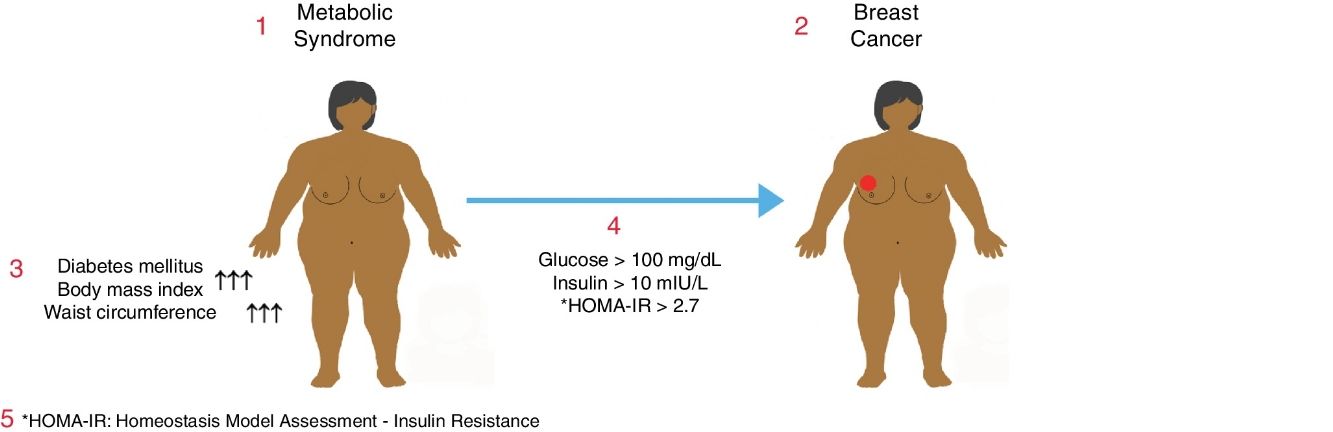
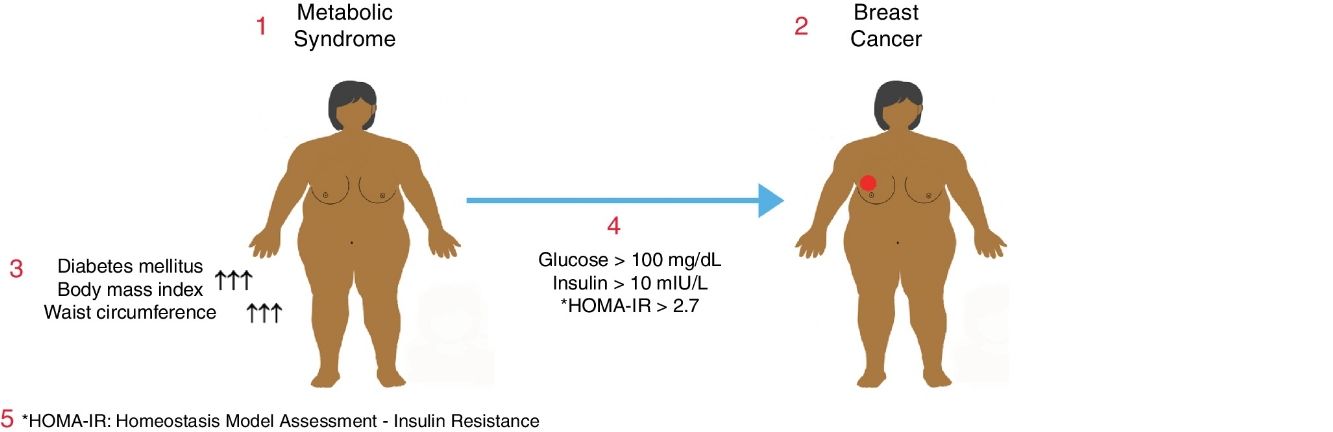
Metabolic syndrome is associated with an increased risk of diabetes mellitus (DM) and coronary heart disease. It may also be associated with a higher risk of some common cancers. The objective of this study was to determine the relationship between metabolic syndrome and breast cancer in postmenopausal women.
