Enhanced recovery after surgery (ERAS) protocols are care programs based on scientific evidence and focused on postoperative recovery. They encompass all aspects of patient care and require multidisciplinary management, with the participation of diverse specialists. The implementation of these protocols is being extended to several abdominal and extra-abdominal surgeries, including bariatric approaches.
Diverse specialists with wide experience in the management of morbidly obese patients have taken part in the working group that developed this protocol. A bibliographic search about ERAS in bariatric surgery in several databases was performed to evaluate the current scientific evidence, establishing evidence levels and recommendations according to the GRADE methodology. The items included in this protocol are separated into preoperative, perioperative and postoperative guidelines.
Los protocolos de rehabilitación multimodal o recuperación intensificada (PRI) son programas de cuidado del paciente, basados en la evidencia científica y orientados a mejorar su recuperación postoperatoria. Abarcan todos los aspectos implicados en el cuidado del paciente y requieren un manejo multidisciplinar, en el que intervienen varios especialistas. La aplicación de estos protocolos se está extendiendo ampliamente por diferentes tipos de cirugías abdominales y extra-abdominales, incluyendo la cirugía bariátrica.
Facultativos de diferentes especialidades, con experiencia en el tratamiento de pacientes obesos mórbidos, han formado parte del grupo de trabajo que desarrolló este protocolo. Para evaluar la evidencia científica actualizada, se realizó una búsqueda bibliográfica sobre PRI en cirugía bariátrica en diferentes bases de datos, estableciendo los niveles de calidad de evidencia y el grado de recomendación según la metodología GRADE. Se agruparon las actuaciones incluidas en la matriz temporal en 3 etapas: Preoperatorio, Perioperatorio y Postoperatorio.
Multimodal rehabilitation or enhanced recovery after surgery (ERAS) protocols are patient care programs based on scientific evidence and aimed at improving postoperative recovery. They cover all aspects of patient care and require multidisciplinary management, in which several specialists are involved. These protocols have been shown to provide clear benefits in postoperative recovery by reducing morbidity and mortality, hospital stay and healthcare costs.1,2 The protocolization of perioperative care has also allowed these processes to become ‘standardized’. This has improved the information provided to patients and family members, while allowing patients to also be an active part of their own recovery.3
Kehlet and Wilmore were the first to apply a series of measures of this type after colorectal surgery in their ‘fast-track’ program.1 Given the excellent results obtained in this type of surgery, it seems logical to extend its application to other abdominal or extra-abdominal surgeries (traumatology, thoracic surgery, etc.).4–6
In 2007, the Grupo Español de Rehabilitación Multimodal (Spanish Multimodal Rehabilitation Group, or GERM) was created in Spain to initially develop protocols for colorectal surgery, whose application showed clear advantages over classic care standards.7–9 In 2014, working groups were created to develop protocols applicable to other abdominal surgeries (gastric surgery,10 esophageal,11 hepatic and bariatric12,13). Based on these protocols, and in collaboration with the Spanish Ministry of Health, Social Affairs and Equality, the Vía clínica de Recuperación Intensificada en Cirugía Abdominal (Enhanced Recovery in Abdominal Surgery, or RICA) was published in 2015.14 Based on the protocol in bariatric surgery, the Vía clínica de cirugía bariátrica (Clinical guidelines for bariatric surgery) was developed in 2017, endorsed by the Spanish Society of Obesity Surgery, the Morbid Obesity Division of the Spanish Association of Surgeons and the GERM.15
In this document, we present the ERAS time matrix for vertical sleeve gastrectomy and Roux-en-Y gastric bypass as bariatric procedures. This time matrix was created with the consensus of all members of the GERM Bariatric Surgery Working Group and is based on an exhaustive review of the evidence available in the literature, with experience-based contributions from the multidisciplinary group of experts of the working group.
MethodsMedical professionals from different specialties, including surgeons, anesthesiologists, endocrinologists, nurses and graduates in sport sciences, with experience in the treatment of morbidly obese patients, have participated in the working group that developed this protocol, creating an initial time matrix at a consensus meeting held in Zaragoza in March 2016. Subsequently, this matrix was reevaluated and updated at a meeting during the 2nd GERM Congress, held in Salamanca in April 2018.
