Morbimortality after bariatric surgery varies according to patient characteristics and associated comorbidities. The aim of this study was to evaluate the usefulness of the obesity surgery mortality risk score scale (OS-MRS) to predict the risk of postoperative complications after bariatric surgery.
MethodsA retrospective study was performed on a prospective series of patients undergoing bariatric surgery in which the OS-MRS scale was applied preoperatively. Postoperative complications were classified as proposed by Dindo–Clavien. We analysed the relationship between the categories of OS-MRS scale: (A) low risk, (B) intermediate risk, and (C) high risk and the presence of complications.
ResultsBetween May 2008 and June 2012, 198 patients were included (85 [42.9%] after gastric bypass and 113 [57.1%] after sleeve gastrectomy). Using the OS-MRS scale, 124 patients were classified as class A (62.6%), 70 as class B (35.4%) and 4 as class C (2%). The overall morbidity rate was 12.6% (25 patients). A significant association between OS-MRS scale and rate of complications (7.3, 20 and 50%, respectively, P=.004) was demonstrated. The gastric bypass was associated with a higher complication rate than sleeve gastrectomy (P=.007). In multivariate analysis, OS-MRS scale and surgical technique were the only significant predictive factors.
ConclusionsThe OS-MRS scale is a useful tool to predict the risk of complications and can be used as a guide when choosing the type of bariatric surgery.
La morbimortalidad tras la cirugía bariátrica varía según las características de los pacientes y las comorbilidades asociadas. El objetivo de este estudio fue evaluar la posible utilidad de la escala Obesity surgery mortality risk score (OS-MRS) para prever el riesgo de complicaciones postoperatorias tras cirugía bariátrica.
MétodosEstudio retrospectivo de una serie prospectiva de pacientes intervenidos de cirugía bariátrica a los que se aplicó antes de la operación la escala OS-MRS. Se clasificaron las complicaciones postoperatorias siguiendo la propuesta de Dindo-Clavien. Se analizó la relación entre las categorías de la escala OS-MRS: (A) bajo riesgo; (B) riesgo intermedio y (C) riesgo elevado, y la presencia de complicaciones.
ResultadosEntre mayo del 2008 y junio del 2012 se incluyó a 198 pacientes (85 [42,9%] a los que se realizó un bypass gástrico y 113 [57,1%] con una gastrectomía vertical). Utilizando la escala OS-MRS, 124 pacientes se clasificaron como clase A (62,6%), 70 como clase B (35,4%) y 4 como clase C (2%). La tasa de morbilidad global fue del 12,6% (25 pacientes). Se demostró una asociación significativa entre la escala OS-MRS y la tasa de complicaciones (7,3, 20 y 50%, respectivamente; p=0,004). El bypass gástrico se asociaba a mayor número de complicaciones que la gastrectomía vertical (p=0,007). En el análisis multivariado, la escala OS-MRS y la técnica quirúrgica fueron los únicos factores con valour predictivo.
ConclusionesLa escala OS-MRS es una herramienta útil para predecir el riesgo de complicaciones y puede orientar a la hora de escoger el tipo de cirugía bariátrica.
Laparoscopy has revolutionised the world of bariatric surgery. Studies to date demonstrate a great many advantages of this type of approach in comparison to open surgery.1,2 Nevertheless, laparoscopic bariatric surgery continues to be associated with post-operative complications and mortality.
The different degrees of obesity and the comorbidities presented by patients who are to undergo bariatric surgery make it difficult to compare the morbi-mortality rates which appear in literature. Having a validated scale to estimate surgical risk could, on the one hand, offer reliable and individualised information for patients about to undergo obesity surgery and, on the other hand, allow those with a high risk of morbi-mortality to be identified preoperatively, which would enable pre and peri-operative treatment to be optimised and perhaps make it possible to choose the most appropriate surgical procedure for each patient.
