This multicentre observational study aimed to determine the anastomotic leak rate in the hospitals included in the Rectal Cancer Project of the Spanish Society of Surgeons and examine whether hospital volume may contribute to any variation between hospitals.
MethodsHospital variation was quantified using a multilevel approach on prospective data derived from the multicentre database of all adenocarcinomas of the rectum operated by an anterior resection at 84 surgical departments from 2006 to 2013. The following variables were included in the analysis; demographics, American Society of Anaesthesiologists classification, use of defunctioning stoma, tumour location and stage, administration of neoadjuvant treatment, and annual volume of elective surgical procedures.
ResultsA total of 7231 consecutive patients were included. The rate of anastomotic leak was 10.0%. Stratified by annual surgical volume hospitals varied from 9.9% to 11.3%. In multilevel regression analysis, the risk of anastomotic leak increased in male patients, in patients with tumours located below 12cm from the anal verge, and advanced tumour stages. However, a defunctioning stoma seemed to prevent this complication. Hospital surgical volume was not associated with anastomotic leak (OR: 0.852, [0.487–1.518]; P=.577). Furthermore, there was a statistically significant variation in anastomotic leak between all departments (MOR: 1.475; [1.321–1.681]; P<.001).
ConclusionAnastomotic leak varies significantly among hospitals included in the project and this difference cannot be attributed to the annual surgical volume.
El objetivo de este estudio observacional multicéntrico fue determinar la tasa de dehiscencia anastomótica en los hospitales que participan en el Proyecto del Cáncer de Recto de la Asociación Española de Cirujanos y evaluar si había diferencias atribuibles al volumen quirúrgico entre los hospitales que participan en él.
MétodosLa variación interhospitalaria se cuantificó mediante un estudio multinivel realizado con una base de datos prospectiva de los pacientes operados por un adenocarcinoma de recto con una resección anterior en 84 hospitales, entre marzo de 2006 y diciembre de 2013. En los análisis se incluyeron: las variables demográficas, la clasificación de la American Society of Anaesthesiologists, la utilización de un estoma de derivación, la localización y el estadio del tumour, la administración de tratamiento neoadyuvante y el volumen quirúrgico anual del hospital.
ResultadosSe analizó a 7.231 pacientes operados consecutivamente. La tasa de dehiscencia anastomótica fue del 10,0%. Los porcentajes de dehiscencia de los hospitales, estratificados por el volumen quirúrgico annual, variaron entre el 9,9 y el 11,3%. En el análisis de regresión multinivel el sexo masculino, los tumores localizados por debajo de 12cm medidos desde el margen anal y los estadios T avanzados favorecieron la aparición de la dehiscencia, mientras que la presencia de un estoma de derivación la previno. El volumen quirúrgico anual del hospital no se asoció con la dehiscencia (OR: 0,852; [0,487–1,518]; P=0,577). Además, se observó una variación significativa de la tasa de dehiscencia entre los hospitales (MOR: 1,475; [1,321–1,681]; P<0,001).
ConclusiónLa dehiscencia anastomótica varía de forma estadísticamente significativa entre los hospitales incluidos en el proyecto, y esta diferencia no se puede atribuir al volumen quirúrgico anual.
The standard treatment for cancer of the rectum, when it is possible, is anterior resection with total mesorectal excision.1 This surgical procedure is associated with anastomotic leakage rate that varies from 10% to 15%.2,3
The contributions of several factors to the appearance of this complication have been studied. Nevertheless, research into the influence of hospital surgical volume and specialisation on the frequency of anastomotic leakage is limited and controversial.4,5 Additionally, the results of Scandinavian records,6,7 which inspired the Rectal Cancer Project of the Spanish Association of Surgeons, are also controversial.
The aim of this study was to determine the rate of anastomotic leakage in the Rectal Cancer Project of the Spanish Association of Surgeons during the period 2006–2013, and to evaluate, using a multivariate study, the influence of hospital surgical volume on anastomotic leakage in the participating hospitals.
MethodsThis observational multicentre study was undertaken using the prospective database of the Rectal Cancer Project of the Spanish Association of Surgeons. This teaching initiative started in 2006 with the aim of firstly introducing mesorectal excision surgery and then extended abdominoperineal amputation to multidisciplinary groups in National Health System hospitals that requested this and fulfilled the requisite conditions: they had to have Coloproctology Units with the indispensible diagnostic technical resources and perform 12 or more colorectal resections per year, which includes the following operations: anterior resection, abdominoperineal amputation, Hartmann's procedure and proctocolectomy.