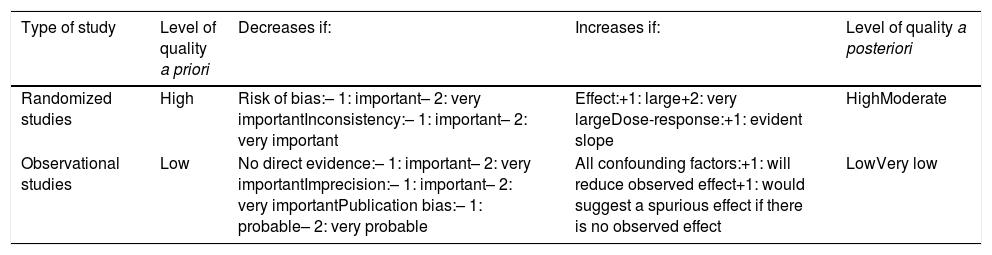
To evaluate the updated scientific evidence, a literature search on multimodal rehabilitation in bariatric surgery was conducted with the Cochrane Library, Medline, EMBASE and Scopus databases, from 1995 to 2018, establishing the quality of evidence levels and the recommendation grades in accordance with the GRADE methodology (Tables 1 and 2).16
GRADE classification—description of the levels of evidence.
| Levels of quality | Definition |
|---|---|
| High | High confidence in the coincidence between the real and estimated effect |
| Moderate | Moderate confidence in the estimation of the effect. There is a possibility that the real effect is unlike the estimated effect. |
| Low | Limited confidence in the estimation of the effect. The real effect can be far from the estimated effect. |
| Very low | Little confidence in the estimated effect. The true effect is quite probably different from the estimated effect |