Different scales have been prepared to estimate the risk of mortality in an attempt to improve information on patients who are to undergo bariatric surgery. In 2007, DeMaria et al. proposed and validated in a multi-centre study, the obesity surgery mortality risk score (OS-MRS) for patients on whom gastric bypass surgery had been performed.3,4 The same scale was used subsequently by Efthimiou5 and Thomas6 with the same objective. In 2011, Sarela7 extended the use of this score to estimate morbidity and applied it to other techniques used in bariatric surgery. Other authors have also proposed their own score systems to predict post-operative morbidity.8,9
The objective of this study was to evaluate the possible use of the OS-MRS score to predict the risk of postoperative complications in morbidly obese patients treated with two laparoscopic bariatric surgery techniques.
Patients and MethodsA retrospective study was undertaken of a prospective and consecutive series of patients on whom laparoscopic bariatric surgery was performed in the Bariatric and Metabolic Surgery Unit of the Hospital del Mar (Barcelona), between May 2008 and July 2012. Two types of bariatric surgery procedures are performed in our centre: laparoscopic vertical gastrectomy (LVG) and laparoscopic gastric bypass (LGB). The preoperative assessment of all the patients was multidisciplinary (Surgery, Endocrinology, Psychiatry, Anaesthesia and Nutrition Departments) and the criteria for inclusion used were as proposed by the National Institute of Health in 1991.10
LVG was indicated in patients with: (a) a body mass index (BMI) between 35 and 39.9kg/m2 with associated comorbidities; (b) with BMI≥50kg/m2; (c) in young patients aged between 18 and 25 or (d) patients ≥55 years of age. For LGB the criteria were: (a) a BMI of between 40 and 50kg/m2; (b) age between 25 and 55 and (c) patients with serious gastro-oesophageal reflux disease or Barrett's oesophagus.
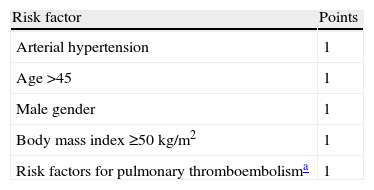
The following variables were obtained for all patients: age, gender, preoperative weight, BMI, the risk rate proposed by the American Society of Anesthesiology (ASA), major comorbidities (type 2 diabetes mellitus [DM2], obstructive sleep apnoea syndrome [OSAS], arterial hypertension [AHT], heart disease, dyslipidaemia, increased risk of pulmonary thromboembolism [PTE]), and a detailed description of the complications occurring in the immediate post-operative period. Following widely accepted definitions it was considered that DM2 was present when the patient presented basal glycaemia ≥110mg/dl or was being medicated; OSAS when the apnoea and hypopnoea index was ≥5episodes/h on nocturnal polysomnography; AHT if systolic blood pressure was ≥160mmHg or diastolic blood pressure was ≥95mmHg; heart disease when there was a history of heart disease of any aetiology (hypertensive, ischaemic, congenital, etc.); dyslipidemia when triglyceride levels were ≥150mg/dl or HDL cholesterol was <35mg/dl (male) or <40mg/dl (female); an increased risk of PTE if there was a previous history of PTE or deep venous thrombosis (DVT), hypoventilation (pCO2 ≥45mmHg), a diagnosis of pulmonary hypertension or the presence of inferior vena cava filter. In addition, the OS-MRS score was calculated based on the presence or absence of ATH, male gender, age ≥45, BMI≥50kg/m2 and the presence of predisposing factors of PTE. The patients were classified into three groups (A, B and C) according to their score (Table 1).
