There is significant controversy in the management of cardiac cancer. It seems unanimous that Siewert type I tumors be operated on as cancer of the esophagus and Siewert type III as gastric cancer. However, for “true” cancer of the gastric cardia or Siewert II, the authors do not agree. There is the obvious need for free proximal and distal margins, as well as correct lymphadenectomy. For some, esophagectomy is necessary to perform correct radical oncological surgery, but other authors defend that an abdominal approach is sufficient to perform total gastrectomy and distal esophagectomy. Recent and older papers published do not clarify this issue, and their results are contradictory. Chemotherapy prior to surgery can reduce the size of the tumor and the presence of lymphadenopathies.
Existe una importante controversia en el manejo quirúrgico del cáncer de cardias. Parece unánime que los tumores tipo i de Siewert se intervengan como un cáncer de esófago y los Siewert III como un cáncer gástrico. Sin embargo, sobre el «verdadero» cáncer de cardias o Siewert II no existe consenso. Es obvia la necesidad de un margen proximal y distal libre, así como una correcta linfadenectomía. Para algunos es necesaria la esofaguectomía para realizar una correcta cirugía oncológica radical, pero otros autores defienden que es suficiente con un abordaje abdominal para realizar una gastrectomía total y esofaguectomía distal. Tanto los trabajos publicados con cierta antigüedad como aquellos más recientes no aclaran este dilema y sus resultados son contradictorios. El hecho de realizar un tratamiento quimioterápico previo a la cirugía, puede reducir el tamaño tumoral y la presencia de adenopatías, por lo que las opciones quirúrgicas pueden haber cambiado en los últimos años.
In Western countries (Europe and North America), the incidence of gastric cancer has been decreasing progressively in recent years,1 especially distally located classic adenocarcinoma (ADC). In contrast, ADC of the proximal stomach with invasion of the cardia is becoming more frequent, and simultaneously there has been increasing incidence of ADC of the cardia and distal esophagus.1–3 This is perhaps because these entities share the same main etiological causes: obesity and gastroesophageal reflux.
The first question that should be asked is: what is cardia cancer? According to the 8th edition of the TNM classification for malignant tumors,4 it is defined as ADC of the esophagogastric junction (EGJ) showing the center of the tumor within 2cm of the cardia, either distally or proximally. In this regard, the Siewert classification5 for EGJ cancer, based on the main location of the tumor, has helped to choose the best surgical treatment. In type I, with most of the tumor in the distal esophagus, the technique of choice would be esophagogastrectomy; in type III, where the tumor is mainly located in the proximal stomach, the technique would be total gastrectomy; and in type II or true cardia cancers, the best surgical option is still debated.
However, this classification has some disadvantages. The tumor is classified according to topographic criteria (endoscopic, radiological and intraoperative), but sometimes it is not easy to define where most of the tumor is located, especially when the tumor is large or there is a hiatal hernia. Another problem with the Siewert classification is that cell origin does not always coincide with tumor origin. In other words, the biopsy of an EGJ tumor may identify signet-ring cell ADC (therefore, it would be gastric in origin, which is more frequent in Asian countries and would be a Siewert III ADC); in contrast, if the biopsy identifies ADC on a Barrett's esophagus, it is logical to think of an esophageal origin, which is more frequent in Western countries, and a Siewert type I ADC.6 Thus, even in the same location, biologically they would be very different tumors.
Until a few years ago, in order to obtain complete resection of a cardia tumor with a wide proximal margin, excision of the distal esophagus (with or without thoracotomy) was necessary. Currently, with the increased use of chemotherapy7 or neoadjuvant radiochemotherapy,8 good responses are achieved with a significant reduction in tumor size; as a result, esophagectomy and thoracotomy can sometimes be avoided.
In any case, radical, potentially curative surgery requires complete resection with free margins (at least 5cm proximally and distally from the tumor, with a circumferential margin greater than 1mm) and a lymphadenectomy that includes at least 15 lymphadenopathies. The various surgical options should be compared in terms of radicality, morbidity and mortality, quality of life and long-term survival (global and disease-free).