Classification of the level of evidence according to the GRADE system.
| Type of study | Level of quality a priori | Decreases if: | Increases if: | Level of quality a posteriori |
|---|---|---|---|---|
| Randomized studies | High | Risk of bias:– 1: important– 2: very importantInconsistency:– 1: important– 2: very important | Effect:+1: large+2: very largeDose-response:+1: evident slope | HighModerate |
| Observational studies | Low | No direct evidence:– 1: important– 2: very importantImprecision:– 1: important– 2: very importantPublication bias:– 1: probable– 2: very probable | All confounding factors:+1: will reduce observed effect+1: would suggest a spurious effect if there is no observed effect | LowVery low |
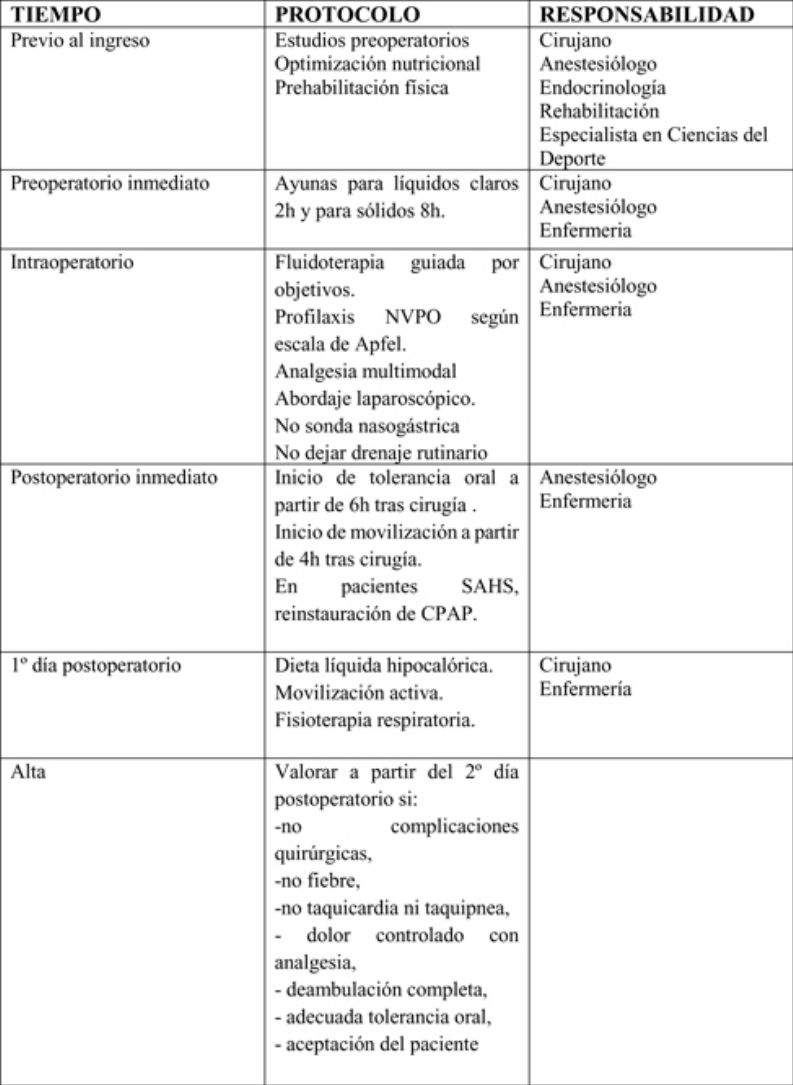
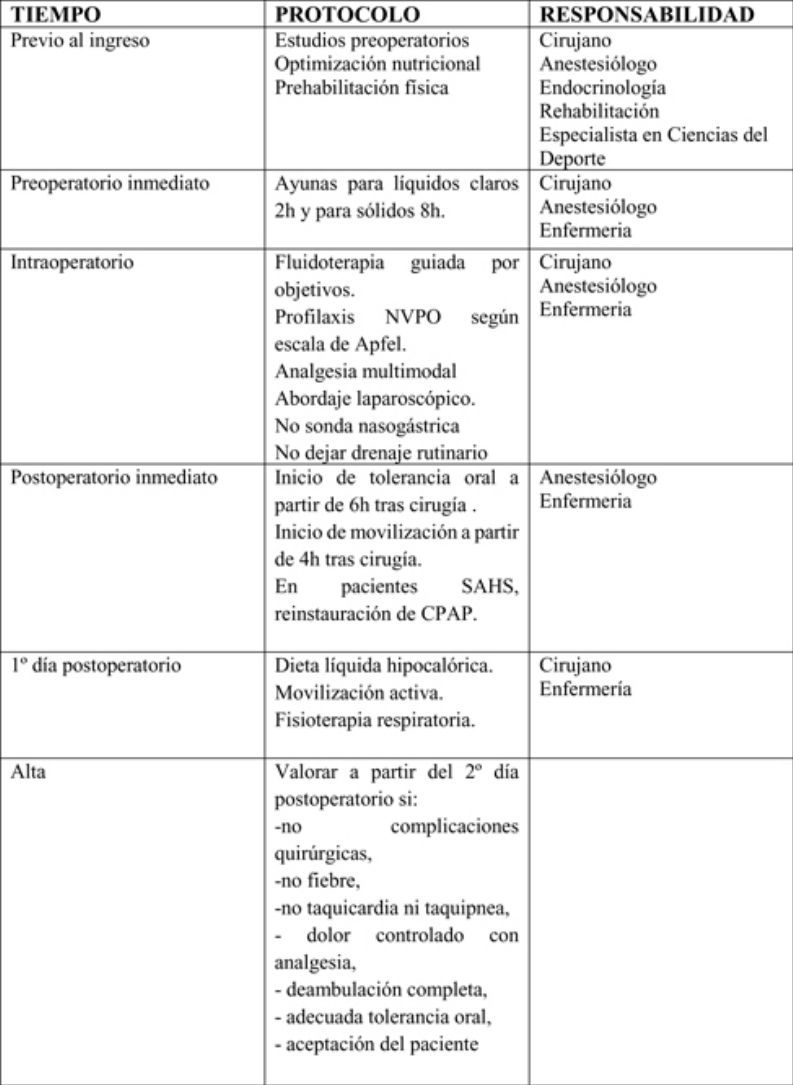
The actions included in the time matrix were grouped into 3 stages: preoperative, perioperative and postoperative.