Risk Factors, Score and Classification Using the Obesity Surgery Mortality Risk Score (OS-MRS).
| Risk factor | Points |
| Arterial hypertension | 1 |
| Age>45 | 1 |
| Male gender | 1 |
| Body mass index≥50kg/m2 | 1 |
| Risk factors for pulmonary thromboembolisma | 1 |
| Risk group | Score | Post-operative mortality |
| A (low risk) | 0–1 | 0.3 |
| B (moderate risk) | 2–3 | 1.7 |
| C (high risk) | 4–5 | 3.2 |
All patients were operated on by the same surgeon and by the same surgical team using a standard technique.11–16 In summarised form: a subcardial gastric reservoir of 20–30ml was created in the LGB with an antecolic and antegastric Roux limb length of 150cm, excluding the first 50cm of jejunum. In the LVG the gastric transaction was started 5cm above the pylorus with a 36F drain. A clinical pathway specifically designed for bariatric surgery was followed for all cases, in which the patient was admitted one day prior to surgery for pre-operative preparation, with fluid intake and mobility started on the 1st day after surgery and discharge on the 3rd postoperative day after removal of intra-abdominal drains.
Postoperative complications in the first 30 days were classified according to Dindo–Clavien17 for minor (grades I and II) and major (grades IIIa, IIIb, IVa, IVb and V) complications.
Statistical AnalysisSPSS software, version 19 (IBM Corp., Chicago, USA) was used for the statistical analysis. The chi-square test or exact Fisher's test were applied to analyse the association between risk factors, type of operation and development of complications. Binary logistic regression analysis was used to analyse the influence of the OS-MRS classes and the type of surgery on the development of complications.
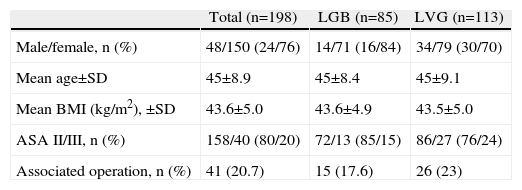
ResultsOne hundred and ninety-eight consecutive patients were included who had undergone bariatric surgery; their demographic and clinical characteristics are summarised in Table 2. The typical patient was female, around 40 years of age, with a BMI slightly above 43kg/m2 and an ASA risk class II. The 41 associated operations were 30 cholecystectomies, nine umbilical hernioplasties, one cholecystectomy plus umbilical hernioplasty and one closure of diaphragm pillars.
Demographic and Clinical Characteristics of the Overall Sample and by Surgical Procedure.
| Total (n=198) | LGB (n=85) | LVG (n=113) | |
| Male/female, n (%) | 48/150 (24/76) | 14/71 (16/84) | 34/79 (30/70) |
| Mean age±SD | 45±8.9 | 45±8.4 | 45±9.1 |
| Mean BMI (kg/m2), ±SD | 43.6±5.0 | 43.6±4.9 | 43.5±5.0 |
| ASA II/III, n (%) | 158/40 (80/20) | 72/13 (85/15) | 86/27 (76/24) |
| Associated operation, n (%) | 41 (20.7) | 15 (17.6) | 26 (23) |
ASA: risk rate according to the American Society of Anesthesiology; LGB: laparoscopic gastric bypass; LVG: laparoscopic vertical gastrectomy; BMI: body mass index.
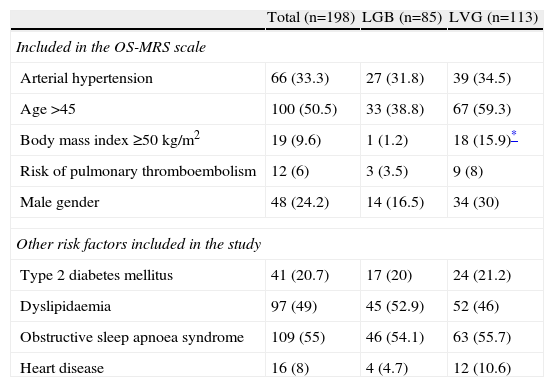
The prevalence of each of the risk factors analysed, whether or not included on the OS-MRS scale, are shown in Table 3 for the whole series and according to the surgical technique used. With the exception of the number and percentage of patients with a BMI≥50kg/m2 which, as was to be expected because of the selection criteria, was significantly higher for the patients treated with LVG; there were no differences between the two populations.