Data gathered prospectively in hospitals by the surgeons in the project were sent to a central registry. This informed each hospital every year of the results of its activity in comparison with all of the other hospitals taking part. A more detailed description of this project has already been published.8,9
Patient Inclusion and Exclusion CriteriaPatients were included who had been operated electively with anterior resection from 1 March 2006 and 31 December 2013 in the 84 hospitals included in the project.
Patients operated as emergencies were excluded, as were those who lacked the results of a relevant variable and those whose results were incongruent.
Surgical TechniqueTotal mesorectal excision was the standard procedure to treat tumours located 12cm or less from the anal margin. Tumours above 12cm were treated using the partial excision of the mesorectum, including the mesorectal fascia up to 5cm below the tumour, or total mesorectal excision at the decision of the surgeon. Whether or not an ileostomy was performed also depended on the surgeon's decision.
Study VariablesLeakage from the anastomosis was the outcome variable of the study. Confusion variables are divided into fixed and random ones. The following demographic variables are considered to be fixed confusion variables: age, categorised into 3 groups (<65, 65–80, >80 years old), and sex; the degree of surgical risk (measured by the ASA degree of anaesthetic risk); the location of the tumour, which was categorised in 3 groups (0–6cm, 7–12cm and 13–15cm); the use of an ileostomy; the pathological stage of the tumour; the use of neoadjuvant treatment and the individual nature of each hospital, categorised in 4 groups according to the median number of patients treated per year using anterior resection: 11, 12–23, 24–35 and >36 patients. The hospital was considered to be a random confusion variable.
DefinitionsRectal tumours (CIE20) were considered to be those located in the last 15cm measured from the anal margin by rigid rectoscopy during withdrawal or by magnetic resonance.10
The pathological tumour stage was classified using the fifth edition of the TNM classification (American Joint Committee on Cancer [AJCC] stages I–IV; 5th edition).11
Leakage from the anastomosis was defined as an event of the anastomosis which required interventional radiology or a postoperative surgical operation. Pelvic abscesses without radiological evidence of leakage and early recto-vaginal fistulas are also considered to be leakages from the anastomosis. Subclinical leakages detected in the soluble contrast enema before closure of the stoma were not considered to be anastomotic leaks, as is the case in the Scandinavian records which this project imitates and with which it compares its results.12,13
As the names of the hospitals and patients included are not given, it was not considered necessary to seek the approval of the Ethics Committees of the hospitals in question, although the project had been approved by them.
Statistical AnalysisCategorical variables were analysed using the χ2 test, while non-parametrical samples were compared using Mann–Whitney's U test.
To determine the variation of the outcome variable of anastomotic leakage among the hospitals included, 3 models were constructed: one model of fixed effects included the fixed confusion variables, one complete model included the fixed confusion variables and the random hospital variable, while one null model exclusively included the random hospital variable. Logistic regression was carried out in the first model, while multilevel logistic regression was applied in the other two models.
Akaike's information criterion (AIC)14 was calculated for each model, together with the deviance test. Random variance (σ2) was calculated in the multilevel models, together with their intervals of confidence, the median odds ratio (MOR)15 and the odds ratio (OR) between the best and worst, excluding 5% of the outlier hospitals. The MOR quantifies the variation between hospitals as medians, comparing randomly selected pairs of patients with the same values of the confusion variables.16
ResultsOf the 7231 patients operated electively using anterior resection in the period studied, 727 (10.0%) presented anastomotic leakage.
