Nodular thyroid disease possesses the potential to harbor malignancy. Our aim was to evaluate the significance of cervical diffusion-weighted magnetic resonance imaging (DW-MRI) for the detection of malignant thyroid nodules.
MethodsSixty-five thyroid nodules from 58 patients who had undergone surgery were evaluated. Preoperative parameters, demographic data, ultrasound findings, fine-needle aspiration biopsy results and apparent diffusion coefficient (ADC) values of the nodules at DW-MRI were compared with the results from postoperative pathology examinations.
ResultsThe “benign group” included 50 (76.9%) nodules, while 15 (23.1%) nodules constituted the “malignancy group”. Minimum, maximum and mean ADC values of the nodules were significantly lower in the malignancy group (P<.05). The best cutoff value for the mean ADC value was 1.33×10−3mm2/s, with a sensitivity of 66.67%, a specifity of 89.13%, a positive predictive value of 53.63% and a negative predictive value of 89.13%. A mean ADC value equal to or lower than 1.33×10−3mm2/s was associated with 9 times higher risk of malignancy (odds ratio: 9.111, 95% confidence interval: 2.49–33.21).
ConclusionsThe ADC value detected by cervical DW-MRI can be considered a predictive parameter for the detection of thyroid cancer.
La enfermedad tiroidea nodular posee el potencial de desarrollar malignidad. El objetivo es evaluar la importancia de la resonancia magnética ponderada por difusión cervical (IRM-DP) para la detección de nódulos tiroideos malignos.
MétodoEl grupo de estudio lo constituyen los pacientes intervenidos por enfermedad tiroidea, en los que preoperatoriamente se les realizó 3 métodos diagnósticos: biopsia guiada por PAAF, US e IRM-DP. El grupo de estudio total se dividió en 2 subgrupos, de acuerdo con la evaluación postoperatoria patológica final de los nódulos en 2 grupos: «grupo benigno» y «grupo de malignidad». Se evaluaron 65 nódulos tiroideos operados, en 58 pacientes. Los parámetros preoperatorios, (datos demográficos, los hallazgos ecográficos, los resultados de la biopsia por aspiración con aguja fina y los valores del coeficiente de difusión aparente (CDA) de los nódulos en IRM-DP), se compararon con los resultados de los exámenes patológicos postoperatorios.
ResultadosEl «grupo benigno» lo constituyen 50 (76,9%) nódulos, mientras que 15 (23,1%) nódulos constituyeron el «grupo de malignidad». Los valores mínimos, máximos y medios de CDA de los nódulos fueron significativamente más bajos en el grupo con malignidad (p<0,05). El mejor valor de corte para el valor medio de CDA fue 1,33×10-3 mm2/s, con una sensibilidad del 66,67%, una especificidad del 89,13%, un valor predictivo positivo del 53,63% y un valor predictivo negativo del 89,13%. Un valor medio de CDA igual o inferior a 1,33 × 10-3 mm2/s se asoció a un riesgo 9 veces mayor de malignidad (p<0,01, odds ratio: 9,111, intervalo de confianza del 95%: 2,49-33,21).
ConclusionesEl valor de CDA detectado en IRM-DP cervical puede considerarse como un parámetro predictivo para la detección de cáncer de tiroides.
Nodular thyroid disease is a common clinical condition which affects approximately 2%–7% of the population and possesses the potential of harboring malignancy.1 Currently, none of the diagnostic tools are capable of definitely excluding thyroid malignancy.
Ultrasonography (US) is the most preferred, first step diagnostic modality for evaluating nodular thyroid disease.2 The structure, dimensions, echogenicity, and the presence of calcifications of a nodule can be visualized by thyriod US, and fine-needle aspiration (FNA) biopsy is performed for nodules with suspicious US findings, in order to either identify, or to rule out malignancy.3 Although this routine diagnostic approach is satisfactory for the follow-up of most cases, FNA can not distinguish between benign and malignant nonpapillary follicular and oxyphilic cell lesions.4–6
Magnetic resonance imaging (MRI) is a cross-sectional radiological diagnostic modality which uses strong magnetic fields and radio waves. The soft tissues are evaluated anatomically via T1 – weighted images, whereas T2 – weighted images are useful for the detection of pathological intensity signals.7,8 Contrast material can also be used to enhance pathologic findings.