This paper analyzes the scientific evidence of the literature on surgical options for cardia cancer, especially based on randomized studies, meta-analyses and reviews.
Siewert I Cancer of the CardiaSince most of the tumor is found on the esophageal side (1–5cm from the cardia), most authors9–11 propose partial esophagogastrectomy with intrathoracic anastomosis following the Ivor-Lewis technique. This approach allows for a sufficient proximal margin to be obtained during resection of the thoracic esophagus, as well as correct mediastinal lymphadenectomy. As an alternative, other authors propose performing transhiatal esophagectomy without thoracotomy and with cervical anastomosis. The disadvantage of this approach is that it is more difficult to correctly perform the mediastinal lymphadenectomy. In addition, if the tumor is voluminous, a wide gastric resection is sometimes necessary to obtain a correct distal margin, therefore the construction of a tubular gastric plasty can be too to be pulled up to the cervical esophagus without tension, in which case we should resort to reconstruction with a cervical coloplasty.12,13
The Dutch randomized study conducted by Hulscher et al. in 20029 compared transthoracic esophagectomy (TTE, n=114) with transhiatal esophagectomy (THE, n=106). Although postoperative mortality was similar in both groups (P=.45), TTE caused greater morbidity, especially pulmonary complications. The 5-year disease-free survival rate was significantly higher with the Ivor-Lewis technique (39% vs 27%), with no significant differences in the 5-year overall survival between the two techniques (39% in TTE vs 29% in THE) When the authors published their long-term results (more than 5 years10), the differences in overall survival did reach significant differences, being 14% higher in the thoracic approach than in the transhiatal approach (51% vs 37%).
However, Davies et al.11 have recently published a comparative cohort study on the transthoracic (n=401) and transhiatal (n=263) approaches in distal esophageal cancer (Siewert I). The authors report that they do not find differences in overall survival (HR 1.07) or recurrence (22.8% vs 24.4%) between the two techniques.
Siewert III Cancer of the CardiaConsidered a gastric cancer, tumors of this presentation are usually treated similarly, using total gastrectomy with excision of the abdominal esophagus and reconstruction by esophagojejunostomy. Signet ring cells are detected in many of these tumors, so tumor aggressiveness and survival prognosis are worse than in other cardia tumors.6,14
Siewert II Cancer of the CardiaThis is what some authors call ‘true’ cardia cancer.15 Given its location and possibilities for dissemination of this type of tumors, the surgical approach is highly debated. The objective of any cancer surgery should be based on 3 pillars: (1) radical R0 resection; (2) complete lymphadenectomy; and (3) low postoperative morbidity and mortality.
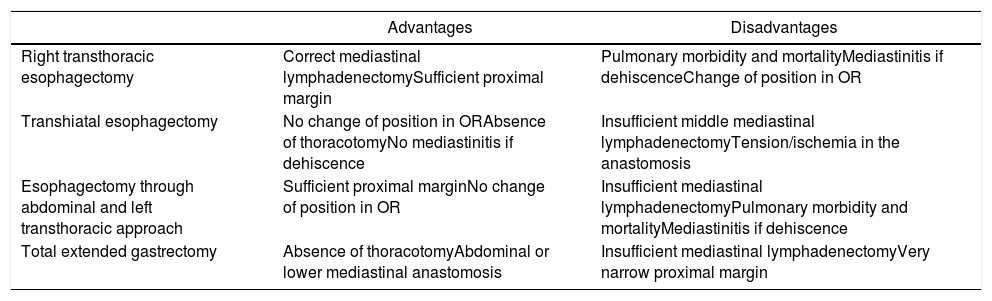
In order to achieve these objectives in these tumors, on the one hand it is argued whether esophagectomy is necessary or a total gastrectomy with an abdominal approach is sufficient. On the other hand, when esophageal resection is associated, the discussion focuses (as in Siewert I) on whether the transthoracic or transhiatal pathway should be chosen (Table 1).
