Early-stage (T1) esophagogastric junction cancer continues to represent 2%–3% of all cases. Adenocarcinoma is the most frequent and important type, the main risk factors for which are gastroesophageal reflux and Barrett's esophagus with dysplasia.
Patients with mucosal (T1a) or submucosal (T1b) involvement initially require a thorough digestive endoscopy, and narrow-band imaging can improve visualization. Endoscopic treatment of these lesions includes endoscopic mucosal resection, radiofrequency ablation and endoscopic submucosal dissection.
Accurate staging is necessary in order to provide optimal treatment. The most precise staging technique in these cases is endoscopic ultrasound.
The suspicion of deep invasion of the submucosa, presence of unfavorable anatomopathological characteristics or impossibility to perform endoscopic resection make it necessary to consider surgical resection.
Los estadios precoces (T1) del cáncer de la unión esofagogástrica continúan representando únicamente el 2-3% de todos ellos. El más frecuente es el adenocarcinoma y el principal factor de riesgo para su desarrollo son el reflujo esofagogástrico y el esófago de Barrett con displasia.
Los pacientes con afectación de mucosa (T1a) o de submucosa (T1b) precisan inicialmente de una endoscopia digestiva minuciosa, pudiendo mejorar la visualización con la cromoendoscopia. El tratamiento endoscópico de estas lesiones incluye la mucosectomía, la ablación con radiofrecuencia y la disección endoscópica de la submucosa.
El tratamiento óptimo precisa una correcta estadificación y la técnica más adecuada para ello es la ultrasonografía endoscópica.
Por otra parte, la sospecha de invasión profunda de la submucosa, la presencia de características anatomopatológicas poco favorables o la imposibilidad de resección endoscópica, obligan a optar por la resección quirúrgica para alcanzar un tratamiento curativo.
Esophagogastric junction cancer (EGJ) is defined as a malignant neoplasm located in the area 2cm above to 2cm below the EGJ. The incidence of this group of tumors has increased significantly in our setting, particularly in association with Barrett's esophagus (BE).1,2 Improved screening programs and a more informed population are responsible for an increase in the incidence of early stages, but these cases only represent 2%–3% of these tumors in our setting.3,4
In this article, we update the diagnosis and treatment of early cancer of the EGJ, with the aim to clarify the management criteria in these patients.
Histological AspectsFrom a pathological point of view, we can differentiate several histological types: adenocarcinoma in BE, adenocarcinoma not associated with BE, and other types of neoplasms, such as squamous carcinoma or neuroendocrine tumors. In this article, we will basically refer to adenocarcinoma.
BE is defined as an intestinal metaplasia that replaces the squamous epithelium of the distal esophagus.1,2 According to the Japanese classification of esophageal cancer, adenocarcinoma that develops in BE has the following differential characteristics: presence of esophageal glands, existence of islands of squamous epithelium within lesions and duplication of the muscularis mucosae below the lesions.5,6 However, the fact that these criteria are not always present can make it difficult to differentiate between adenocarcinoma in BE and without BE. Consequently, both types are included together in most studies on adenocarcinoma of the EGJ.7
EpidemiologyThe main risk factors for the development of adenocarcinoma of the EGJ are gastroesophageal reflux and the presence of BE associated with dysplasia.8 Other related factors are: increased body mass index, high-fat diet, male sex and tobacco use.9 The progression of adenocarcinoma in patients with low-grade dysplasia is 0.12% per year, which increases to 6% per year in those with high-grade dysplasia.10 For this reason, this progression from intestinal metaplasia to dysplasia and adenocarcinoma necessitates the design and application of rigorous screening programs, involving periodic endoscopic monitoring for early diagnosis and the application of endoscopic techniques to improve survival.