Overall Prevalence of Surgical Risk Factors Analysed in This Study and in the Subgroups of Patients With Laparoscopic Gastric Bypasses or Vertical Gastrectomies.
| Total (n=198) | LGB (n=85) | LVG (n=113) | |
| Included in the OS-MRS scale | |||
| Arterial hypertension | 66 (33.3) | 27 (31.8) | 39 (34.5) |
| Age>45 | 100 (50.5) | 33 (38.8) | 67 (59.3) |
| Body mass index≥50kg/m2 | 19 (9.6) | 1 (1.2) | 18 (15.9)* |
| Risk of pulmonary thromboembolism | 12 (6) | 3 (3.5) | 9 (8) |
| Male gender | 48 (24.2) | 14 (16.5) | 34 (30) |
| Other risk factors included in the study | |||
| Type 2 diabetes mellitus | 41 (20.7) | 17 (20) | 24 (21.2) |
| Dyslipidaemia | 97 (49) | 45 (52.9) | 52 (46) |
| Obstructive sleep apnoea syndrome | 109 (55) | 46 (54.1) | 63 (55.7) |
| Heart disease | 16 (8) | 4 (4.7) | 12 (10.6) |
LGB: laparoscopic gastric bypass; LVG: laparoscopic vertical gastrectomy.
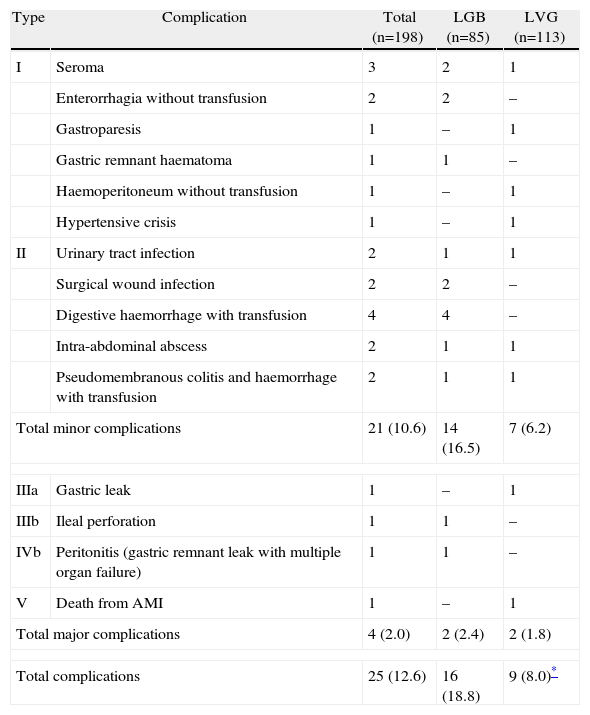
Twenty-five patients of the series (12.6%) presented a complication of some type during the 30 days following surgery. These are detailed according to the Dindo–Clavien classification in Table 4. One patient died due to cerebral hypoxia 45 days after surgery following an acute myocardial infarction on the second postoperative day. Three patients needed reoperation (two in the LGB and one in the LVG group) to treat their complications. The rate of complications was significantly higher in patients undergoing an LGB than for those on whom an LVG was performed (18.8% vs 8.0%, respectively; P=.007).
Postoperative Complications Classified According to Dindo–Clavien's Proposal for the Series as a Whole and According to the Surgical Procedure Performed.
| Type | Complication | Total (n=198) | LGB (n=85) | LVG (n=113) |
| I | Seroma | 3 | 2 | 1 |
| Enterorrhagia without transfusion | 2 | 2 | – | |
| Gastroparesis | 1 | – | 1 | |
| Gastric remnant haematoma | 1 | 1 | – | |
| Haemoperitoneum without transfusion | 1 | – | 1 | |
| Hypertensive crisis | 1 | – | 1 | |
| II | Urinary tract infection | 2 | 1 | 1 |
| Surgical wound infection | 2 | 2 | – | |
| Digestive haemorrhage with transfusion | 4 | 4 | – | |
| Intra-abdominal abscess | 2 | 1 | 1 | |
| Pseudomembranous colitis and haemorrhage with transfusion | 2 | 1 | 1 | |
| Total minor complications | 21 (10.6) | 14 (16.5) | 7 (6.2) | |
| IIIa | Gastric leak | 1 | – | 1 |
| IIIb | Ileal perforation | 1 | 1 | – |
| IVb | Peritonitis (gastric remnant leak with multiple organ failure) | 1 | 1 | – |
| V | Death from AMI | 1 | – | 1 |
| Total major complications | 4 (2.0) | 2 (2.4) | 2 (1.8) | |
| Total complications | 25 (12.6) | 16 (18.8) | 9 (8.0)* | |
LGB: laparoscopic gastric bypass; LVG: laparoscopic vertical gastrectomy; AMI: acute myocardial infarction.