Diffusion-weighted magnetic resonance imaging (DW-MRI) derives its image contrast from differences in the motion of water molecules between tissues. The degree of restriction to water diffusion in biologic tissue is inversely correlated to tissue cellularity and integrity of cell membranes.9,10 The motion of water molecules is more restricted in tissues with a high cellular density associated with numerous intact cell membranes (i.e. tumoral tissue).11 By contrast, in areas of low cellularity or where the cellular membrane has been breached, the motion of water molecules is less restricted. Apparent diffusion coefficient (ADC) is defined as a measure of the magnitude of diffusion (of water molecules) within tissues. ADC is calculated for each pixel of the image and is displayed as a parametric map. By drawing regions of interest (ROIs) on these maps, the ADC values of different tissues can be derived.11,12 Areas of restricted diffusion in highly cellular areas show low ADC values compared with less cellular areas that return higher ADC values.11
Although DW-MRI is not used routinely for the diagnostic evaluation of thyroid pathologies, there are studies revealing that this imaging modality may give information about the malignancy potential of a thyroid nodule.6,13–15 In the present study, our aim was to evaluate the diagnostic value of DW-MRI and the ADC value for the detection of malignant nodules in nodular thyroid disease.
MethodsThe study group consisted patients having undergone thyroid surgery due to nodular thyroid disease that had been detected preoperatively via all three diagnostic methods including US, US guided FNA biopsies, and DW-MRI. The total study group was divided into two subgroups according to the postoperative final pathological evaluations of the nodules as the “benign group”, and the “malignancy group”.
Patients having undergone a completion thyroidectomy, or surgery for recurrent nodular thyroid disease, cases with poor quality MRI images, presence of a previous history of radiation therapy in the neck, or a past history of neck surgery were accepted to be the exclusion criteria.
Parameters of the study group including the demographical data of the patients, US findings, FNA cytology results, and the ADC values of the nodules at DW-MRI were recorded. Approval for this study was gained from the Ethics Committee of our institution, and signed informed consent forms were obtained from all patients.
The MRI study was performed using a 1.5 – T MRI unit (GE Signa HDxt, GE Healthcare, General Electric Company, UK). The patients were positioned in the supine position and images were obtained with a neck array coil. Spin echo sequence was used to acquire T1 weighted (TR: 560, TE: 10ms) and T2 weighted (TR: 3000, TE: 100ms) axial images for the evaluation of the relevant anatomy and to determine cystic components. A section thickness of 3mm and intersection gap of 1mm, with a field of view (FOV) of 15cm was chosen for these sequences. Diffusion weighted images were obtained using single shot echo-planar imaging sequence (TR: 5000, TE: 60ms) with a b-value of 1000s/mm2, slice thickness of 2–4mm, a FOV of 26cm and matrix size of 192×256. The ADC values of the nodules were calculated by the same radiologist who was blinded for the FNA cytology results. In the T1- and T2-weighted sequences, the ROIs for both the nodules and the extranodular normal thyroid tissues were drawn by the radiologist taking care of excluding large cystic areas if present. The ROI areas placed in the nodules and normal areas of thyroid tissues measured between 25–50mm2. Following the determination of ROIs, the minimum, maximum, and mean ADC values were calculated automatically by the software of the system.
Comparisons of the minimum, maximum, and mean ADC values either between malignant and benign nodules, or between the pathological nodules and the extranodular normal thyroid tissues were carried out to obtain the results of the study.
Mann–Whitney U test, Pearson's chi-squared test, Fisher's exact test, Fisher-Freeman-Halton test, and Yates’ correction for continuity were used for statistical analyses. Differences were considered statistically significant when P<.05.
ResultsThe study group consisted of 65 thyroid nodules of 58 patients having undergone thyroid surgery. Fifty two patients (87.9%) were women and seven (12.1%) were men. The mean age was 49.00±12.02 (range: 18–71). According to the final pathological evaluations of the postoperative specimens, the “benign group” consisted of 50 (76.9%) nodules, while 15 (23.1%) nodules constituted the “malignancy group”.