MethodsWe present a prospective cohort study of postmenopausal women. This cohort was divided into two groups: the “benign diagnosis group”, including women who were studied after breast cancer screening; and the “malignant tumor group”, including patients with breast cancer that had been diagnosed by biopsy. Age, weight, height, body mass index (BMI), abdominal perimeter, serum glucose, LDL, HDL and insulin levels were analyzed as variables under study. The HOMA-IR homeostatic model formula was used to assess insulin resistance. The differences were considered statistically significant when P<.05.
ResultsTwo hundred women with a mean age of 61.5±9.6 (range: 37–93) were enrolled in the study, consisting of 150 (75%) patients with a benign diagnosis and 50 (25%) patients with a malignant tumor. BMI and abdominal perimeter were higher in the group with a malignant tumor (P<.05). The incidence of DM and metabolic syndrome was higher in the malignant tumor group (P<.005). In the malignant tumor group, much higher incidences correlated with fasting glycemic levels >100mg/dL, insulin levels >10mIU/L and HOMA-IR scores >2.7 (P<.05).
ConclusionsThere is a relationship between metabolic syndrome and postmenopausal breast cancer. More studies are needed to establish methods for the prevention of breast cancer in women with metabolic syndrome.
El síndrome metabólico (SM) se asocia con un aumento del riesgo de diabetes mellitus (DM) y cardiopatía coronaria. El SM también puede estar asociado con un aumento del riesgo de algunos cánceres frecuentes. El objetivo de este estudio fue determinar la relación entre el SM y el cáncer de mama en mujeres posmenopáusicas.
MétodosEstudio de cohortes prospectivo de mujeres posmenopáusicas. Dicha cohorte se dividió en dos grupos: el «grupo con un diagnóstico benigno», formado por mujeres a las cuales se les realizó un seguimiento por cribado del cáncer de mama, y el «grupo con un tumor maligno», formado por pacientes con cáncer de mama diagnosticado por biopsia. Se analizaron como variables a estudio la edad, peso, altura, índice de masa corporal (IMC), perímetro abdominal, glucosa sérica, LDL, HDL y niveles de insulina. Se utilizó la fórmula del modelo homeostático HOMA-IR para evaluar la resistencia a la insulina. Las diferencias se consideraron estadísticamente significativas cuando p<0,05.
ResultadosDoscientas mujeres con una media de edad de 61,5±9,6 años (rango: 37-93) se inscribieron en el estudio que consta de 150 (75%) pacientes en el grupo con un diagnóstico benigno y 50 (25%) pacientes en el grupo con un tumor maligno. El IMC y el perímetro abdominal fueron mayores en el grupo con un tumor maligno (p<0,05). La incidencia de DM y SM fue mayor en el grupo con un tumor maligno (p<0,005). En el grupo con un tumor maligno se detectaron incidencias mucho más altas en relación con los niveles glucémicos en ayunas >100mg/dl, los niveles de insulina >10mUI/l y puntuaciones en el HOMA-IR>2,7 (p<0,05).
ConclusionesExiste relación entre el SM y el cáncer de mama posmenopáusico. Son necesarios más estudios para establecer métodos de protección para la prevención del cáncer de mama en mujeres con SM.
Metabolic syndrome (MetS) is a group of diseases affected by genetic and environmental etiologies and includes more than one cardiovascular risk factors. Hyperglycemia, hypertension (HT), dyslipidemia, visceral obesity and hypercoagulability constitute the components of MetS. The main pathophysiological mechanism in MetS is the resistance of the target tissues to the use of glucose induced by insulin. MetS has become epidemic in developed and developing countries where lifestyle changes are variable.1 Another definition of MetS is that, it is a mortal endocrinopathy in which abdominal obesity induced by insulin resistance, glucose intolerance, dyslipidemia, hypertension (HT) and coronary artery disease (CAD) are combined together.2
The prevalence of MetS is variable in different countries. Prevalence varies depending on the ethnic characteristics of the society, nutrition and lifestyle and the diagnostic criteria used for the screening of this disease.3,4 In recent years, a significant increase in the number of patients with MetS has been observed worldwide, directly proportional with the increase in the prevalence of obesity and diabetes mellitus (DM).5 It has been determined that MetS plays a role in the development of various cancer types including breast cancer, which is the most common cancer type in women, although the mechanism is yet unclear.6 Additionally, in women, breast cancer is the most common life-threatening cancer among all.7,8 The overall incidence of breast cancer is increasing worldwide, and from 1970s to the present time this rate has altered from 1/18 to 1/8 women.9 When breast cancer age distribution is examined in our country, more than half of the cases are found to be over the age of 50, and 55% are postmenopausal.10 The etiology of breast cancer is unknown in most cases.11 Various risk factors have been defined for breast cancer the most important of which are female gender and advanced age. In recent studies, obesity and obesity related conditions have been shown to play role in the breast cancer development. Besides, it has been shown that obesity and insulin resistance are associated with poor prognosis in advanced breast cancer.12,13
In this study, our aim was to determine the relationship between breast cancer and MetS which consists of conditions including insulin resistance, abdominal obesity, dyslipidemia, and glucose intolerance or DM among postmenopausal women.