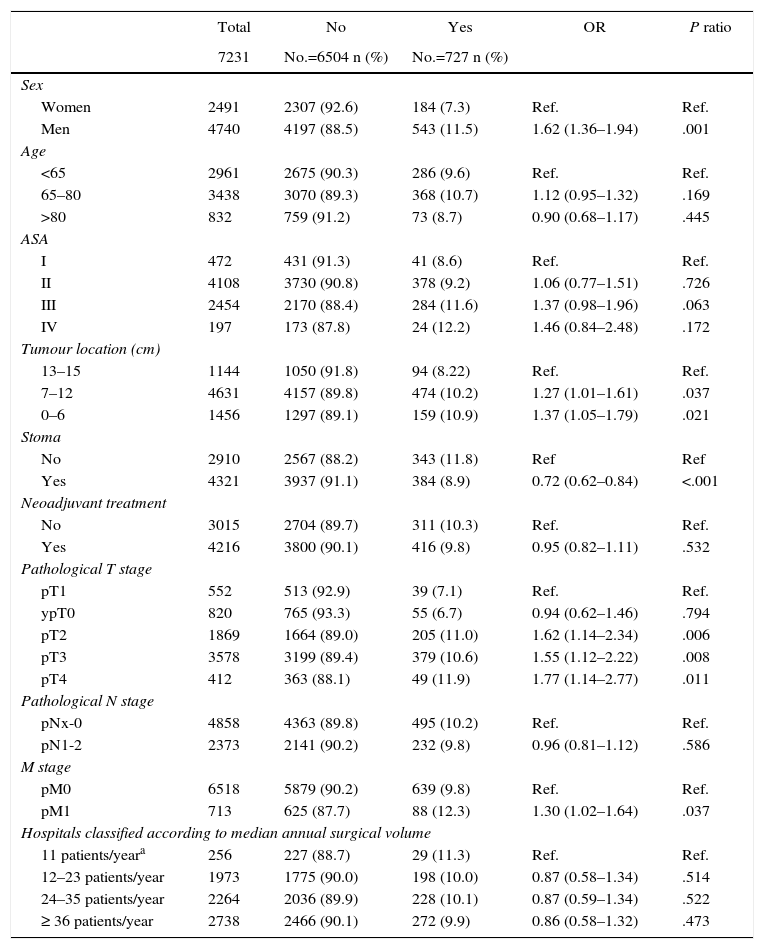
Table 1 describes the characteristics of the population studied and the risk of leakage expressed in OR for each one of the variables studied using univariate analysis. It shows the percentages of anastomotic leakage in the hospitals grouped into categories according to surgical volume, from 9.9% to 11.3%.
Description and Results of Univariate Analysis of the Population Sample Studied.
| Total | No | Yes | OR | P ratio | |
|---|---|---|---|---|---|
| 7231 | No.=6504 n (%) | No.=727 n (%) | |||
| Sex | |||||
| Women | 2491 | 2307 (92.6) | 184 (7.3) | Ref. | Ref. |
| Men | 4740 | 4197 (88.5) | 543 (11.5) | 1.62 (1.36–1.94) | .001 |
| Age | |||||
| <65 | 2961 | 2675 (90.3) | 286 (9.6) | Ref. | Ref. |
| 65–80 | 3438 | 3070 (89.3) | 368 (10.7) | 1.12 (0.95–1.32) | .169 |
| >80 | 832 | 759 (91.2) | 73 (8.7) | 0.90 (0.68–1.17) | .445 |
| ASA | |||||
| I | 472 | 431 (91.3) | 41 (8.6) | Ref. | Ref. |
| II | 4108 | 3730 (90.8) | 378 (9.2) | 1.06 (0.77–1.51) | .726 |
| III | 2454 | 2170 (88.4) | 284 (11.6) | 1.37 (0.98–1.96) | .063 |
| IV | 197 | 173 (87.8) | 24 (12.2) | 1.46 (0.84–2.48) | .172 |
| Tumour location (cm) | |||||
| 13–15 | 1144 | 1050 (91.8) | 94 (8.22) | Ref. | Ref. |
| 7–12 | 4631 | 4157 (89.8) | 474 (10.2) | 1.27 (1.01–1.61) | .037 |
| 0–6 | 1456 | 1297 (89.1) | 159 (10.9) | 1.37 (1.05–1.79) | .021 |
| Stoma | |||||
| No | 2910 | 2567 (88.2) | 343 (11.8) | Ref | Ref |