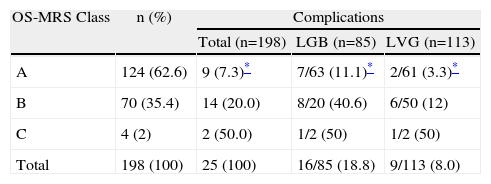
Applying the score system proposed by the OS-MRS scale, 124 patients were classified as class A (62.6%); 70 as class B (35.4%) and 4 as class C (2%). The rate of complications was 7.3%, 20% and 50% respectively and there was a statistically significant association between the OS-MRS class and the rate of complications (P=.004) (Table 5). This same association was demonstrated on analysis of each surgical technique (P=.003 for LGB and P=.010 for LVG).
Distribution of Patients and Rate of Complications According to the OS-MRS Scale for the Series as a Whole and According to the Surgical Procedure Used.
| OS-MRS Class | n (%) | Complications | ||
| Total (n=198) | LGB (n=85) | LVG (n=113) | ||
| A | 124 (62.6) | 9 (7.3)* | 7/63 (11.1)* | 2/61 (3.3)* |
| B | 70 (35.4) | 14 (20.0) | 8/20 (40.6) | 6/50 (12) |
| C | 4 (2) | 2 (50.0) | 1/2 (50) | 1/2 (50) |
| Total | 198 (100) | 25 (100) | 16/85 (18.8) | 9/113 (8.0) |
LGB: laparoscopic gastric bypass; LVG: laparoscopic vertical gastrectomy.
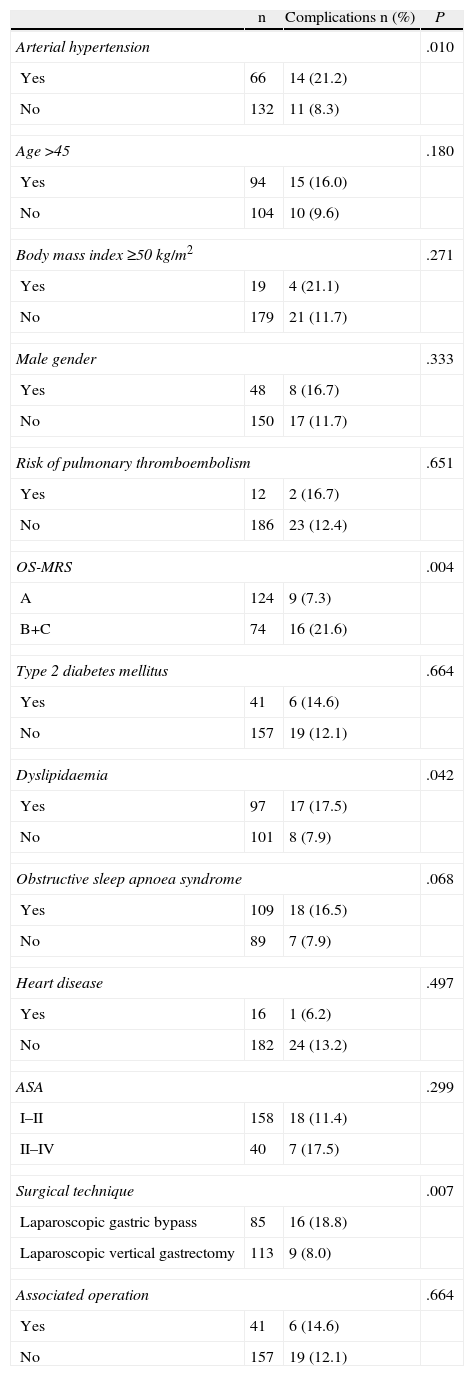
On individual analysis of each of the risk factors included in the study, it was observed that the presence of AHT (P=.010), the OS-MRS scale (P=.004), dyslipidaemia (P=.040) and an LGB having been performed (P=.007) reached statistically significant values with regard to the development of postoperative complications (Table 6). Conversely, age >45, male gender, BMI≥50kg/m2, risk of developing DVT, the presence of DM2, OSAS, heart disease, ASA class or the need for associated surgery were not associated with a greater risk of complications. In the multivariate analysis, only the OS-MRS scale (95% CI: 1.994–14.259; P=.0001) and the surgical procedure (95% CI: 1.994–14.259; P=.001) remained as factors of predictive value.