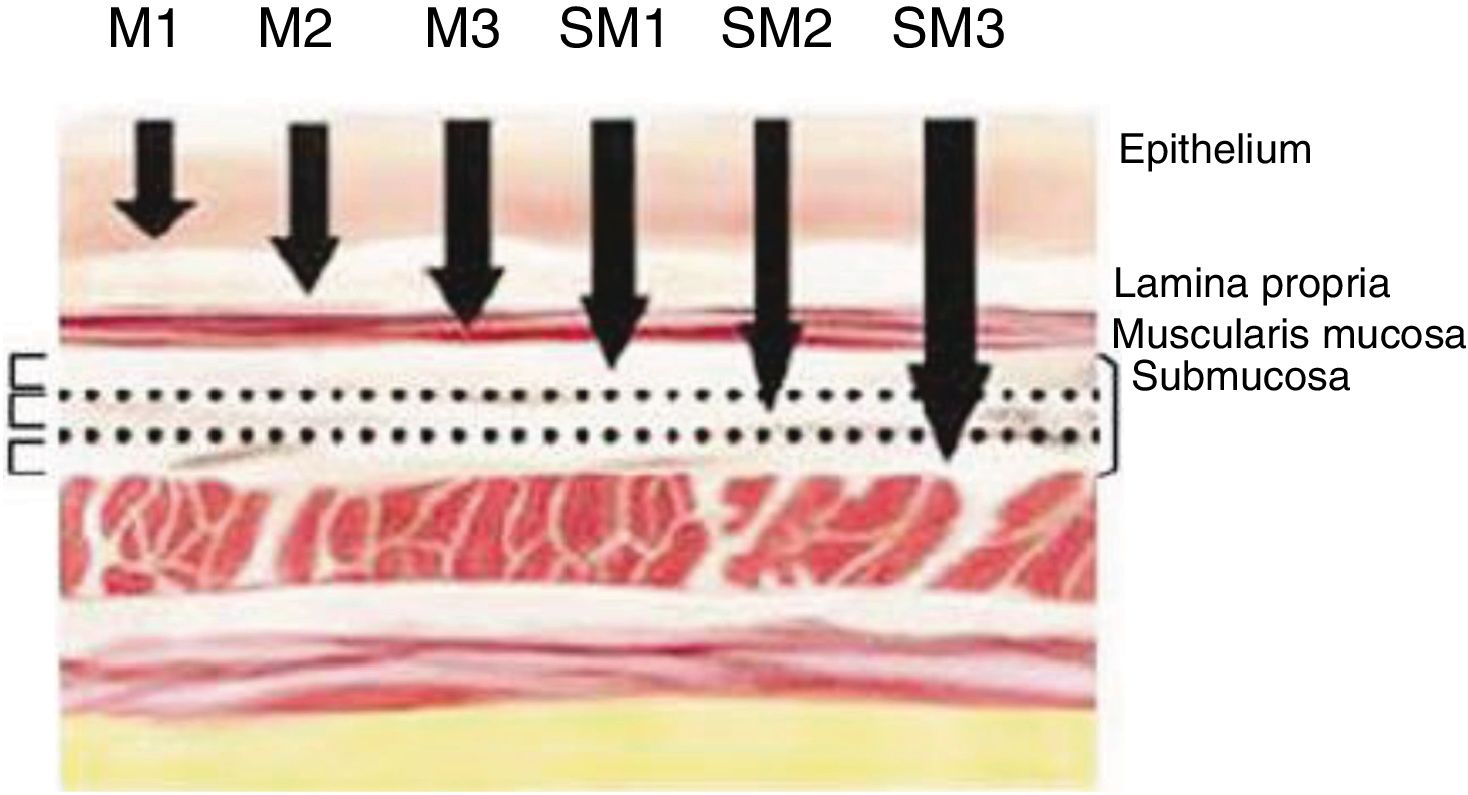
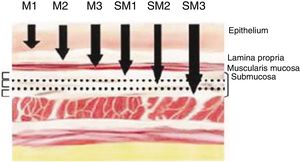
Definition of Early Cancer; TNM ClassificationEarly cancers are defined as tumors that invade the mucosa or submucosa, regardless of lymph node involvement. These lesions are category T1 of the TNM classification – 8th Edition.11 Invasion of the mucosa layer is classified as type T1a, and of the submucosa as T1b. Likewise, tumors that affect the mucosa can be subdivided into m1, m2 and m3. M1 are intraepithelial tumors (carcinomas in situ), m2 invade the lamina propia of the mucosa and m3 invade the muscular layer of the mucosa. On the other hand, tumors that affect the submucosa (T1b) are classified as sm1 (invasion of the upper third of the submucosa, <500μ of invasion), sm2 (invasion of the middle third of the submucosa) and sm3 (invasion of the lower third of the submucosa) (Fig. 1).