ResultsIndications and contraindicationsCandidates for the application of the recommended measures are patients who will be treated with a vertical gastrectomy or a Roux-en-Y gastric bypass, complying with the general indications of these procedures: body mass index (BMI) > 40 kg/m2 or BMI > 35 kg/m2 associated with comorbidities derived from obesity.
In addition, the following criteria must be met:
- –
Age over 18
- –
Adequate cognitive state
- –
ASA I, II and III
Patients treated with other bariatric procedures, as well as patients treated with these techniques as revision procedures after other previous bariatric techniques, were excluded from the application of this protocol.
Preoperative periodSurgeons, anesthesiologists, endocrinologists, rehabilitators and sports science specialists participate in this period and apply the following measures:
- •
Detailed oral and written information of the entire process: this reduces patient anxiety, promotes tolerance to oral intake and early mobilization, and improves pain control. (Strong recommendation; Moderate level of evidence)17
- •
Preoperative studies:
- none◦
Cardiological evaluation if > 3 cardiovascular risk factors: according to the American Heart Association, every patient who is a candidate for bariatric surgery should have a part of the medical history and examination focused on discovering heart disease. However, the physical examination usually underestimates the cardiac dysfunction of obese patients. (Strong recommendation; Level of evidence: high)
- none◦
As associated tests, all patients should have a 12-lead electrocardiogram and chest x-ray. Risk factors for coronary disease should be studied, including: major abdominal surgery, history of heart disease, heart failure or strokes, treatment with insulin and preoperative serum creatinine > 2.0 mg/dL. If there are 3 or more risk factors, a stress test, echocardiogram, stress echocardiogram, transesophageal echocardiogram with dobutamine or isotopic ventriculography should be performed. (Strong recommendation; High level of evidence)18
- none◦
Screening for sleep apnea-hypopnea syndrome (SAHS): The prevalence of SAHS among patients indicated for bariatric surgery is 76–96%, but only 15–20% are diagnosed. In patients with SAHS diagnosis in whom CPAP is indicated, its use is essential for at least 4–6 weeks because it reduces the incidence of cardiopulmonary complications. Polysomnography should not be done indiscriminately. There are different questionnaires for SAHS assessment. The STOP-BANG test has the highest sensitivity (100%) and negative predictive value (100%) for an apnea-hypopnea index > 30. SAHS screening with the STOP-BANG test is recommended. A score equal to or greater than 3 is an indication for polysomnography. (Strong recommendation; High level of evidence)19–21
- none◦
Spirometry: Spirometry is recommended only if there are pulmonary risk factors identified with other studies or when conducting a medical history. (Strong recommendation; High level of evidence)22
- none◦
Laboratory studies: there is no evidence of any benefit in determining preoperative vitamin and mineral levels. (Weak recommendation; Low level of evidence)23
- none◦
In all obese patients, a lipid profile should be obtained. (Strong recommendation; High level of evidence)23
- none◦
A renal function assessment is recommended for all patients, especially if they are diabetic and hypertensive. (Strong recommendation; High level of evidence)24
- none◦
In diabetic patients, it is recommended to determine glycosylated hemoglobin (HbA1c) levels, which is able to assess mid-term glycemic control (3 months). Before surgery, baseline blood glucose ≤ 110 mg/dL, postprandial blood glucose ≤ 140 mg/dL and HbA1c 6.5%-7% are recommended, although HbA1c 7%-8% is accepted in patients with difficulties to achieve good control. (Weak recommendation; Low level of evidence)23
- none◦
Upper digestive endoscopy or esophageal-gastroduodenal study: it is common for morbidly obese patients to present with gastritis or gastroesophageal reflux, which is not always symptomatic and may affect patient progress after the intervention. In symptomatic patients, upper gastrointestinal endoscopy should be performed prior to surgery. (Strong recommendation; High level of evidence)25,26
- none◦