| Yes | 4321 | 3937 (91.1) | 384 (8.9) | 0.72 (0.62–0.84) | <.001 |
| Neoadjuvant treatment | |||||
| No | 3015 | 2704 (89.7) | 311 (10.3) | Ref. | Ref. |
| Yes | 4216 | 3800 (90.1) | 416 (9.8) | 0.95 (0.82–1.11) | .532 |
| Pathological T stage | |||||
| pT1 | 552 | 513 (92.9) | 39 (7.1) | Ref. | Ref. |
| ypT0 | 820 | 765 (93.3) | 55 (6.7) | 0.94 (0.62–1.46) | .794 |
| pT2 | 1869 | 1664 (89.0) | 205 (11.0) | 1.62 (1.14–2.34) | .006 |
| pT3 | 3578 | 3199 (89.4) | 379 (10.6) | 1.55 (1.12–2.22) | .008 |
| pT4 | 412 | 363 (88.1) | 49 (11.9) | 1.77 (1.14–2.77) | .011 |
| Pathological N stage | |||||
| pNx-0 | 4858 | 4363 (89.8) | 495 (10.2) | Ref. | Ref. |
| pN1-2 | 2373 | 2141 (90.2) | 232 (9.8) | 0.96 (0.81–1.12) | .586 |
| M stage | |||||
| pM0 | 6518 | 5879 (90.2) | 639 (9.8) | Ref. | Ref. |
| pM1 | 713 | 625 (87.7) | 88 (12.3) | 1.30 (1.02–1.64) | .037 |
| Hospitals classified according to median annual surgical volume | |||||
| 11 patients/yeara | 256 | 227 (88.7) | 29 (11.3) | Ref. | Ref. |
| 12–23 patients/year | 1973 | 1775 (90.0) | 198 (10.0) | 0.87 (0.58–1.34) | .514 |
| 24–35 patients/year | 2264 | 2036 (89.9) | 228 (10.1) | 0.87 (0.59–1.34) | .522 |
| ≥ 36 patients/year | 2738 | 2466 (90.1) | 272 (9.9) | 0.86 (0.58–1.32) | .473 |
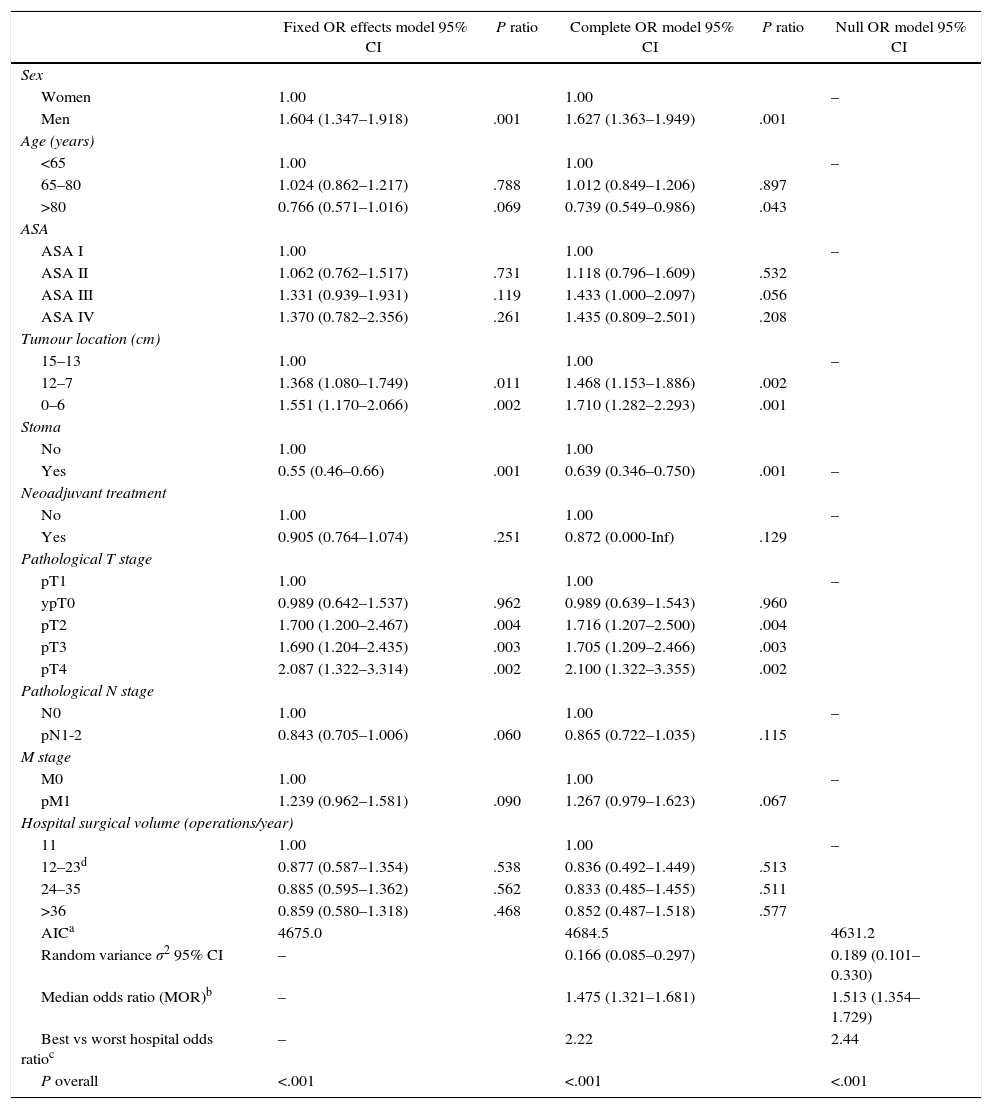
