientific societies, have issued clinical guidelines and recommendations to try to standardize practices at this critical moment, aimed at protecting both patients and healthcare professionals.42–44
Around the world, the available evidence has been used to dynamically update treatment guidelines in moderately and critically ill patients, diagnostic testing, tracing contacts, as well as the necessary protective measures for healthcare workers depending on the type of care or procedure to be carried out.45 Any procedure with increased exposure to respiratory secretions (intubation, tracheostomy, ENT examination, etc.) is considered high risk. Other body fluids that may present viral load, such as saliva, feces and blood, in which viral load has been detected to a greater or lesser extent, are also considered a risk.46
Regarding the possibility of aerosolization in surgical procedures (electric scalpel and other electronic devices, laparoscopic smoke, etc.), various studies have been carried out by surgical teams, and specific proposals have been made to reduce this aerosolization (smoke filtration with a water seal and bleach, suction of smoke from the electric scalpel, etc.). In any case, and given the possibility of this infection route, it is essential that all operating room personnel have adequate and complete personal protective equipment, especially face protection (FFP2/FFP3/N95 masks, closed goggles and shields when needed).
After the experience in China and Italy, some important measures have been proposed for laparoscopy:47 minimize the dispersion of aerosols, avoiding uncontrolled leakage of gas/fluids through trocars or incisions; use closed filtration and evacuation systems for smoke; use the lowest possible gas pressure and the lowest intensity programs for electrocoagulation. It is also essential to adequately train and instruct operating room staff in this regard.
Specific Considerations for the Field of SurgeryElective SurgeryUnder the circumstances of a pandemic, the high number of patients affected simultaneously saturates healthcare services. In the case of COVID-19 (due to its ability to produce severe respiratory symptoms), there is an increased demand for ventilators and hospital or ICU beds for these patients. Therefore, in anticipation of the need for these resources, it has been proposed to delay all elective surgical procedures when possible, always considering the local epidemiological situation and the resources of each hospital. Patients with a benign pathological process and/or with a low risk of complications may be postponed without a problem. In other elective cases in which there is some risk of complication due to not performing the intervention, the urgency of each case will be assessed individually (considering the benefit/risk), but postponing is recommended as long as possible until the current epidemiological situation is normalized.48,49
Oncology PatientsOncology patients can be especially affected during the pandemic period for several reasons50:
- •
Greater susceptibility to infection due to the baseline disease or secondary to immunosuppression of adjuvant treatments (chemotherapy).
- •
Alteration of their treatment regimens (chemotherapy and radiotherapy) or inability to conduct scheduled surgical procedures, which may affect the course of their disease and/or prognosis.
- •
Complications of oncological disease that require urgent treatment or hospitalization and increase the risk of SARS-CoV-2 infection (bleeding, perforation or obstruction due to untreated stenosing tumors, thrombosis, other infections, etc.).
In case of suspicion and symptoms compatible with COVID-19, multiple international societies, such as the European Cancer Organization, advocate the prioritization of SARS-CoV-2 detection tests in these patients.51
Decisions on whether or not to proceed with elective surgery in cancer patients currently depend on the local epidemiological situation, availability of operating rooms and ICU at the corresponding hospital, disease status and the risk of progression or complications (individualized), assessment of surgical risk and potential complications of the procedure. In addition, specific assessment is necessary of possible pre/postoperative COVID-19 infection as well as the individual risk in terms of the comorbidities of the patient, who must also be informed.52 Individualized decision-making is promoted by multidisciplinary committees,53 and the creation of ‘clean’ hospitals where cancer patients can be referred has been proposed in high-incidence areas. In some cases, and depending on the type of tumor, even alternative treatments (such as extra cycles of chemotherapy) are proposed to control progression and/or delay surgery.54
Urgent SurgeryDisease requiring urgent surgical management cannot be delayed or canceled despite the pandemic situation. In cases of pathological processes requiring surgery that cannot be postponed (appendicitis, peritonitis, etc.), several aspects must be evaluated when making decisions:
- •
Patient COVID-19 status: does the patient present a concomitant viral infection in addition to the surgical disease? This determination is essential for the patient’s prognosis and surgical risk as well as for the surgical team, who must be adequately protected.