The European Association for Endoscopic Surgery advises the evaluation of the upper digestive tract by endoscopy or esophago-gastro-duodenal transit study for all patients. (Weak recommendation; Low level of evidence)27
- none◦
Screening for Helicobacter pylori (H. pylori) and preoperative eradication (if there is gastric exclusion): the American Society for Gastrointestinal Endoscopy (ASGE) recommends testing for H. pylori in all patients and eradication in positive cases in patients in whom surgery will involve gastric exclusion. (Weak recommendation; Low level of evidence)28
- none◦
- •
Optimized nutrition:
- none◦
Weight loss before surgery: weight loss prior to surgery has demonstrated benefits (decreased liver volume, better glycemic control, less visceral fat, lower risk of conversion, improved patient compliance with postoperative treatment) and it is advisable to help the patient achieve a 5%-10% weight loss before surgery. The best strategies to achieve this weight loss are low-calorie diets (800-1,500 kcal/day) for 6–12 weeks before the intervention, or very low-calorie diets (600 kcal/day) for 2–6 weeks. Commercial products that substitute a meal are usually used, as these provide reproducible results. (Weak recommendation; Low level of evidence)29,30
- none◦
Assess coadjuvant methods: an gastric balloon can be used for 6–12 months before the intervention, or pharmacological treatment with sibutramine can be prescribed for 6 months. These methods together with a very low-calorie diet can produce better weight loss and visceral volume results. (Weak recommendation; Low level of evidence)29,30
- none•
Respiratory physiotherapy: the preoperative training of the inspiratory muscles in patients treated with bariatric surgery improves the strength and resistance of these muscles with an increase in the maximum inspiratory pressure, enabling early recovery of lung function. The isolated use of an incentive spirometer is not useful to prevent the deterioration of lung function after bariatric surgery. Preoperative and postoperative respiratory physiotherapy is recommended. (Strong recommendation; Moderate level of evidence)31
- none◦
- •
Physical pre-habilitation
Morbidly obese patients usually have a sedentary lifestyle and low fitness levels. A low cardiorespiratory capacity is associated with a greater number of postoperative complications (pulmonary, thromboembolic, etc.) and a longer hospital stay.
Along with this, preoperative diets, in addition to reducing fat mass, will also reduce lean mass, which can be avoided by strength training.
When the functional capacity of the patient allows, it is advisable to establish an exercise program focused on improving the patient’s physical condition (especially cardiorespiratory capacity), maximizing the loss of fat mass and reducing the loss of lean mass. It is recommended to implement an exercise program 3–6 months prior to surgery that combines cardiorespiratory and strength training. It should start with a frequency of 2 days per week and progress to 4 days per week. Start at moderate intensities, for both cardiorespiratory training (40%-50% peak oxygen consumption) and strength training (∼50% of a maximum repetition), and progress to higher intensities (depending on patient tolerance). In patients that cannot withstand such exertion, we should insist on them increasing the number of steps walked per day: start with 5000 steps/day and work up to 10,000 steps/day. (Strong recommendation; Low level of evidence)32–34
Perioperative periodSurgeons, anesthesiologists and nursing staff should participate in this period to apply the following measures:
- •
Thromboprophylaxis: patients treated with bariatric surgery have a high risk of venous thromboembolic events in the postoperative period, and pulmonary embolism is one of the main causes of mortality. Thromboembolic prophylaxis is routinely recommended in all patients, along with early mobilization, the use of intermittent pneumatic compression devices and pharmacological prophylaxis with low-molecular-weight heparin. Intermittent pneumatic compression should be initiated in the operating room and maintained until the patient is completely mobile. (Strong recommendation; Moderate level of evidence)35
- •
Pharmacological antithrombotic prophylaxis should be considered, following the thromboprophylaxis protocols at each hospital (Strong recommendation; Moderate level of evidence)35