MethodsThis prospective cohort study was performed in the Department of General Surgery concurrently with the Department of Family Medicine of the same single institution in a one-year-period. All procedures performed were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was obtained from the Clinical Research Ethics Committee of the institution and informed consent was obtained from all individual participants included in the study.
All patients were detected, diagnosed and treated in the same unit, and were divided into two groups as the “benign group” and the “malignancy group”. The “benign group” consisted of randomly selected voluntary postmenopausal women who were followed-up for breast cancer screening in the Department of Family Medicine with routine periodic outpatient office visits without any evidence of the development of breast cancer. The “malignancy group” consisted of patients who received surgical treatment with the diagnosis of biopsy-proven breast cancer in the Department of General Surgery, the postoperative specimens of whom were also confirmed to be consistent with breast malignancy histopathologically. These two groups were composed separately as none of the patients in the “benign group” shifted into the “malignancy group” due to any newly developed malignacy.
MetS in a patient was defined as the presence of at least one of either DM, glucose intolerance, or insulin resistance accompanied by any two of the contributing conditions including HT, dyslipidemia, and abdominal obesity.
Premenopausal women, women having undergone surgical menopause, individuals younger than the age of 18, men, individuals having a prior history of breast cancer surgery and/or neaodjuvant/adjuvant therapy were excluded.
In addition to the demographic data, parameters including age, weight, height, body mass index (BMI), waist circumference, serum glucose, LDL cholesterol, HDL cholesterol and insulin levels were recorded and analyzed. The Homeostasis Model Assessment – Insulin Resistance (HOMA-IR) formula, calculated with the equation [HOMA-IR=Fasting serum insulin×Fasting serum glucose/405], was used to determine the presence of insulin resistance. The normal value for HOMA-IR has been reported as <2.7 where individuals with a HOMA-IR level ≥2.7 are considered as harboring various levels of insulin resistance.14
The Number Cruncher Statistical System 2007 (Kaysville, Utah, USA) program was utilized for statistical analysis. As well as the descriptive statistical methods (mean, median, frequency, odds ratio, minimum, maximum), the Pearson's chi square test, Fisher's exact test, Fisher–Freeman–Halton test, and Yates's continuity correction were used for the comparison of qualitative data. A value of P<.05 was considered as statistically significant.
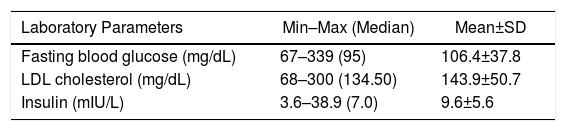
ResultsA cohort of 200 postmenopausal women with a mean age of 61.5±9.6 (range: 37–93) years were enrolled to the study consisting of 150 (75%) patients in the benign group, and 50 (25%) patients in the malignancy group. The demographic values of the entire study group including age as well as weight and height measurements are summarized in Table 1. Additionally, the average values of the laboratory results of all patients in the present study are shown in Table 2.
Laboratory Parameters Among the Overall Group of Patients.
| Laboratory Parameters | Min–Max (Median) | Mean±SD |
|---|---|---|
| Fasting blood glucose (mg/dL) | 67–339 (95) | 106.4±37.8 |
| LDL cholesterol (mg/dL) | 68–300 (134.50) | 143.9±50.7 |
| Insulin (mIU/L) | 3.6–38.9 (7.0) | 9.6±5.6 |
Min: minimum, Max: maximum, SD: standard deviation.