The results of the fixed effects models, the complete model and the null model are shown in Table 2. This shows the variables that had a significant influence on anastomotic leakage. Male sex, the distance from the tumour to the anal margin and advanced T stages made it more probable; while ileostomy prevented it. Hospital surgical volume did not influence the rates of anastomotic leakage. Additionally, the complete and null models showed that leakage from the anastomosis differed significantly between the hospitals studied.
Analytical Results of the 3 Models.
| Fixed OR effects model 95% CI | P ratio | Complete OR model 95% CI | P ratio | Null OR model 95% CI | |
|---|---|---|---|---|---|
| Sex | |||||
| Women | 1.00 | 1.00 | – | ||
| Men | 1.604 (1.347–1.918) | .001 | 1.627 (1.363–1.949) | .001 | |
| Age (years) | |||||
| <65 | 1.00 | 1.00 | – | ||
| 65–80 | 1.024 (0.862–1.217) | .788 | 1.012 (0.849–1.206) | .897 | |
| >80 | 0.766 (0.571–1.016) | .069 | 0.739 (0.549–0.986) | .043 | |
| ASA | |||||
| ASA I | 1.00 | 1.00 | – | ||
| ASA II | 1.062 (0.762–1.517) | .731 | 1.118 (0.796–1.609) | .532 | |
| ASA III | 1.331 (0.939–1.931) | .119 | 1.433 (1.000–2.097) | .056 | |
| ASA IV | 1.370 (0.782–2.356) | .261 | 1.435 (0.809–2.501) | .208 | |
| Tumour location (cm) | |||||
| 15–13 | 1.00 | 1.00 | – | ||
| 12–7 | 1.368 (1.080–1.749) | .011 | 1.468 (1.153–1.886) | .002 | |
| 0–6 | 1.551 (1.170–2.066) | .002 | 1.710 (1.282–2.293) | .001 | |
| Stoma | |||||
| No | 1.00 | 1.00 | |||