Analysis of the Association Between the Risk Factors Independently Included in the OS-MRS Classification and Development of Complications.
| n | Complications n (%) | P | |
| Arterial hypertension | .010 | ||
| Yes | 66 | 14 (21.2) | |
| No | 132 | 11 (8.3) | |
| Age>45 | .180 | ||
| Yes | 94 | 15 (16.0) | |
| No | 104 | 10 (9.6) | |
| Body mass index≥50kg/m2 | .271 | ||
| Yes | 19 | 4 (21.1) | |
| No | 179 | 21 (11.7) | |
| Male gender | .333 | ||
| Yes | 48 | 8 (16.7) | |
| No | 150 | 17 (11.7) | |
| Risk of pulmonary thromboembolism | .651 | ||
| Yes | 12 | 2 (16.7) | |
| No | 186 | 23 (12.4) | |
| OS-MRS | .004 | ||
| A | 124 | 9 (7.3) | |
| B+C | 74 | 16 (21.6) | |
| Type 2 diabetes mellitus | .664 | ||
| Yes | 41 | 6 (14.6) | |
| No | 157 | 19 (12.1) | |
| Dyslipidaemia | .042 | ||
| Yes | 97 | 17 (17.5) | |
| No | 101 | 8 (7.9) | |
| Obstructive sleep apnoea syndrome | .068 | ||
| Yes | 109 | 18 (16.5) | |
| No | 89 | 7 (7.9) | |
| Heart disease | .497 | ||
| Yes | 16 | 1 (6.2) | |
| No | 182 | 24 (13.2) | |
| ASA | .299 | ||
| I–II | 158 | 18 (11.4) | |
| II–IV | 40 | 7 (17.5) | |
| Surgical technique | .007 | ||
| Laparoscopic gastric bypass | 85 | 16 (18.8) | |
| Laparoscopic vertical gastrectomy | 113 | 9 (8.0) | |
| Associated operation | .664 | ||
| Yes | 41 | 6 (14.6) | |
| No | 157 | 19 (12.1) | |
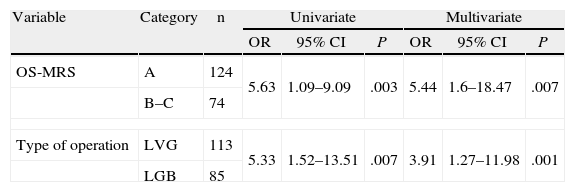
Therefore, and as the last stage of the study, a univariate binary logistic regression was used between these two factors (Table 7). When the patients classified as class A with those of classes B and C (grouped together because of the small sample size of class C) were compared; it was observed that the risk of presenting complications was multiplied by 5 (OR: 5.63 with 95% CI [1.09–9.09]) in patients of the B–C group. Analysing the type of bariatric surgery, if the patient had undergone an LGB the risk of presenting complications was also five times greater than if an LVG had been performed (OR: 5.33 with 95% CI [1.52–13.51]). When we analysed the influence of presenting the two factors simultaneously with a bivariate analysis, we observed that statistical significance persisted.