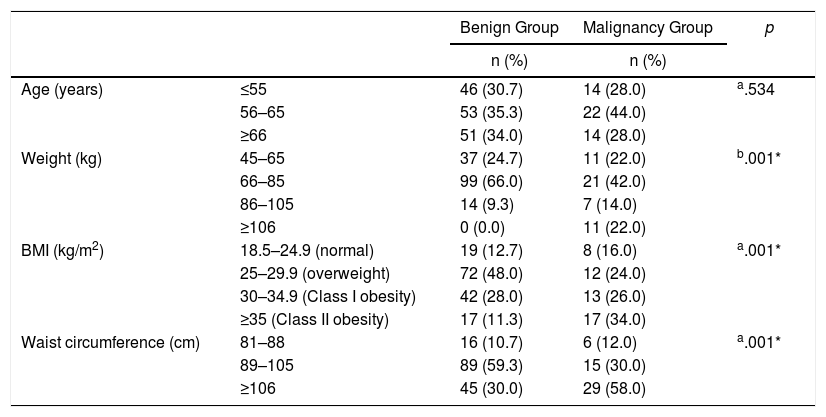
When the weight of the participants were examined, it was observed that 99 (66%) patients in the benign group weighed between 66–85kg and this rate was significantly higher than the malignancy group (P=.005). Conversely, the number of patients that weighed higher than 106kg was significantly increased in the malignancy group (P=.001). According to the BMI values of the participants, it was found that, 72 (48%) patients in the benign group had BMI values between 25 and 29.9kg/m2 and this rate was significantly higher than the malignancy group (P=.005), whereas 17 (34%) patients in the malignancy group had BMI values above 35kg/m2 which was significantly increased when compared to the benign group (P=.001). The number of patients with a waist circumferences between 89 and 105cm was significantly higher in the benign group (P=.001), whereas the waist circumferences were above 106cm in 29 (58%) patients in the malignancy group which revealed a significantly higher rate than the benign group (P=.001) (Table 3).
Comparisons of Age and the Paramaters of Obesity Between the Study Groups.
| Benign Group | Malignancy Group | p | ||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Age (years) | ≤55 | 46 (30.7) | 14 (28.0) | a.534 |
| 56–65 | 53 (35.3) | 22 (44.0) | ||
| ≥66 | 51 (34.0) | 14 (28.0) | ||
| Weight (kg) | 45–65 | 37 (24.7) | 11 (22.0) | b.001* |
| 66–85 | 99 (66.0) | 21 (42.0) | ||
| 86–105 | 14 (9.3) | 7 (14.0) | ||
| ≥106 | 0 (0.0) | 11 (22.0) | ||
| BMI (kg/m2) | 18.5–24.9 (normal) | 19 (12.7) | 8 (16.0) | a.001* |
| 25–29.9 (overweight) | 72 (48.0) | 12 (24.0) | ||
| 30–34.9 (Class I obesity) | 42 (28.0) | 13 (26.0) | ||
| ≥35 (Class II obesity) | 17 (11.3) | 17 (34.0) | ||
| Waist circumference (cm) | 81–88 | 16 (10.7) | 6 (12.0) | a.001* |
| 89–105 | 89 (59.3) | 15 (30.0) | ||
| ≥106 | 45 (30.0) | 29 (58.0) |
n: number of patients, BMI: body mass index.
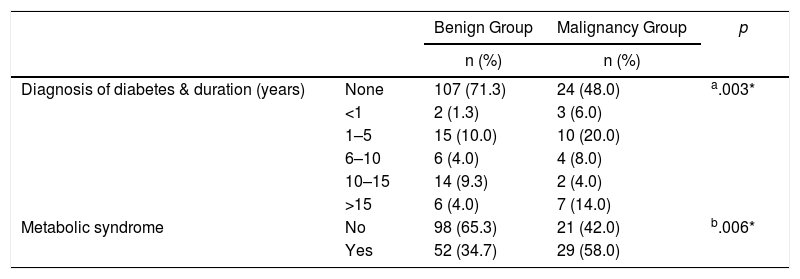
The incidence of DM in the benign group was significantly lower than the malignancy group (P=0.005). In the malignancy group, the number of patients having the diagnosis of DM for a period of more than 15 years was significantly higher than the benign group (P=0.02). When the patients having fulfilled the MetS criteria were analyzed, it was found that 29 (58%) patients were diagnosed with MetS in the malignancy group while 52 (35%) patients received this diagnosis in the benign group revealing that the incidence of MetS was significantly higher among breast cancer patients (P=.006). Likewise, the number of non-MetS patients in the benign group was significantly higher than the malignancy group (P=0.006) (Table 4).