DiagnosisPatients with early EGJ cancer do not present specific symptoms. Therefore, the impact of early diagnosis on patients with BE is essential, thereby improving survival and optimizing the national healthcare system's economic resources.
Early esophageal cancers present as superficial erythematous plaques, nodules or ulcerations, and a thorough digestive endoscopy with white light is the initial study used for diagnosis.
Regarding BE, this entity has a characteristic appearance of salmon-colored mucosa with circumferential involvement or digital projections, which contrasts with the more whitish appearance of the normal esophageal mucosa.
Detailed Endoscopic ExaminationSystematic endoscopic inspection should be performed, including an active search for elevations, ulcerations and nodules or small irregularities of the mucosa, both during insufflation and during aspiration maneuvers.
In patients with BE, this should be measured according to the Prague classification, which evaluates the circumferential and longitudinal extent of the visualized segment.12 Special attention should be paid to the area between 12 and 6 o’clock (clockwise), where most neoplastic lesions are found.
In patients with BE and high-grade dysplasia (HGD) it is recommended to follow the Seattle biopsy protocol,13 obtaining targeted biopsies of all visible anomalies as well as randomly in the four quadrants every 1cm from the top of the gastric folds to the most proximal extension of the BE (squamocolumnar junction). Some 80%–90% of diagnoses are made with targeted biopsies,14 and random biopsies will be required for the diagnosis of up to 20% of non-visible lesions.
Chromoendoscopy (NBI)Narrow-band imaging (NBI) chromoendoscopy is a high-resolution endoscopic technique that improves the visualization of the mucosa surface without the use of dyes. It is based on the depth of light penetration according to wavelength, providing better visualization of mucosal and surface vascularization patterns. Hence, electronic chromoendoscopy with NBI is able to detect lesions compatible with early dysplasia or neoplasm by analyzing glandular and vascular patterns.15
In a meta-analysis that included 446 patients and 2194 lesions, Buskens et al.16 showed that the combined sensitivity and specificity of NBI to detect BE mucosa were 95% and 65%, respectively. Likewise, the sensitivity and specificity for the detection of high-grade dysplasia were 96% and 94%. These findings suggest that the NBI is useful for the detection of mucosa with BE and HGD15 and has become a tool to target biopsies in areas with suspicious surface morphology. Furthermore, the NBI has the advantage of being able to alternate with the standard vision under white light, without requiring the use of conventional chromoendoscopy dyes.
More recently, another electronic chromoendoscopy technique has been reported, Blue Light Imaging, which allows us to improve the detection of early adenocarcinoma in BE.17 Likewise, chromoendoscopy using dyes like indigo carmine or acetic acid can also sometimes be useful to detect such lesions.
Staging and Patient SelectionThe possibility of lymph node involvement is one of the determining factors for the selection of optimal treatment in this type of tumors, so adequate tumor staging is necessary.
The risk of lymph node involvement increases with deep invasion16 and varies according to the histological type. In well-differentiated sm1 tumors it is less than 3% and reaches more than 20% when the lesion is classified as sm218 (Fig. 2). This deep invasion and its possible lymph node involvement will determine the need for an endoscopic or surgical approach to achieve radical oncological treatment.