The mean size of the nodules was 27.00±8.80 (range: 10–56) mm. According to the preoperative US and fine-needle aspiration (FNA) biopsy results, 44 (67.7%) nodules were determined to be benign, while 9 (13.8%) to be cancer or follicular/Hurthle cell neoplasia, 7 (10.8%) to be atypia of undetermined significance, and 5 (7.7%) nodules were determined as nondiagnostic cytology.
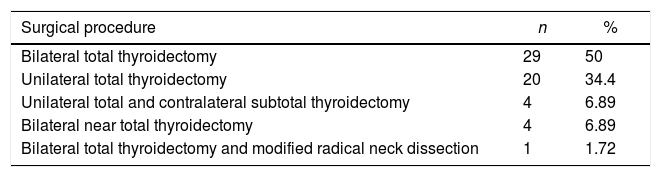
All patients underwent thyroid surgery due to either suspicious US findings (i.e. solid nodule with irregular margins, increased nodule size, hypoechogenicity, presence of calcifications, etc.), or FNA biopsy results. The distribution of the surgical procedures among the entire study group is summarized in Table 1.
The Distribution of the Surgical Procedures Among the Entire Study Group.
| Surgical procedure | n | % |
|---|---|---|
| Bilateral total thyroidectomy | 29 | 50 |
| Unilateral total thyroidectomy | 20 | 34.4 |
| Unilateral total and contralateral subtotal thyroidectomy | 4 | 6.89 |
| Bilateral near total thyroidectomy | 4 | 6.89 |
| Bilateral total thyroidectomy and modified radical neck dissection | 1 | 1.72 |
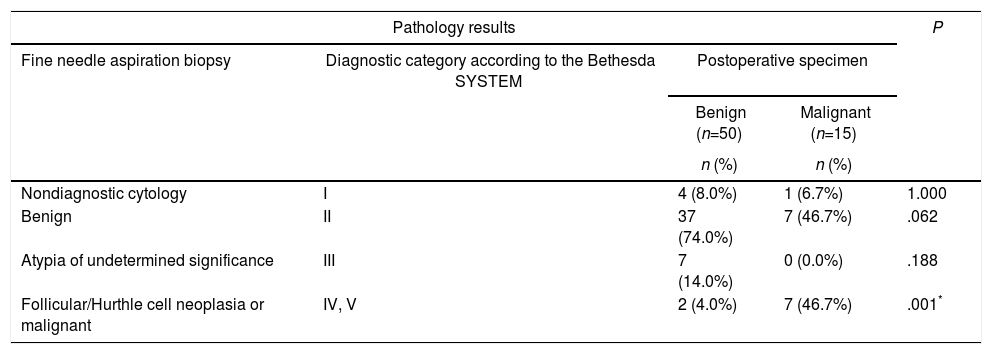
No statistically significant differences were detected between the benign group and the malignancy group in concern of either nondiagnostic cytology, benign cytology, or atypia of undetermined significance obtained via preoperative FNA biopsies (P>.05). The rates of the preoperative FNA results revealing cancer or follicular/Hurthle cell neoplasia were significantly higher in the malignancy group when compared to the benign group (P=.001; P<.01). The comparisons between the preoperative FNA biopsy results and the postoperative final pathological examination results of all nodules in the study group are summarized in Table 2.
The Comparison of the Fine Needle Aspiration Biopsy Results With the Postoperative Patological Evaluations.
| Pathology results | P | |||
|---|---|---|---|---|
| Fine needle aspiration biopsy | Diagnostic category according to the Bethesda SYSTEM | Postoperative specimen | ||
| Benign (n=50) | Malignant (n=15) | |||
| n (%) | n (%) | |||
| Nondiagnostic cytology | I | 4 (8.0%) | 1 (6.7%) | 1.000 |
| Benign | II | 37 (74.0%) | 7 (46.7%) | .062 |
| Atypia of undetermined significance | III | 7 (14.0%) | 0 (0.0%) | .188 |
| Follicular/Hurthle cell neoplasia or malignant | IV, V | 2 (4.0%) | 7 (46.7%) | .001* |
Fisher's exact test.