Comparisons of the Parameters of Diabetes and Metabolic Syndrome Between the Study Groups.
| Benign Group | Malignancy Group | p | ||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Diagnosis of diabetes & duration (years) | None | 107 (71.3) | 24 (48.0) | a.003* |
| <1 | 2 (1.3) | 3 (6.0) | ||
| 1–5 | 15 (10.0) | 10 (20.0) | ||
| 6–10 | 6 (4.0) | 4 (8.0) | ||
| 10–15 | 14 (9.3) | 2 (4.0) | ||
| >15 | 6 (4.0) | 7 (14.0) | ||
| Metabolic syndrome | No | 98 (65.3) | 21 (42.0) | b.006* |
| Yes | 52 (34.7) | 29 (58.0) |
n: number of patients.
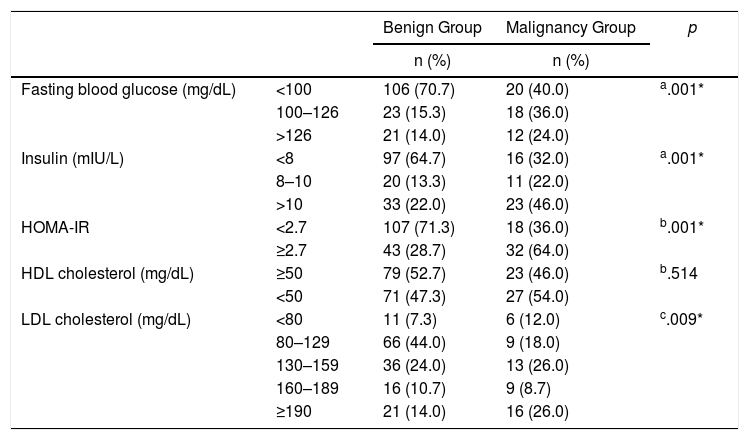
Regarding fasting glucose levels, values <100mg/dL were more prevalent in the benign group (P=0.001). On the other hand, in the malignancy group, most of the patients were detected to have glucose levels >100mg/dL (P=.003). The number of patients having basal serum insulin levels <8mIU/L was significantly higher in the benign group, whereas the number of patients with insulin levels >10mIU/L was significantly increased in the malignancy group (P=.001 and P=.002, respectively). HOMA-IR levels were also examined, which showed that the HOMA-IR >2.7 was significantly more prevalent in the malignancy group (P=.001). There was no difference with regard to HDL cholesterol levels between the study groups (P>.05). When the LDL cholesterol levels of both groups were examined, it was detected that 16 (26%) patients in the malignancy group had LDL≥190mg/dL revealing a significantly higher rate than the benign group (P=.009). The majority of the benign group had LDL levels between 80 and 129mg/dL (P=.023) (Table 5).
The Distribution of Laboratory Findings Among the Study Groups.