Advantages and Disadvantages of the Different Surgical Approaches in Cancer of the Cardia.
| Advantages | Disadvantages | |
|---|---|---|
| Right transthoracic esophagectomy | Correct mediastinal lymphadenectomySufficient proximal margin | Pulmonary morbidity and mortalityMediastinitis if dehiscenceChange of position in OR |
| Transhiatal esophagectomy | No change of position in ORAbsence of thoracotomyNo mediastinitis if dehiscence | Insufficient middle mediastinal lymphadenectomyTension/ischemia in the anastomosis |
| Esophagectomy through abdominal and left transthoracic approach | Sufficient proximal marginNo change of position in OR | Insufficient mediastinal lymphadenectomyPulmonary morbidity and mortalityMediastinitis if dehiscence |
| Total extended gastrectomy | Absence of thoracotomyAbdominal or lower mediastinal anastomosis | Insufficient mediastinal lymphadenectomyVery narrow proximal margin |
In 2018, Blank et al.15 prospectively analyzed the postoperative and long-term results of 2 surgical techniques in Siewert II cardia cancer. The 242 patients were subjectively selected with no standardized criteria for a TTE group treated with the Ivor-Lewis technique (n=56; 23%) and abdominal/thoracic D2 lymphadenectomy. Reconstruction was done with tubular gastroplasty in 77% of cases. In the second group (n=186; 77%), only one laparotomy was performed for total extended gastrectomy (TEG) with distal esophagectomy as well as D2 abdominal and lower mediastinal lymphadenectomy. In all patients, the tract was reconstructed by Roux-en-Y esophagojejunostomy. Neoadjuvant chemotherapy treatment was administered in 50% of patients (similar in both groups); the other half initially went to surgery. Patients over 70 were normally selected for abdominal surgery, but there were no prior differences between the two groups regarding other characteristics (ASA, neoadjuvant therapy, TNM). The authors reported similar postoperative results with both techniques (TTE vs TEG) in terms of morbidity (57% vs 47%), anastomotic dehiscence (14% vs 12%), pulmonary complications (33% vs 28%), hospital mortality (5% vs 4%), R0 resections (84% vs 86%) and number of resected lymph nodes (24 vs 24). Most cases in the two groups were stages pT3 (46% vs 60%) and N1+(66% vs 71%). Regarding survival, the authors reported longer survival (43 months) in patients undergoing TTE than in patients with TEG (33 months, P=.02). Likewise, the 5-year survival was higher in the Ivor-Lewis group than in the TEG group (57% vs 40%, P=.02). In the multivariate study, the type of surgery was included as an independent prognostic factor (P=.005) with a hazard ratio of 2.5 in patients treated with an abdominal approach. Despite the age bias (older patients in the group did not have thoracotomy), the authors analyzed the subgroups of patients under 70, with similar results in favor of TTE. Nonetheless, the scientific evidence is based on a retrospective study, so the authors are developing a randomized prospective study with the same surgical techniques and objectives.
In another recent article, Martin et al.16 conducted a thorough analysis of 2 large national databases in the US: the American College of Surgeons-National Surgical Quality Project (ACS-NSQIP), and the Surveillance Epidemiology and End Results (SEER). The objective was to select patients with Siewert II cardia cancer who had undergone gastrectomy or esophagectomy. The short-term (morbidity and mortality) and long-term (overall survival) results were analyzed. Using ACS-NSQIP data in a matched cohort study, they compared postoperative results in a group of 214 patients undergoing total abdominal gastrectomy with another group of 967 patients undergoing esophageal resection. They did not find a higher percentage of complications in either group in terms of dehiscence (1.4% vs 1.6%), morbidity (33.2% vs 35%), pneumonia (13.1% vs 13%), 30-day mortality (3.7% vs 2.4%) or hospital stay (10 vs 10.5 days). In the multivariate mortality study, the surgical technique did not acquire statistical significance as a predictive factor (OR 0.54; P=.221). After the analysis of the SEER data on survival, the overall results showed greater survival in patients after esophagectomy (26 vs 21 months, P=.025). These data were considered biased due to the greater use of neoadjuvant radiotherapy in the former. After the multivariate study, the type of surgery was not statistically significant as an independent factor of overall survival (HR 0.95; P=.259). With these results, the authors concluded that the decision to perform esophagectomy or gastrectomy in patients with cardia cancer should be based on tumor extension, the use of oncological protocols and, above all, on the experience in both surgical techniques of each group.