Univariate and Multivariate Analysis of Factors Associated With the Development of Complications in Bariatric Surgery.
| Variable | Category | n | Univariate | Multivariate | ||||
| OR | 95% CI | P | OR | 95% CI | P | |||
| OS-MRS | A | 124 | 5.63 | 1.09–9.09 | .003 | 5.44 | 1.6–18.47 | .007 |
| B–C | 74 | |||||||
| Type of operation | LVG | 113 | 5.33 | 1.52–13.51 | .007 | 3.91 | 1.27–11.98 | .001 |
| LGB | 85 | |||||||
CI: confidence interval; OR: odds ratio.
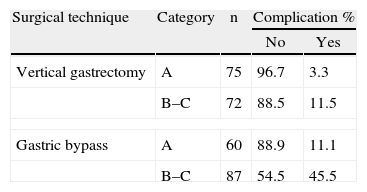
When these two variables are related with the rate of complications, three groups of patients are clearly defined (Table 8). The first group was with a very low complications rate, comprising the patients on whom an LVG was performed who belonged to class A. A second group was with intermediate morbidity, combining patients on whom an LVG was performed, classified as B and C and patients who had undergone an LGB and were included under class A. Finally, a group with a very high rate of morbidity was defined comprising those patients on whom an LGB was performed and who had been included in class C.
DiscussionOur work demonstrates that the OS-MRS scale is useful for estimating the risk of morbidity in patients requiring bariatric surgery and suggests that it could also be important in the selection of patients for either technique depending on the score obtained.
There have been several attempts made to obtain a method for predicting morbi-mortality after bariatric surgery. The first proposal was by DeMaria et al.3 in 2007, which was initially limited to estimating the mortality rate and was validated in this area by the author himself4 and others5,6 and then was extended to predicting morbidity in 2011 by Sarela et al.7 Other proposals have followed Flum et al.18 based on the results of the longitudinal assessment of bariatric surgery (LABS), of Blackstone et al.8 who propose the use of the metabolic acuity score, or those of Gupta et al.19 and Turner et al.9 developed from the National Surgical Quality Improvement Program (NSQIP) database of the American College of Surgeons. All of these have been constructed from very large populations and have demonstrated their capacity for predicting the possibility of developing a complication. However, defining or obtaining some of the variables included could either impede or limit their applicability in certain scenarios.
The great advantage of the OS-MRS scale is that it is a very simple tool, which uses five clinical variables that are very easy to achieve and which are very simple to apply and interpret. One disadvantage which could be attributed to it is that it was developed for the analysis of mortality and for gastric bypass3 in particular, although it was used later in analysing morbidity.7
No information can be extracted from our study to ratify this scale as a predictor of mortality since only one patient died in our series (a mortality rate of 5/1000) and did so 45 days after surgery after suffering an acute myocardial infarction in the immediate postoperative period. This patient did belong to group C of the OS-MRS scale, a group which a recent systematic revision that included 6 studies with 9382 patients attributed a rate of mortality of 4.3%.6 This same study confirms that the OS-MRS scale is useful, as significant differences were found in postoperative mortality between classes A, B and C (0.2%, 1.3% and 4.3%, respectively).6
Use of the OS-MRS scale to predict morbidity rate and applying it to patients who are to undergo surgical techniques other than gastric bypass or the laparoscopic approach has been much more recent and there is little data available in literature.7 In our study we have attempted to scrutinise the possible usefulness of this scale in two different procedures (gastric bypass and vertical gastrectomy) which are always performed laparoscopically. Similarly the role that other comorbidities associated with obesity might play which are not included on the OS-MRS scale, such as DM2, OSAS, heart disease and dyslipidaemia was also studied.