| Benign Group | Malignancy Group | p | ||
|---|---|---|---|---|
| n (%) | n (%) | |||
| Fasting blood glucose (mg/dL) | <100 | 106 (70.7) | 20 (40.0) | a.001* |
| 100–126 | 23 (15.3) | 18 (36.0) | ||
| >126 | 21 (14.0) | 12 (24.0) | ||
| Insulin (mIU/L) | <8 | 97 (64.7) | 16 (32.0) | a.001* |
| 8–10 | 20 (13.3) | 11 (22.0) | ||
| >10 | 33 (22.0) | 23 (46.0) | ||
| HOMA-IR | <2.7 | 107 (71.3) | 18 (36.0) | b.001* |
| ≥2.7 | 43 (28.7) | 32 (64.0) | ||
| HDL cholesterol (mg/dL) | ≥50 | 79 (52.7) | 23 (46.0) | b.514 |
| <50 | 71 (47.3) | 27 (54.0) | ||
| LDL cholesterol (mg/dL) | <80 | 11 (7.3) | 6 (12.0) | c.009* |
| 80–129 | 66 (44.0) | 9 (18.0) | ||
| 130–159 | 36 (24.0) | 13 (26.0) | ||
| 160–189 | 16 (10.7) | 9 (8.7) | ||
| ≥190 | 21 (14.0) | 16 (26.0) |
n: number of patients.
Along with its increasing incidence, MetS is a group of risk factors for DM and cardiovascular disease which constitutes an important problem worldwide.15 These risk factors include obesity, glucose intolerance, HT, elevated triglyceride levels, and low levels of HDL cholesterol.16 MetS is also associated with the increased risk of certain types of cancers.17 In this study, we found that there was an increased risk of breast cancer development in the presence of underlying MetS in postmenopausal women. In addition, the investigations of the components of MetS revealed significant differences when compared between the benign and malignancy groups of our study.
Obesity might cause altered levels of circulating hormones and growth factors, which may lead to enhanced carcinogenesis.18 In a cohort study which compared postmenopausal breast cancer and obesity through a 5-year follow-up, there was a proportional relationship between breast cancer and having high BMI values from the age of 18.19 There are also studies revealing increased breast cancer risks in postmenopausal women in addition to poorer clinical outcomes at all ages associated with high BMI values.18 In the present study, according to the BMI values, the number of patients in the malignancy group with a BMI≥35 was significantly higher than the benign group.
Some studies in postmenopausal women reported increased breast cancer risk in relation with higher waist circumference values which puts forth the fact that, as a marker of metabolic consequences of obesity, the presence of abdominal obesity appears to influence breast cancer risk.20 Although some controversy exists, certain cohort and case-control studies support these findings.21,22 In most of those studies, the upper limit of waist circumference was determined as 88cm according to established guidelines.23 In the present study, the number of patients with a waist circumferences between 89 and 105cm was significantly higher in the benign group, whereas, in the malignancy group, the number of patients with a waist circumference ≥106cm were significantly higher.
Chronic hyperglycemia in patients with DM develops as a result of insulin resistance which reduces glucose uptake in the muscle tissue and reduces glucose storage in the liver leading to elevated blood glucose levels.20 In cancer biology, since neoplastic cells use glucose for proliferation, it can be considered that higher levels of circulating glucose may stimulate carcinogenesis.20 Along with increased levels of oestrogens or insulin-like growth factor I, the development of insulin resistance may constitute a risk factor for breast cancer.24 Insulin resistance has been found to be associated with obesity, HT, dyslipidemia and glucose intolerance and a relationship between fasting blood glucose levels and breast cancer has been shown in some studies.24 A similar relationship was also found in our study as in the malignancy group, the number of diabetic patients were significantly higher than the benign group. DM, being one of the most common diseases in the postmenopausal ages among the normal population, was found in high rates in the breast cancer group, not only in our study but also in many other similar ones.25 Thus, management of DM has been proven to be essential, once again.
In addition to the metabolic effects of insulin, it also induces mitogenic activity that can cause proliferation of the normal breast epithelial cells and increased proliferation due to hyperinsulinemia might lead to the development of breast cancer.26 There are studies revealing the association between hyperinsulinemia and increased risk of breast cancer.20 Coherently, in the present study, the number of patients having basal serum insulin levels <8mIU/L was significantly higher in the benign group, whereas the number of patients with insulin levels >10mIU/L was significantly higher in the malignancy group.
The normal value of the HOMA-IR formula has been reported as <2.7 where individuals with a HOMA-IR level ≥2.7 are considered as harboring various levels of insulin resistance.14 It has been reported that higher HOMA-IR values were associated with increased breast cancer risk in postmenopausal women, however, in breast cancer diagnosed before 55 years of age, no significant association was observed.27 In our study, a value of HOMA-IR >2.7 was significantly more prevalent in the malignancy group. The results in this study were similar to others in which the cutoff value of the HOMA-IR score was set at 2.5, although we determined 2.7 to be the cutoff value based on the latest guidelines.14,16,27 Since both fasting glucose and insulin values are used for the calculation of the HOMA-IR formula, a positive correlation with HOMA-IR scores and breast cancer was determined.