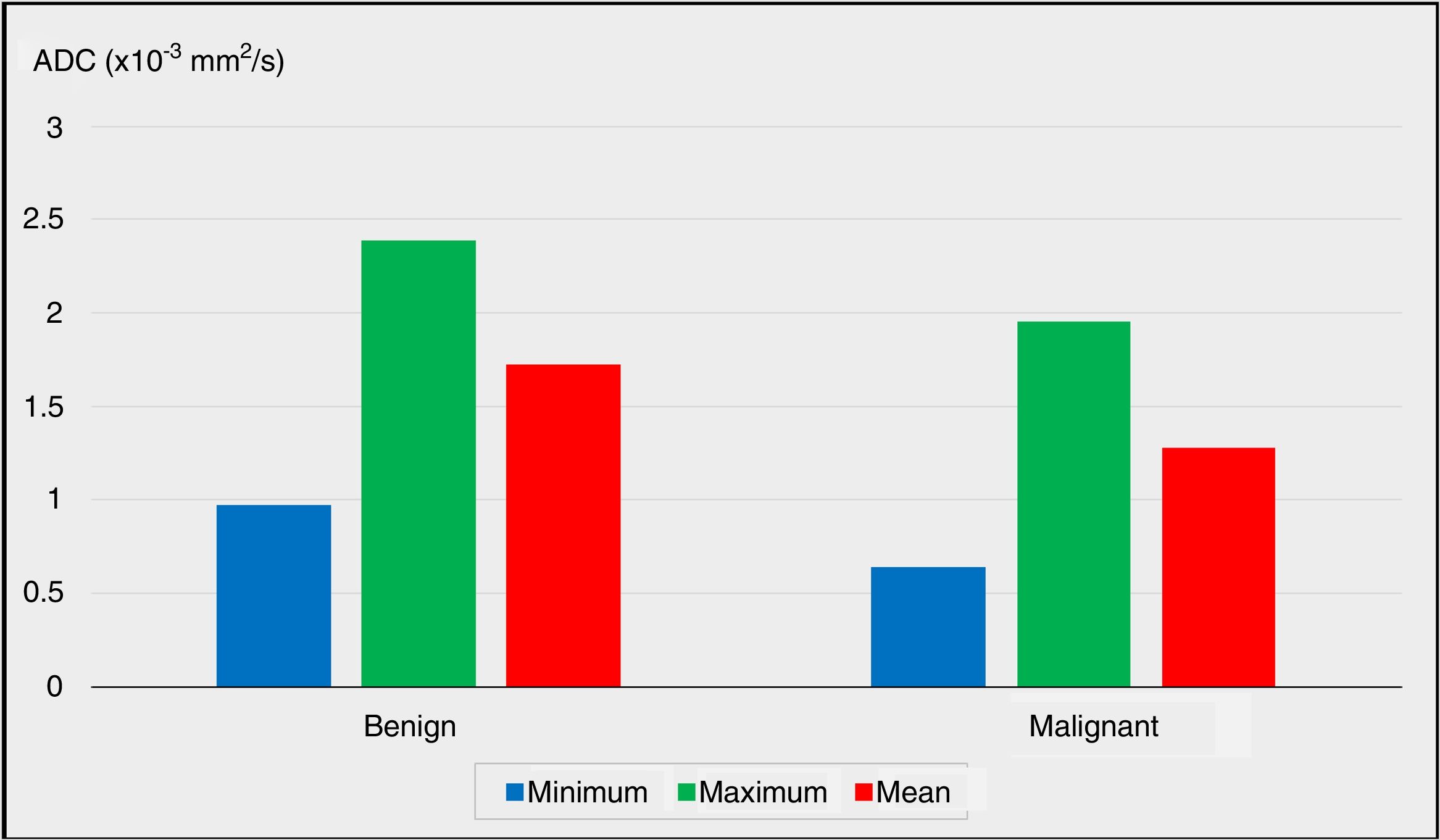
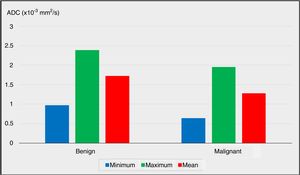
The preoperative DW-MRI findings were radiologically evaluated to calculate the ADC values of the nodules. The minimum and maximum ADC values of the nodules were significantly lower in the malignancy group when compared to the benign group (P=.011 and P=.013; P<.05 and P<.05, respectively).
The comparison of the mean ADC values between the benign and malignancy groups revealed advanced level of significance (P=.001). The mean ADC value of the nodules was significantly lower in the malignancy group (P<.01).