The published morbidity rate after bariatric surgery is very disparate, but recent studies place it at between 4% and 15%, depending on the type of procedure used and the characteristics of the patients.19–26 This great variability is determined by important differences in the follow-up and collection, definition and classification of complications. In our work, unlike others, the follow-up of the patients was exhaustive, clear definitions were used and, as far as we are aware, this is the first time that complications have been classified using a perfectly validated scale such as the Dindo–Clavien classification. Against this background, the morbidity rate was 12.5% (25 patients), but only four major complications were detected (Clavien>2) which means a 2% rate of serious complications. Our work confirms the findings of Sarela et al.7 in the sense that the OS-MRS scale is useful for defining the patients who are more likely to suffer complications and, more importantly, for providing patients with accurate information when obtaining informed consent.
From the individualised analysis of risk factors, of those included in the OS-MRS scale or others defined in the literature, only AHT, the OS-MRS scale itself, dyslipidaemia and surgical technique are related to the rate of complications in our study. Other authors have also identified AHT as a risk factor for complications,27,28 along with coagulation disturbances, ASA classification, diabetes and congestive heart failure.18,29,30 However, it is the first time that dyslipaemia appears as a risk factor for bariatric surgery complications.
From the multivariate analysis only the OS-MRS scale and surgical technique are clearly defined as predictive factors related to the rate of complications. In the first case and placing class B and C into a single group because there were so few patients in class C, the probability of presenting complications in this group was multiplied by 5. This data coincides with that obtained by Sarela et al.7 Therefore, with regard to surgical technique, the probability of suffering complications after LGB is also five times higher than after LVG. This finding is novel since, to date, no series have been studied which solely cover LGB and LVG – the techniques that are most used in bariatric surgery in our field.
An element which is of particular relevance in our work, and which is also without precedent, is that when a correlation between the OS-MRS scale and surgical technique was made with the probability of developing complications, three very well defined groups were identified. One of the groups, comprising patients classified as class B or C on the OS-MRS scale who had undergone an LGB, present an associated morbidity which appears unacceptable (45.5%), although a good many of these complications are minor.
A possible limitation of the study is that it might incorporate morbidity which could be attributed to our learning curve. Our study includes patients from 2008, when a clinical pathway was first introduced for obesity surgery in which the score system of the OS-MRS scale was included. But this point does not coincide with the start of the bariatric surgery programme launched in 2004. Since then until the start of the study motivating this work, 92 bariatric operations had been performed (68 LGB and 24 LVG). Although the number of estimated procedures in order to conclude a learning curve is subject to discussion, if we consider it to be 100, we could attribute some of our complications to the fact that the team was still in a learning curve period. In this regard, the fact that the 3 major complications which required surgical treatment (the fourth described in the text is an acute myocardial infarction) occupy positions 97, 106 and 136 of our series as a whole and positions 72 and 75 with regard to LGB and 47 with regard to LVG, could endorse this argument. Nevertheless, we believe that the results described are representative and reproducible for other teams.
In conclusion, the OS-MRS scale provides useful information on the probability of developing postoperative complications after bariatric surgery which, together with the surgical technique to be used, makes it possible, on the one hand, to provide the patient with appropriate information and, on the other hand, perhaps, to avoid certain procedures for selected populations.
Conflict of InterestsThe authors have no conflict of interests to declare.
To Sergi Mojal, Statistical Support Unit, Institut Hospital del Mar d’Investigacions Mèdiques (IMIM), for his collaboration with the statistical analysis.
Please cite this article as: Lorente L, Ramón JM, Vidal P, Goday A, Parri A, Lanzarini E, et al. Utilidad de la escala Obesity surgery mortality risk score en la predicción de complicaciones tras cirugía bariátrica por vía laparoscópica. Cir Esp. 2014;92:316–323.
The preliminary data from this study were presented at the Congreso Nacional de Cirug%3Fiacute;a, Madrid 2012 (National Surgery Conference in Madrid, 2012) and in the Congreso Nacional de la Sociedad Espa%3Fntilde;ola de Cirug%3Fiacute;a de la Obesidad, Madrid 2013 (National Conference of the Spanish Society of Obesity Surgery).