Over the past 20 years, many studies comparing the 2 approaches have been published. The retrospective study by Siewert et al.,17 published in 2000 and based on 1000 operated patients with EGJ cancer, is already a classic. In the Siewert II subgroup, 271 patients were included who underwent surgery using 2 surgical techniques: TTE (n=48) or TEG (n=223). Radical R0 resection was the main independent prognostic factor in the multivariate study for long-term survival. When the surgery was complete (R0), the authors found no differences between the two techniques in terms of overall 5-year survival. In contrast, patients who underwent TTE had higher postoperative morbidity and mortality rates.
Another alternative to right thoracotomy-esophagectomy is the proposal by the Japan Clinical Oncology Group,18 which published in 2006 the results of a randomized study comparing total extended gastrectomy with abdominal esophagectomy (TEG, n=82) versus esophagogastrectomy using the left thoracoabdominal approach (n=85) in patients with Siewert II (n=95) and Siewert III (n=63) cardia cancer. The study was canceled after the initial interim analysis of the results due to the clear disadvantages of the thoracic approach, based on a higher percentage of pneumonia (13% vs 4%), weight loss and loss in vital capacity, with no clear improvement in 5-year survival. Even in the subsequent study of the results after 10 years of follow-up,19 the left thoracoabdominal approach showed similar 5-year (42% vs 50%, P=.496) and 10-year (37% vs 24%, P=.060) survival rates in patients with Siewert II. In these tumors, the authors recommend performing total gastrectomy by laparotomy, with resection of the abdominal esophagus and inferior mediastinal lymphadenectomy using the transhiatal route.
Transthoracic vs Transhiatal EsophagectomyIf we decide to perform esophagectomy, it could be transthoracic (TTE) or transhiatal (THE), as in Siewert I.
In 2007, results were published from the Dutch study mentioned above10 about these 2 surgical approaches after a minimum follow-up of 5 years. In the group of patients with EGJ Siewert II cancer (n=115), 52 patients were randomized for the transhiatal approach and 63 patients for the transthoracic route. None of the patients received neoadjuvant treatment. The authors reported no differences between the two surgical approaches in terms of 5-year survival (31% in THE and 27% in TTE).
However, a more recent meta-analysis20 reports different results. After studying 805 potential articles between 1996 and 2012 with keywords “cancer”, “esophagus” and “esophagogastric junction”, only 6 were finally selected (only 2 were prospective) with 647 patients diagnosed with Siewert I and II ADC of the cardia, 281 treated with TTE and 366 THE. When the short- and long-term results were compared, there were no differences between the two techniques in terms of postoperative mortality, pulmonary complications, anastomotic dehiscence, R1-R2 palliative resections or hospital stay. In contrast, the thoracic approach demonstrated a statistically significant greater number of resected lymph nodes (P=.001), higher 5-year overall survival (P=.03) and 5-year disease-free survival (P=.05). In short, the authors report that in distal esophageal and cardia tumors, TTE is oncologically superior to THE, with similar results in terms of postoperative morbidity and mortality.
Therefore, the results are currently still contradictory and both approaches are considered valid, depending on the characteristics of the patient and the experience of the surgeon.