Serum lipid profile changes including decreased levels of HDL cholesterol and increased levels of total cholesterol, LDL cholesterol, and triglycerides are referred to as dyslipidemia. Since cholesterol is a precursor of steroid hormones, breast cancer risk is considered to increase in dyslipidemic women, especially with elevated LDL cholesterol levels.20 On the contrary, it has been detected that women with increased HDL-cholesterol levels are at lower the risk for breast cancer.20 Having investigated the relation between serum cholesterol levels and breast cancer risk, several studies revealed that elevated total cholesterol and LDL cholesterol and decreased HDL cholesterol levels posed a higher risk for breast cancer, besides, a stronger association among postmenopausal women was reported.18,20,28 In the present study, LDL cholesterol levels were found to be significantly higher in the malignancy group although LDL cholesterol solely is not a diagnostic criteria for MetS. Our interpretation is that elevated LDL levels might be due to other environmental factors reflecting a shift to western lifestyle habits in our country such as altered dietary habits and lower level of physical activity.
In their prospective study of 4888 women, Kabat et al. reported that 165 patients developed breast cancer after an 8-years follow-up period, and they concluded that there was no relationship between an underlying MetS and breast cancer.29 On the contrary, in a case-control study performed by Rosato et al., 3869 postmenopausal breast cancer patients and a control group of 4082 postmenopausal women were evaluated. MetS was diagnosed with the presence of at least three of the criteria including DM, HT, hyperlipidemia, and obesity. The number of patients who met at least three of these criteria was significantly higher in the breast cancer group.28 In a case-control study conducted by Wang et al., among 43 postmenopausal breast cancer patients and 86 control group patients, women who met four of the MetS criteria showed greater risk for postmenopausal breast cancer.18 In our study, the incidence of MetS in the malignancy group was found to be significantly higher compared to the benign group.
Having been conducted at a single center, the relatively small sample size, the neglected ethnical properties which may affect the different diagnostic and definitive criteria for MetS, and not investigating the life style properties of the patients may be considered as the drawbacks of our study.
In conclusion, the significantly higher incidence of MetS in the malignancy group of our study reflects an association between MetS and postmenopausal breast cancer development. It should be kept in mind that it is an important task for the physician to inquire the diagnostic criteria of MetS, to apply a holistic approach without evaluating the diseases separately and to interfere at the earlier stages of this syndrome in each patient. We consider that further studies are needed for establishing protective methods to prevent the development of breast cancer in women with MetS.
FundingAll authors declare that no source of funding exists, and that they have no direct or indirect commercial financial incentive associated with publishing this article.
Author ContributionsStudy design: Ozgur Ekinci, Tunc Eren, Melike Kurtoglu Yakici, Zuhal Aydan Saglam, Orhan Alimoglu.
Literature search: Ozgur Ekinci, Tunc Eren, Melike Kurtoglu Yakici.
Data collection: Ozgur Ekinci, Melike Kurtoglu Yakici, Aman Gapbarov, Muhammet Ali Aydemir.
Data analysis: Ozgur Ekinci, Melike Kurtoglu Yakici, Aman Gapbarov, Muhammet Ali Aydemir, Zuhal Aydan Saglam, Orhan Alimoglu.
Writing of the manuscript: Ozgur Ekinci, Tunc Eren, Melike Kurtoglu Yakici, Aman Gapbarov.
Critical revision: Ozgur Ekinci, Tunc Eren, Zuhal Aydan Saglam, Orhan Alimoglu.
Conflict of InterestThe corresponding author, and all co-authors declare that no conflict of interest exists.
Please cite this article as: Ekinci O, Eren T, Kurtoglu Yakici M, Gapbarov A, Aydemir MA, Saglam ZA, et al. Relación del síndrome metabólico con el cáncer de mama posmenopáusico. Cir Esp. 2020. https://doi.org/10.1016/j.ciresp.2019.12.015