- •
Fasting: fasting generates a catabolic response characterized by dehydration, insulin resistance, postoperative hyperglycemia and a depressed immune response. A fasting period for clear liquids (water, juice without pulp, carbohydrate drinks, light tea and coffee) of 2 h is recommended, and 8 h for solids. (Strong recommendation; Moderate level of evidence)14,36
- •
Antibiotic therapy: the administration of systemic antibiotic prophylaxis is recommended within the 120 min prior to anesthetic induction. The recommended first-choice drugs are second-generation cephalosporins and, in case of beta-lactam allergy, fluoroquinolones or the association of vancomycin and gentamicin, or clindamycin and aztreonam are recommended. The doses administered should be adjusted to the patient’s weight. (Strong recommendation. High level of evidence)37
- •
Invasive monitoring: invasive monitoring is not routinely indicated, although invasive arterial cannulation is useful in selected patients (those with severe cardiorespiratory disorders, who may present postoperative problems). (Strong recommendation; Low level of evidence)14,38
- •
Central venous catheter is not routinely indicated: the use of central venous catheters should be limited to patients with respiratory diseases who are predicted to require the administration of vasopressors or inotropic agents in continuous infusion. (Strong recommendation; Low level of evidence)14
- •
Bladder catheterization is not routinely indicated: bladder catheter placement should be limited to patients in whom prolonged surgery is expected or in patients at high risk of presenting cardiorespiratory or renal complications during the intra- or postoperative period. If done, it is recommended to remove the catheter 24 h after surgery, if the patient’s circumstances allow. (Strong recommendation; Low level of evidence)14
- •
Opioid-free anesthesia: Opioids have many adverse effects, some of which are responsible for the appearance of postoperative complications (increased nausea and vomiting, respiratory complications and opioid-induced hyperalgesia). Patients with SAHS are even more at risk due to respiratory depression caused by these drugs. Opioid-free anesthesia improves postoperative recovery and reduces the degree of immunosuppression; it is also associated with lower opioid requirements in the postoperative period.
Indications for opioid-free anesthesia include addiction or intolerance to narcotics, patients with hyperalgesia, immunosuppressed patients, those with SAHS or other lung diseases, or a history of postoperative nausea or vomiting. Alterations of the autonomic nervous system and patients treated with beta blockers or heart blocks are relative contraindications. The most commonly used drugs in opioid-free anesthesia are dexmedetomidine, lidocaine, ketamine, magnesium, clonidine and ketorolac. (Strong recommendation; Moderate level of evidence)39
- •
Objective-guided fluid therapy: the aim of fluid therapy is to maintain an adequate circulatory volume, avoiding overload as much as possible. The use of objective-guided fluid therapy has been shown to reduce morbidity, mortality and hospital stay. It is recommended to monitor the systolic volume or systolic volume variation with validated devices to guide the intraoperative administration of fluids. Esophageal Doppler monitoring or methods based on pulse contour analysis are preferred. (Strong recommendation; High level of evidence)14
- •
If these devices are not available, restrictive fluid therapy based on the ideal weight is recommended. Most studies that apply objective-guided fluid therapy in bariatric surgery limit fluid infusion, at a rate of 600−800 mL/h. (Strong recommendation; Moderate level of evidence)40,41
- •
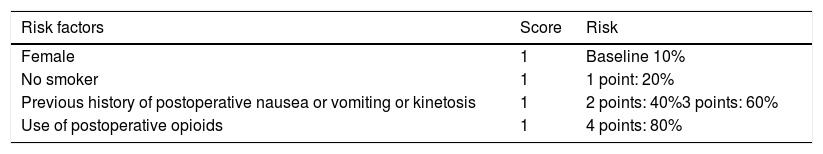
Prophylaxis for postoperative nausea and vomiting (PONV), according to the modified Apfel scale: postoperative nausea and vomiting are the first cause of delayed tolerance to oral intake after surgery and hospital discharge. The risk of PONV occurrence should be stratified according to the Apfel scale (Table 3). (Strong recommendation; High level of evidence)42
Table 3.Apfel scale for the stratification of the risk for postoperative nausea or vomiting.