- •
Is there sufficient protective material (personal protective equipment [PPE])? Are the facilities satisfactory to operate safely?
- •
Are there any alternative treatments other than surgery that would be safe for the patient?
For this reason, medical societies like the AEC55 and the American College of Surgeons56 suggest determining the patient’s COVID-19 status before proceeding with the surgical intervention in order to act in the safest way. All available means will be used to make this determination, ideally including RT-PCR and chest radiology (preferably CT scan). When it is not possible to determine COVID-19 status, or in uncertain cases, proceed with surgery under the assumption that the patient is positive, providing adequate protection for operating room personnel with complete PPE.
Some extra measures have also been suggested, such as delaying the entry of staff into the operating room as much as possible, that the procedure be carried out by the most experienced surgeon available and that all non-essential personnel leave the operating room before extubation.
As already mentioned, the use of the laparoscopic approach has generated great controversy because of possible aerosolization with the use of pneumoperitoneum. The Association of Surgeons of the United Kingdom and Ireland even recommended avoiding this route to only perform urgent open surgery in the context of the pandemic, which they subsequently rectified.57 Others have debated that depriving the patient of the advantages of laparoscopy (less invasive access, shorter hospital stay and therefore less likelihood of infection in negative COVID patients, etc.) is not justified. At the present time, treatment should be individualized as much as possible, taking into consideration the patient’s surgical needs, condition, COVID-19 status, available therapeutic alternatives and the hospital resources available (PPE, operating room, intensive care units, etc).
Health Personnel and Individual ProtectionThe availability of adequate material and sufficient PPE has been one of the most relevant issues since the start of the pandemic because of the global shortage and the many infections (and deaths) occurring among front-line healthcare workers.58 The experience in Italy has been especially important in this regard, since a much higher number of infections was detected among healthcare personnel than in China, even leading to the closure of hospitals due to lack of personnel and the death of 25 doctors.59,60
Unfortunately, the situation in Spain has developed in a way similar to Italy, and we are currently the country with the most infected health personnel (around 14%), which in many cases is secondary to the lack of adequate protection.
It is essential for any surgeon working with COVID-19 to have sufficient, quality PPE available.
ConclusionsThe current SARS-CoV-2 coronavirus pandemic is the greatest challenge that contemporary medicine has ever experienced. It directly affects all surgeons, requiring their role within health institutions to be redefined and their usual therapeutic strategies to be rethought, while having to adapt to unfavorable working conditions in which surgeons may be seriously affected.
The very high incidence of SARS-CoV-2 infection requires surgeons to have an in-depth understanding of the disease, including daily tasks and medical treatments with which they are not familiar. Likewise, they need to adapt their practice to a critical situation, prioritizing safety in the work environment and care for the most serious patients, while considering options they usually do not consider, especially in emergencies. The need to know and implement strategies aimed at patients whose surgeries cannot be delayed, especially cancer patients, is essential to avoid what are called secondary and tertiary victims of the pandemic: infected healthcare workers and, above all, patients who ultimately receive suboptimal treatment.
It is essential to highlight that the current situation has made decision-making necessary in a scenario full of uncertainties, where the scientific evidence to support these decisions is scarce. Thus, although the effort of the health community continues to be intense, it is essential to constantly reevaluate each of the measures adopted. Being a surgeon in the era of COVID-19 requires maximum versatility, which means accepting that today’s decisions may not be tomorrow’s. Again, this is an opportunity to renew Deaver’s aphorism that a surgeon is always more than just a doctor, yet never anything less.
Conflict of InterestsThe authors have no conflict of interests to declare.
The authors would like to dedicate this article to the memory of Dr. Joaquín Diaz Domínguez, Head of General Surgery at the Hospital Universitario La Paz, in Madrid. May he rest in peace.
Esteban Martin-Antona, Estíbaliz Álvarez Peña, Alejandra Garcia-Botella, Elena Martín Pérez, Mario Álvarez Gallego, Sagrario Martínez Cortijo, Isabel Pascual Migueláñez, Lola Pérez Díaz, José Luis Ramos Rodríguez, Eloy Espín-Basany, Raquel Sánchez Santos, Xavier Guirao Garriga, José Manuel Aranda Narváez and Salvador Morales-Conde.