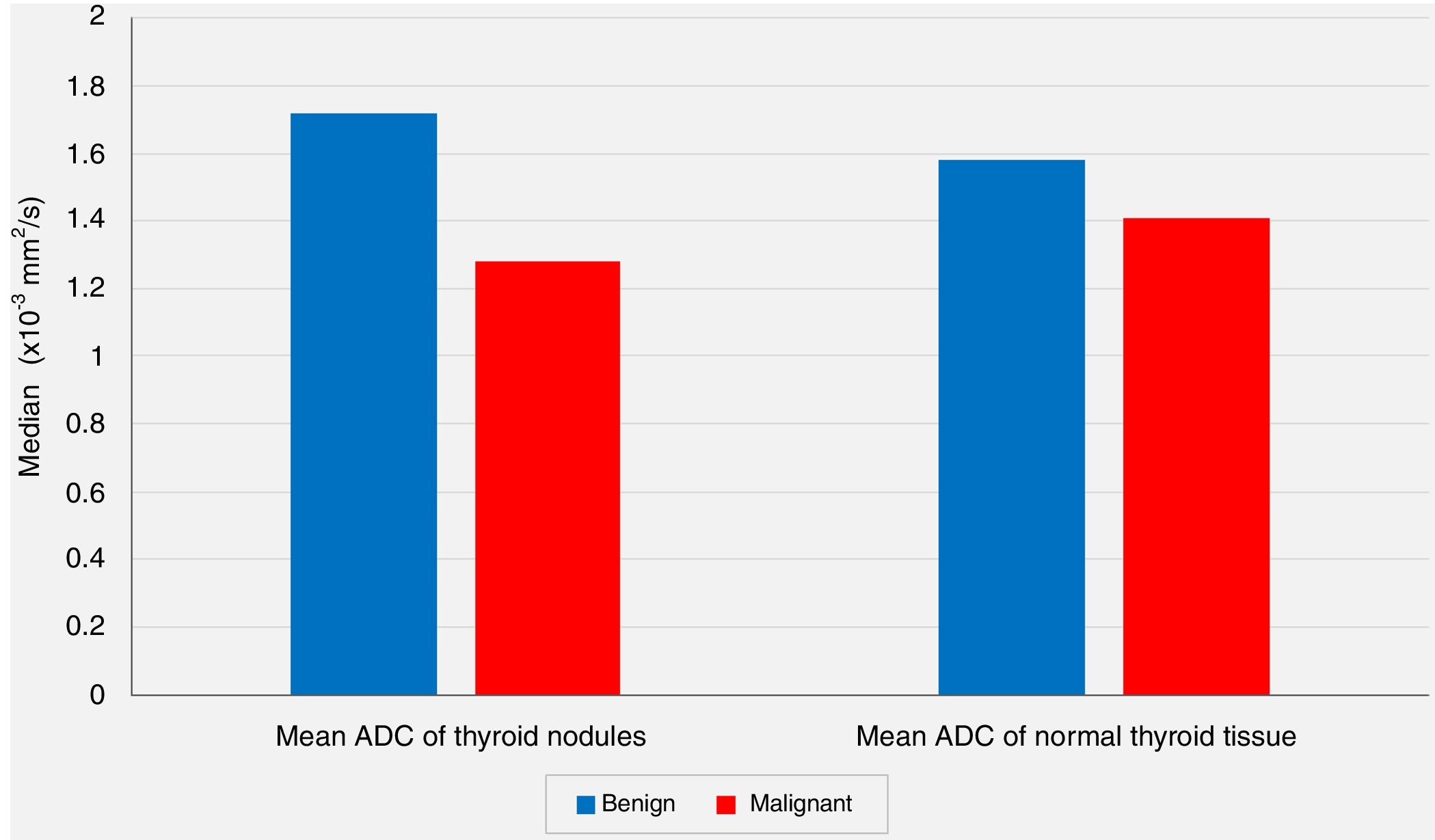
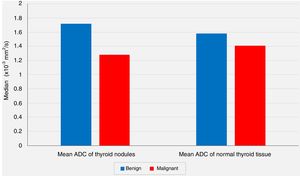
The comparisons of the mean ADC values of the extranodular normal thyroid tissues of the same thyroid lobe that harbored the nodule revealed no statistically significant differences between the benign group and the malignancy group (P=.213, P>0.05).