Risk factors Score Risk Female 1 Baseline 10% No smoker 1 1 point: 20% Previous history of postoperative nausea or vomiting or kinetosis 1 2 points: 40%3 points: 60% Use of postoperative opioids 1 4 points: 80% Low risk (0–1 point, 10–20%); moderate (2 points, 40 %); high (3–4 points, 60–80%).
General measures to reduce the risk of PONV should be taken in moderate-high-risk patients: avoid halogenated anesthetics, use propofol for induction and maintenance, avoid the use of protoxide, and minimize the use of perioperative opioids (Strong recommendation; High level of evidence)43–45
Depending on the score obtained on the Apfel scale, the drugs used include dexamethasone, ondansetron and droperidol, which are administered individually, or in double/triple therapy. (Strong recommendation. High level of evidence)46
- •
Multimodal analgesia: there is scientific evidence about the superiority, both in analgesic quality and in the lower rate of postoperative complications, of epidural analgesia used in open abdominal surgery. Anesthesia should be conducted in combination with epidural analgesia in all patients treated with open abdominal surgery. (Strong recommendation; High level of evidence)47
In laparoscopic surgery, there are no significant differences in postoperative complications, reduction of hospital stay or recovery of early intestinal function. Despite the better analgesic profile, given the risk/benefit relationship of the technique, epidural analgesia is not recommended as a routine analgesic method in laparoscopic surgery. (Strong recommendation. High level of evidence)48
The transverse abdominis plane (TAP) block provides analgesia for skin, muscles and parietal peritoneum from T7 to L1. The use of the ultrasound-guided technique reduces complications and the failure rate. However, the distorted anatomy of the morbidly obese complicates the technique, even with ultrasound. Laparoscopy-guided TAP is an alternative for the effective performance of the technique. In no case does this practice exceed the analgesic efficacy of epidural analgesia. Patients in whom epidural analgesia is contraindicated may benefit from bilateral TAP as part of multimodal analgesia (Weak recommendation; Low level of evidence)49–51
There is insufficient evidence to demonstrate that infiltration with local anesthetic of trocar wounds improves postoperative pain, so its routine use cannot be recommended. (Weak recommendation; Low level of evidence)52,53
- •
No nasogastric tube (intraoperative only to empty stomach): the use of a nasogastric tube should not be routine, since it has not been shown to prevent complications and it increases hospital stay (Strong recommendation; Moderate level of evidence)54,55
- •
Drain tubes: if used, they must be closed, unidirectional and connected to a vacuum system. Recent studies show that the use of drain tubes does not prevent leaks or bleeding, nor do they facilitate an early diagnosis. Their routine use is not recommended. (Strong recommendation; Moderate level of evidence)56
Surgeons, anesthesiologists and nursing staff should all participate in this phase. The following measures should be mentioned:
- •
Start of early oral tolerance: the main objective of initial oral tolerance after bariatric surgery is to minimize possible early side effects after surgery (nausea, vomiting, diarrhea, abdominal pain, etc.). It is recommended to initiate oral tolerance to liquids in the first 6 postoperative hours. (Strong recommendation; High level of evidence)23,57
- •
It is recommended that the postoperative diet the first 2 weeks should be a liquid diet (preferably commercial) that provides > 60 g protein/day. (Strong recommendation; Moderate level of evidence)58,59
- •
Early mobilization after surgery: early postoperative mobilization reduces the risk of complications, such as pulmonary thromboembolism, pressure ulcers and pneumonia, and therefore reduces hospital stay. The start of mobilization will be indicated 4 h after the end of the surgery. Active exercises of the lower extremities and circulatory exercises while sitting will be emphasized. (Strong recommendation; High level of evidence)60,61
- •
In patients with SAHS, early reinstatement of CPAP: postoperative CPAP improves arterial blood gas and reduces the need for intubation, while it does not increase the risk of postoperative fistula. In patients with SAHS, when indicated, early reinstatement of CPAP use is recommended in the postoperative period (Weak recommendation; Low level of evidence)62,63
- •
Complementary tests: there is no consensus about the need for routine imaging studies after bariatric surgery. Radiological studies with oral contrast have low sensitivity to rule out anastomotic leaks. It is recommended to reserve imaging studies for the clinical suspicion of anastomotic leakage, which should include computed tomography with oral contrast, either with or without associated endoscopic studies. (Weak recommendation; Moderate level of evidence)64
The hospital discharge criteria will be: absence of surgical complications, fever, tachycardia or tachypnea, pain controlled with analgesia, complete ambulation, adequate oral tolerance, acceptance by the patient and understanding of the information given, possibility of follow-up after discharge and availability of home support and coordination with Primary Care. (Strong recommendation. Strong level of evidence)65 ERAS in bariatric surgery provides patients with information about the main warning signs of possible complications (fever, tachycardia, increased abdominal pain, respiratory distress, vomiting or gastrointestinal bleeding), given which they should immediately contact their Primary Care Physician or go to the Emergency Department of their referral hospital.