LymphadenectomyAnother important point of debate between the abdominal and thoracic approaches is the quality and quantity of the lymph node dissection. Obviously, if only an abdominal approach is performed, the possibility of removing the mediastinal nodes is limited to the lower mediastinum through the hiatus. In contrast, with the thoracic approach, we can also perform middle and even upper mediastinal lymphadenectomy. Whether this influences survival or not is unclear, but it is currently known that type II cardia tumors, which mostly metastasize in the abdominal lymph nodes (71%), also frequently metastasize to the mediastinum (30%). Even in Siewert III (gastric origin), 9% of patients can develop tumor lymphadenopathies at this location.21,22
In 2015, Parry et al.23 published a comparative study between esophagectomy (n=155) and gastrectomy (n=21) in patients with Siewert II cardia cancer. The study did not show differences in 5-year overall survival (P=.606), disease-free survival (P=.251) or recurrence percentage (P=.669). Nevertheless, the authors found 11% of tumor lymphadenopathies in the upper mediastinum, for which the authors propose that TTE should be the treatment of choice in these tumors. Other authors24 agree with this fact, reporting 22% Siewert II patients with positive mediastinal lymphadenopathies (subcarinal, paratracheal and aortopulmonary) after TTE. Logically, these patients had a statistically lower survival rate (P=.009) than patients without these affected lymph nodes.
Japanese studies25,26 show less mediastinal involvement in these tumors. A multicenter retrospective study in 315 patients with Siewert II (pT2-4) cancer of the cardia reports tumor lymph node invasion of only 3.8% in the upper mediastinum and 7% in the middle mediastinum.
Quality of LifeOne aspect to highlight between the two approaches, abdominal and thoracic, is the quality of life. A recent German study27 has compared both procedures based on quality-of-life questionnaires previously validated by the European Organization for Research and Treatment of Cancer (EORTC), the QLQ C-30 and the specific cancer module, QLQ OG-25. The authors report a lower incidence of pulmonary symptoms (P<.05) and reflux (P<.05) in gastrectomized patients with Siewert II. In addition, other authors28,29 have also reported that, although they do not find differences in long-term survival between esophagectomy and gastrectomy, quality of life is more seriously compromised with esophageal resection.
Special Situations- 1.
Extension of the tumor along the gastric wall impedes construction of a gastric tube with the residual stomach. Total gastrectomy is necessary. For reconstruction, there are 2 options:
- A.)
Abdominal approach: distal esophagectomy and Roux-en-Y esophagojejunostomy
- B.)
Abdominal and right thoracic approach: thoracic esophagectomy and thoracic coloplasty
- C.)
Abdominal and left cervical approach: transhiatal esophagectomy and cervical coloplasty
- A.)
- 2.
The respiratory function of the patient rules out thoracotomy.
- A.)
Abdominal approach: total gastrectomy, distal esophagectomy and Roux-en-Y esophagojejunostomy
- B.)
Abdominal approach: proximal partial gastrectomy, distal esophagectomy+esophagogastrostomy
- C.)
Abdominal and left cervical approach: proximal gastrectomy, transhiatal esophagectomy and cervical gastroplasty
- A.)
- 3.
Early-stage ADC over Barrett's esophagus
In type II cancer of the cardia, the abdominal or thoracic surgical approaches do not entail significant differences in terms of long-term survival. It is clear that thoracotomy provides for a better mediastinal lymphadenectomy and a greater proximal margin, but this does not always result in a cure of the disease. Abdominal gastrectomy usually has fewer associated complications and better quality of life, so a priori it may be preferred. As seen throughout the chapter, treatment of these tumors continues to be controversial. But, lastly, surgical treatment should always be individualized and take into consideration the patient's surgical risk and the locoregional esophageal, gastric, mediastinal and abdominal extension of the tumor.
Conflict of InterestsThis article has received no funding, and the authors have no conflict of interests to declare.
Please cite this article as: Munitiz V, Ortiz A, Ruiz de Angulo D, Martinez de Haro LF, Parrilla P. Resultados de las diferentes opciones quirúrgicas para el tratamiento del cáncer de la unión esofagogástrica. Revisión de la evidencia. Cir Esp. 2019;97:445–450.