The comparisons of age, nodule size, and ADC values between the benign group and malignancy group are summarized in Table 3.
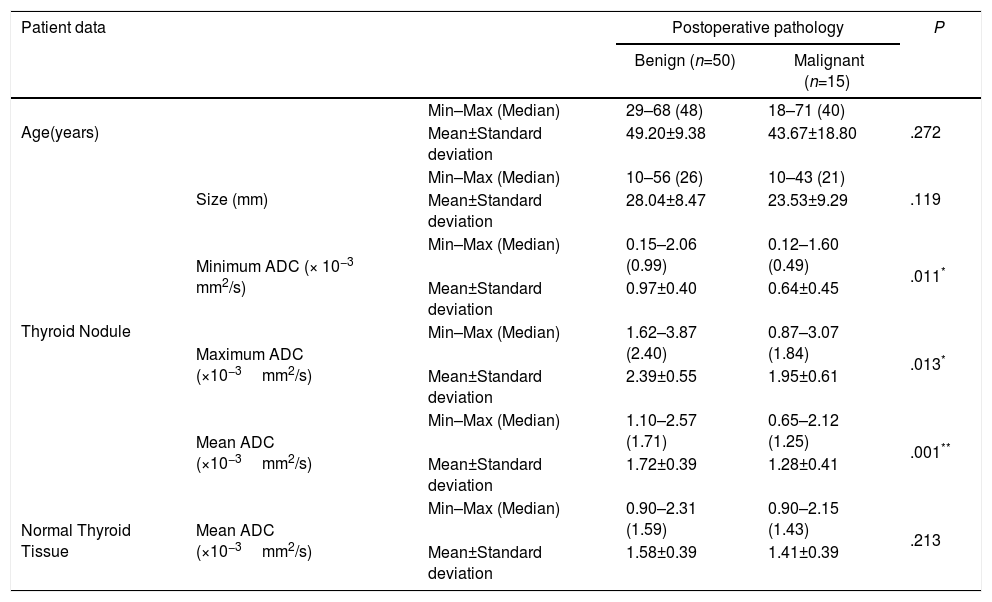
The Comparisons of Age, Nodule Size, and Apparent Diffusion Coefficient (ADC) Values Between the Benign and Malignancy Groups.
| Patient data | Postoperative pathology | P | |||
|---|---|---|---|---|---|
| Benign (n=50) | Malignant (n=15) | ||||
| Age(years) | Min–Max (Median) | 29–68 (48) | 18–71 (40) | .272 | |
| Mean±Standard deviation | 49.20±9.38 | 43.67±18.80 | |||
| Thyroid Nodule | Size (mm) | Min–Max (Median) | 10–56 (26) | 10–43 (21) | .119 |
| Mean±Standard deviation | 28.04±8.47 | 23.53±9.29 | |||
| Minimum ADC (× 10−3 mm2/s) | Min–Max (Median) | 0.15–2.06 (0.99) | 0.12–1.60 (0.49) | .011* | |
| Mean±Standard deviation | 0.97±0.40 | 0.64±0.45 | |||
| Maximum ADC (×10−3mm2/s) | Min–Max (Median) | 1.62–3.87 (2.40) | 0.87–3.07 (1.84) | .013* | |
| Mean±Standard deviation | 2.39±0.55 | 1.95±0.61 | |||
| Mean ADC (×10−3mm2/s) | Min–Max (Median) | 1.10–2.57 (1.71) | 0.65–2.12 (1.25) | .001** | |
| Mean±Standard deviation | 1.72±0.39 | 1.28±0.41 | |||
| Normal Thyroid Tissue | Mean ADC (×10−3mm2/s) | Min–Max (Median) | 0.90–2.31 (1.59) | 0.90–2.15 (1.43) | .213 |
| Mean±Standard deviation | 1.58±0.39 | 1.41±0.39 | |||
Mann–Whitney U Test.
The comparisons of the minimum, maximum and mean ADC values between the benign group and the malignancy group are plotted in Fig. 1 (P<.05, P<.05 and P<.01, respectively).