After hospital dischargeThe postoperative follow-up should be guided by surgeons, endocrinologists, nursing staff and specialists in sports science:
- •
Thromboprophylaxis: pharmacological thromboprophylaxis should be prolonged at least 7–10 days after discharge, and extended treatment for 4 weeks should be considered in high-risk patients. (Weak recommendation; Low level of evidence)35,66
- •
Postoperative exercise program: one year after surgery, bone mineral density of the hip drops by 8%-11%, which increases the risk of fractures. Similarly, reductions in lean mass occur (˜10 kg one year after surgery). This will decrease the basal metabolic rate, which will predispose the body to weight gain.
The main objectives of exercise in this phase will be to reduce the loss of lean mass and bone mineral density, as well as to improve the functional capacity of the patient. One month after surgery, it is recommended to initiate a cardiorespiratory and strength training program. The program should be started at moderate intensities and progress, based on the evolution of the patient, to higher intensities, especially in strength training. Start with 2 days a week and progress to at least 4 days a week. (Strong recommendation; Low level of evidence)31–33
Although these recommendations were initially focused on patients treated with vertical sleeve gastrectomy and Roux-en-Y gastric bypass, at the last meeting of the bariatric surgery working group of the GERM, it was discussed whether to evaluate the application of this ERAS to patients treated with other bariatric procedures, such as the one-anastomosis gastric bypass or other malabsorptive procedures. Although theoretically the vast majority of these measures would be applicable to all bariatric procedures, there is not enough evidence in the literature to support the use of ERAS in these techniques.
The implementation of these measures should be adapted to the organization of each hospital, although it is recommended to apply most of them together. The multidisciplinary work of various specialists is necessary to obtain a well-structured sequence of care, which will allow all parts of the process to happen more quickly and effectively. One of the purposes of the GERM is to periodically evaluate the results from the application of the protocols and update them in accordance with new scientific evidence.
Conflict of interestsThe authors have no conflict of interests to declare.
Eva Alarcón, Ricardo Belda, Manuel Ferrer, Antonio Morandeira, José Luis Muñoz, Pablo Royo, Elisabeth Redondo, Manuel Duran, Alejandro Garcia, Carlos Ferrigni, Camilo Castellón, Irene Portero, Carmen Vallejo, Damian Garcia-Olmo, Miguel Angel Bielsa, Jose Miguel Candeal, Maria Jose Palacios, Ana Navarro, Azucena Gonzalo, Diana Berrio, Maria Diez, Nuria Esteve, Elena Miranda, Lorea Zubiaga, Antonio Arroyo, Carolina Llavero, Juan Carlos Ruiz de Adana, Javier Martin Ramiro, José María Calvo, Salud García and Fátima Sabench
Please cite this article as: Ruiz-Tovar J, Sanchez-Santos R, Martín-García-Almenta E, García Villabona E, Hernandez AM, Hernández-Matías A, et al. Rehabilitación multimodal en cirugía bariátrica. Cir Esp. 2019;97:551–559.
Members of the Bariatric Surgery Working Group of the Grupo Español de Rehabilitación Multimodal (GERM) can be found in Appendix A.