| Yes | 0.55 (0.46–0.66) | .001 | 0.639 (0.346–0.750) | .001 | – |
| Neoadjuvant treatment | |||||
| No | 1.00 | 1.00 | – | ||
| Yes | 0.905 (0.764–1.074) | .251 | 0.872 (0.000-Inf) | .129 | |
| Pathological T stage | |||||
| pT1 | 1.00 | 1.00 | – | ||
| ypT0 | 0.989 (0.642–1.537) | .962 | 0.989 (0.639–1.543) | .960 | |
| pT2 | 1.700 (1.200–2.467) | .004 | 1.716 (1.207–2.500) | .004 | |
| pT3 | 1.690 (1.204–2.435) | .003 | 1.705 (1.209–2.466) | .003 | |
| pT4 | 2.087 (1.322–3.314) | .002 | 2.100 (1.322–3.355) | .002 | |
| Pathological N stage | |||||
| N0 | 1.00 | 1.00 | – | ||
| pN1-2 | 0.843 (0.705–1.006) | .060 | 0.865 (0.722–1.035) | .115 | |
| M stage | |||||
| M0 | 1.00 | 1.00 | – | ||
| pM1 | 1.239 (0.962–1.581) | .090 | 1.267 (0.979–1.623) | .067 | |
| Hospital surgical volume (operations/year) | |||||
| 11 | 1.00 | 1.00 | – | ||
| 12–23d | 0.877 (0.587–1.354) | .538 | 0.836 (0.492–1.449) | .513 | |
| 24–35 | 0.885 (0.595–1.362) | .562 | 0.833 (0.485–1.455) | .511 | |
| >36 | 0.859 (0.580–1.318) | .468 | 0.852 (0.487–1.518) | .577 | |
| AICa | 4675.0 | 4684.5 | 4631.2 | ||
| Random variance σ2 95% CI | – | 0.166 (0.085–0.297) | 0.189 (0.101–0.330) | ||
| Median odds ratio (MOR)b | – | 1.475 (1.321–1.681) | 1.513 (1.354–1.729) | ||
| Best vs worst hospital odds ratioc | – | 2.22 | 2.44 | ||
| P overall | <.001 | <.001 | <.001 | ||
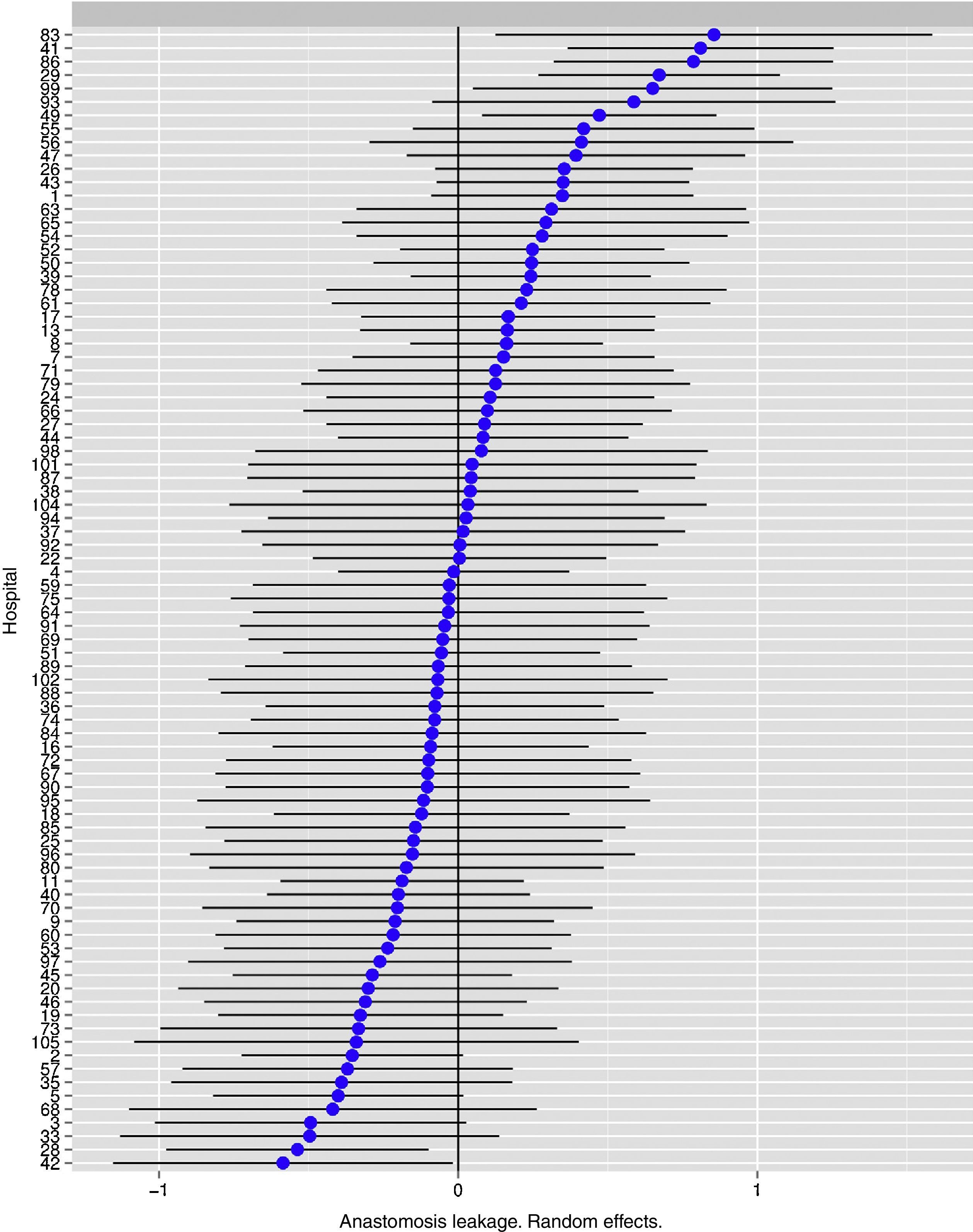
Fig. 1 shows the differences between the hospitals.