In spite of the significant difference between the two groups concerning the mean ADC values of the nodules, the comparisons of the mean ADC values of the extranodular normal thyroid tissues of the same thyroid lobe harboring the nodule revealed no statistically significant differences between the benign group and the malignancy group (P<.05 and P>.05, respectively) (Fig. 2).
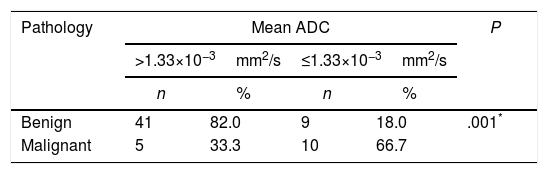
According to the postoperative final pathology results, the best cutoff for the mean ADC value was calculated as 1.33×10−3mm2/s with a sensitivity of 66.67%, a specifity of 89.13%, a positive predictive value of 53.63%, and a negative predictive value of 89.13%. The odds ratio for the pathological results was found as 9.111 (95% confidence interval: 2.49–33.21). It was determined that the detection of a mean ADC≤1.33×10−3mm2/s was associated with 9 times higher risk of malignancy (P=.001, P<.01) (Table 4).
The Cutoff Value for Apparent Diffusion Coefficient (ADC) Was Calculated to Be 1.33×10−3mm2/s, and Significantly Increased Risk of Malignancy Was Detected With Values Equal or Below This Level.
| Pathology | Mean ADC | P | |||
|---|---|---|---|---|---|
| >1.33×10−3mm2/s | ≤1.33×10−3mm2/s | ||||
| n | % | n | % | ||
| Benign | 41 | 82.0 | 9 | 18.0 | .001* |
| Malignant | 5 | 33.3 | 10 | 66.7 | |
Yates’ correction for continuity.
Since nodular thyroid disease is a common clinical condition, its diagnosis and management remains to be an important issue.1,16
US is the most commonly used diagnostic tool for the detection of nodular thyroid disease, and US guided FNA biopsy is performed for further evaluation of the thyroid nodules.2,4,17–19 In their study of 6226 patients, Amrikachi et al. have given sensitivity, and specifity rates of 93%, and 96%, respectively for FNA biopsies.20 In our series, the rates of FNA results revealing benign cytology, atypia of undetermined significance, and nondiagnostic cytology did not reveal any significant difference between the benign and malignancy groups (P>.05). On the other hand, the number of nodules determined with either cancer or follicular/Hurthle cell neoplasia via preoperative FNA was significantly higher in the malignancy group (P<.01). We consider that the variabilities in FNA results are due to the limited number of patients in our study group which consisted of specific patients who had been evaluated via both US and DW-MRI preoperatively.
MRI has also been used for the diagnosis of thyroid malignancies. At dynamic MRI, the images taken before and after the administration of the contrast agent are compared, and interpretations are made according to the wash out patterns. Dynamic MRI gives adequate information about the thyroid gland, and the cervical soft tissues. In their study on 57 thyroid nodules of 26 patients, Tunca et al. evaluated the dynamic contrast-enhanced MRI findings of the nodules.14 Eight nodules were detected to be malignant according to the postoperative pathology results, and the MRI findings of all of these malignant nodules revealed significantly delayed wash out patterns (P<.01). Tunca et al. stated that in cases when other diagnostic methods ended up to be inconclusive, dynamic contrast-enhanced MRI was significantly superior to US guided FNA biopsy for the evaluation of thyroid nodules.14
In their study on 42 thyroid nodules of 38 patients, Nakahira et al. investigated the efficacy of DW-MRI on distinguishing malignant thyroid nodules.6 They found that the mean ADC value for the benign nodules was 1.93±0.37×10−3mm2/s, and the mean ADC value for the malignant nodules was 1.20±0.25×10−3mm2/s. They concluded that the mean ADC value of the malignant nodules was significantly lower than the benign nodules (P<.01).6 In another study, El-Hariri et al. evaluated 56 thyroid nodules of 37 patients with nodular goiter via DW-MRI, and they found the mean ADC value to be 1.85±0.24×10−3mm2/s for the benign nodule group, whereas this value was calculated to be 0.89±0.27×10−3mm2/s for the malignant nodule group, once again, demonstrating that the mean ADC value of the malignant nodules was significantly lower (P<.01).15
In our series, according to the preoperative DW-MRI, the mean ADC value for the benign group was calculated as 1.72±0.39×10−3mm2/s, whereas this value was 1.28±0.41×10−3mm2/s for the malignancy group, and the statistical analysis showed that the mean ADC value of the nodules was significantly lower in the malignancy group when compared to the benign group (P<.01). On the other hand, the comparisons of the mean ADC values of the extranodular normal thyroid tissues of the same thyroid lobe that harbored the nodule revealed no statistically significant differences between the benign group and the malignancy group (P>.05). However, although not statistically significant, the mean ADC value of the benign nodules (1.72±0.39×10−3mm2/s) being higher than the mean ADC of the extranodular normal thyroid tissue of the same lobe (1.58±0.39×10−3mm2/s), and the mean ADC value of the malignant nodules (1.28±0.41×10−3mm2/s) being lower than the extranodular normal thyroid tissue of the same lobe (1.41±0.39×10−3mm2/s) were considered to be remarkable findings.
In their series of 42 thyroid nodules, Nakahira et al. reported that the best cutoff value for ADC was 1.60×10−3mm2/s in order to distinguish malignant nodules from benign thyroid nodules with a sensitivity of 94.73%, a specifity of 82.60%, a positive predictive value of 81.82%, and a negative predictive value of 95.00%.6 Likewise, El-Hariri et al. evaluated 56 thyroid nodules in their study, and stated that they calculated the best cutoff value for ADC to be 1.5×10−3mm2/s.15 In our series, according to the postoperative final pathology results, the best cutoff for the mean ADC value was calculated as 1.33×10−3mm2/s with a sensitivity of 66.67%, a specifity of 89.13%, a positive predictive value of 53.63%, and a negative predictive value of 89.13%. It was determined that the detection of mean ADC≤1.33×10−3mm2/s was associated with 9 times higher risk of malignancy (P<.01, Odds ratio: 9.111, 95% confidence interval: 2.49–33.21).
In conclusion, as a non-invasive and inexpensive modality, US remains to be the most important and initial step for the evaluation of thyroid nodules. However, only US examination alone is inadequate for reaching the definitive diagnosis in most cases, and for this reason, US guided FNA biopsy and cytopathological evaluation is indicated in most cases that possess suspicious US findings. Although cytopathological results are obtained via FNA, these results may not always reflect the features of the overall thyroid tissue. Controversies still exist for the evaluation and management of thyroid nodules diagnosed with either atypia of undetermined significance, or indeterminate cytology as results of FNA biopsies.3 In these cases, further imaging modalities such as MRI and DW-MRI with ADC measurements may aid the clinician through the evaluation of thyroid nodules, and may avoid repeated FNA biopsies. In the present study, according to the results of cervical DW-MRI, the mean ADC values were detected to be significantly lower for malignant nodules, and a mean ADC≤1.33×10−3mm2/s was associated with 9 times higher risk of malignancy. The ADC value determined at cervical DW-MRI may be considered as a predictive parameter for the detection of thyroid cancer.
Authors StatementAccording to the findings of the present study, the authors conclude that the apparent diffusion coefficient (ADC) value determined at cervical diffusion-weighted magnetic resonance imaging (DW-MRI) may be considered as a predictive parameter for the detection of malignant thyroid nodules. These findings are considered to represent a meaningful diagnostic advance in endocrine surgery for thyroid cancer.
Approval for this study was gained from the Ethics Committee of Istanbul Medeniyet University School of Medicine, Goztepe Training & Research Hospital, and signed informed consent forms were obtained from all patients. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Conflict of InterestOzgür EKINCI, Sumeyra Emine BOLUK, Tunc EREN, Ibrahim Ali OZEMİR, Salih BOLUK, Artur SALMASLIOGLU, Metin LEBLEBICI and Orhan ALIMOGLU declare that they have no conflict of interest.
The authors would like to thank Bulent TASEL, MDα for the radiological evaluations, and Mrs. Emire BORβ for the statistical work-up of this study.
Please cite this article as: Ekinci O, Boluk SE, Eren T, Ozemir IA, Boluk S, Salmaslioglu A, et al. Utilidad de la resonancia magnética ponderada por difusión cervical en la detección del cáncer tiroideo. Cir Esp. 2018;96:620–626.