The risk of anastomotic leakage in hospitals, obtained by multilevel logistic regression, considering the hospital variable to be a random effect to correct the non-independence of data. The hospitals are represented along the vertical axis by their project code number. The value of the random constant for each hospital is shown. The hospitals are ranked from lower to higher constant value: positive constant values show the worst results.
The results of this study indicate that in the Rectal Cancer Project of the Spanish Association of Surgeons anastomotic leakage does not depend on hospital surgical volume, and that rates vary between the hospitals included in a statistically significant way.
The greatest weakness of this study is connected with the voluntary nature of the inclusion of data in the Colorectal Cancer Project of the Spanish Association of Surgeons, above all when it is compared with records in Scandinavian countries,17,18 where it is obligatory to include data in the registry. Nevertheless, as has already been stated in greater detail,8,9 several measures were applied to prevent voluntary or involuntary distortion in inclusion as well as in the data. Unfortunately, due to the anonymous nature of the data and the lack of other sources to compare information in our country, it is not possible to state with certainty that there are no such data. Lastly, in the worst-case scenario the data in this study indicate minimum rates of anastomotic leakage.
Another weakness of this study is that the project database does not include several variables that may influence rates of anastomotic leakage, such as obesity or albumin levels, etc. Nevertheless, the aim of the project is for hospitals to know the immediate and long-term results of their work in comparison with those of all the hospitals. As the project is implemented in 15 autonomous communities, it is improbable that there will be differences that can be attributed to socio-economic factors, as has been proven in Denmark.19
The rate of anastomotic leakage observed in this study, 10%, is between the rates found in Norway17 (7%), Sweden20 (8.7%) and Denmark18 (12.7%), according to the latest reports and studies published by these registries. It is far higher than the rate shown in the Irish programme that centralises data on this disease (4.3%).5
Three of these registries evaluated the influence of hospital surgical volume on the occurrence of leakage, with contradictory results. Univariate analyses in Ireland5 and Norway6 showed differences that could be attributed to volume, while multivariate analysis in Denmark7 showed no such differences, as is the case in this study which used multilevel statistical analysis. The discrepancy between these 4 studies may therefore be explained by the difference in statistical methodology. Furthermore, multivariate analysis which makes it possible to adjust results according to multiple variables in the sample is far more solid, together with multilevel analysis.
It is a natural tendency to use national registry data to establish activity outcome indicators, as was the case in Scandinavia, as these make it possible for hospitals to try to correct variability. Unfortunately, although the departments taking part in the Rectal Cancer Project of the Spanish Association of Surgeons have similar characteristics to those of the Scandinavian hospitals, and the size of the sample of patients analysed in this study is reasonably large, it is impossible to use it as a national outcome indicator. The reason for this, as has been found for the voluntary records of the National Bowel Cancer Project in Great Britain21 and the Belgian rectal cancer PROCARE22 project, is that when hospitals do not send in all of their data and thereby distort the information, or if they fail to take part in the registry at all, the rates of surgical mortality are higher for the patients not included in the registry than they are for those who are included. This leads to an underestimation of surgical mortality at a national level, preventing exact estimations of the activity indicators.
To conclude, the results of this study indicate that in the Rectal Cancer Project of the Spanish Association of Surgeons the rate of anastomotic leakage is similar to those of the Scandinavian registries. It also varies in a statistically significant way between the hospitals included, and this difference cannot be explained by surgical volume.
FinancingThis project was financed by the following research grants: FIS number: PI11/00010 and the Board of Health, Government of Navarre: 20/11.
Conflict of InterestThe authors have no conflict of interests to declare.
The collaborating Group of the Colorectal Cancer Project of the Spanish Association of Surgeons (2006–2013).
Virgen de la Arrixaca (Juan Luján), Bellvitge (Doménico Fraccalvieri, Sebastiano Biondo), Complejo Hospitalario de Navarra (Miguel Á. Ciga), Clínico de Valencia (Alejandro Espí), Josep Trueta (Antonio Codina), Sagunto (María D. Ruiz), Vall d’Hebrón (Eloy Espin, F. Vallribera), La Fe (Eduardo García-Granero), Complejo Hospitalario Ourense (Alberto Parajo), Germans Trias i Pujol (Ignasi Camps, Marta Piñol), Lluís Alcanyis (Vicent Viciano), Complejo Asistencial Burgos (Evelio Alonso), Hospital del Mar (Miguel Pera), Meixoeiro (Nieves Cáceres), Complejo Asistencial Salamanca (Jacinto García), Gregorio Marañón (Marcos Rodríguez), Torrecárdenas (Ángel Reina), General de Valencia (Verónica Gumbau), Txagorritxu (José Errasti), Donostia (José A. Múgica), Reina Sofía (José Gómez), Juan Ramón Jiménez (Ricardo Rada, Mónica Orelogio), Arnau de Vilanova de Valencia (Natalia Uribe), General de Jerez (Juan de Dios Franco), Arnau de Vilanova de Lérida (José Enrique Sierra), Santa Creu i Sant Pau (Pilar Hernández), Clínico de Santiago de Compostela (Jesús Paredes), Universitario de Jaén (Gabriel Martínez), Clínico San Carlos (Mauricio García), Cabueñes (Guillermo Carreño), General de Albacete (Jesús Cifuentes), Miguel Servet (José Monzón), Xeral de Lugo (Olga Maseda), Universitario de Fuenlabrada (Daniel Huerga), Clínico y Provincial de Barcelona (Luis Flores), Joan XXIII (Fernando Gris), Virgen de las Nieves (Inmaculada Segura, Pablo Palma), Nuestra Señora de la Candelaria (José G. Díaz), Complejo Hospitalario de Badajoz (Jesús Salas), Clínico Universitario San Cecilio (Francisco Pérez, Benito Mirón), General Universitario de Alicante (Félix Lluis), Virgen Macarena (Luis Capitán, Javier Valdés), Xeral-Cies de Vigo (Nieves Cáceres), Infanta Sofía (Javier Martínez), Policlínica de Vigo (Alfredo Estévez), Virgen del Rocío de Sevilla (José Díaz, María V. Maestre), San Juan de Dios del Aljarafe (Antonio Amaya, Mónica Reig), Nuestra Señora de Sonsoles (Antonio Carmona), Universitario de Getafe (Francisco Javier Jiménez), H. Granollers (Didac Ribe), Universitario de La Paz (Isabel Prieto), Rafael Méndez (Ginés Sánchez, Sergio del Valle), General Universitario Reina Sofía (Pedro A. Parra), San Pedro de Alcántara (Francisco Romero), Torrevieja Salud (Alessandro Garcea), Santa María de Lérida (Xavier Rodamilans), Virgen del Puerto (José A. Pérez), Segovia (Guillermo Asís), Reus (Santiago Blanco), Instituto Valenciano de Oncología (Alfonso García, Rafael Estévan), Viladecans (Albert Sueiras), Cruces (Alberto Lamíquiz, José M.ª García), Ramón y Cajal (Javier Die), Manises (Amparo Solana), La Ribera Alzira (Francisco J. Blanco), Nuestra Señora del Rosell (Ana Lage), Mérida (José Domínguez), Universitario Fundación Alcorcón (Paula Dujovne), Henares Coslada (Natividad Palencia), Vinaroz (Raúl Adell), Onkologikoa de San Sebastián (Roberto Martínez), Consorci Sanitari Integral (Luis Ortiz de Zarate), Complejo Hospitalario Palencia (Ana M.ª Huidobro), Fundación Jiménez Díaz (Carlos Pastor), Torrejón (Jesús Á. Garijo), Puerto Real (M.ª del Coral de la Vega), Espíritu Santo (Manuel López).
Please cite this article as: Ortiz H, Biondo S, Codina A, Ciga MÁ, Enríquez-Navascués J, Espín E, et al. Variabilidad interhospitalaria de la dehiscencia anastomótica en el Proyecto del Cáncer de Recto de la Asociación Española de Cirujanos: La influencia del volumen quirúrgico. Cir Esp. 2016;94:213–220.
Appendix A presents a list of collaborators.