Endoscopic ultrasound (EUS) is the most accurate technique for locoregional staging of esophageal cancer. It is able to differentiate between T1 (mucosa/submucosa involvement) and T2 (invasion of the muscular layer) with great precision.
EUS has a negative predictive value greater than 95% for the absence of tumor invasion in the deepest wall layers and local lymph nodes.
Endoscopic mucosal resection (EMR) plays a primary role, not only as a treatment for early cancer but also as a staging procedure,12,19–21 since the histopathological evaluation of the resected sample is able to evaluate the depth of infiltration.
This approach is consistent with the guidelines of the American Society of Gastrointestinal Endoscopy (ASGE 2013), which recommend EMR for the treatment and staging of nodular BE and the suspicion of early adenocarcinoma of the EGJ.22 If the endoscopic appearance of the lesion does not create suspicion of deep submucosal invasion, the tumor can be removed by EMR.
Although CT and PET/CT are necessary diagnostic tests to complete the staging of EGJ cancer, in patients with HGD or early cancer with no signs of deep submucosal invasion or suspicious lymph nodes found on EUS23 they are of less importance given the low risk of distant metastasis.
Endoscopic TreatmentEndoscopic treatment of premalignant lesions and early EGJ tumors is increasingly widespread and includes mucosectomy (or EMR), radiofrequency ablation (RFA) and endoscopic submucosal dissection (ESD) (preferably in Asian countries and not so widespread in our environment).
Patients who meet the following criteria will be candidates for these techniques:
- -
Limited mucosa or superficial submucosa involvement (sm1)
- -
No vascular or lymphatic involvement
- -
No lymph node involvement
- -
If there is underlying BE, its complete eradication is necessary.
With these criteria,24 patients with the lowest risk of lymph node dissemination can be selected, with an incidence of lymph node involvement of 1.3%. Patients with deeper layer involvement are candidates for surgical treatment.
Unlike other gastrointestinal locations, the degree of tumor differentiation has not been identified as an independent risk factor for lymph node metastasis or tumor recurrence.16 This finding may be related to the fact that most undifferentiated tumors have already invaded the submucosa at the time of diagnosis. However, there are limited data evaluating the relevance of histopathological differentiation, especially given the small number of early undifferentiated lesions in the studies available.
Endoscopic Resection of the Mucosa or MucosectomyThis is the most frequently used technique in our setting in the treatment of early cancer of the EGJ that meets the aforementioned criteria.
It consists of the creation of a pseudopolyp that encompasses the lesion and its subsequent resection. There are 2 types:
- -
‘inject-and-cut’ EMR (EMRc): the lesion is lifted by injecting a dye (usually indigo carmine) into the submucosa, followed by traction and resection with a diathermy loop that is incorporated in the cap.25
- -
EMR with bands (EMRb): suction of the lesion into the endoscopic cap and placement of an elastic band for subsequent resection with a diathermy loop. Unlike those previously mentioned, this technique is faster and less expensive as it does not require the injection of substances.
Both techniques are effective and safe, with a low rate of complications. Although EMRc enables larger specimens to be obtained, there are no differences in the depth of the pieces obtained between the two techniques.19 One of the most relevant studies evaluating the efficacy of EMR in patients with early esophageal adenocarcinoma included 1000 patients who were followed up for an average of 56.6 months.26 Complete remission was achieved in 96% of patients. Recurrent or metachronous lesions developed in 140 patients (15%), 115 (82%) of which were successfully treated endoscopically. Overall, the long-term complete remission rate is around 94%.26
Endoscopic Dissection of the SubmucosaThis is used in the management of lesions larger than 2cm, and ulcerated lesions, with higher en bloc resection rates than EMR. However, ESD is more complex and requires more experience.27 Its learning curve is longer, as is the completion time of the technique, and it entails a higher incidence of perforations (4.5% vs 1%).28
It was initially developed for the treatment of early gastric cancer, and its use was subsequently extended to other locations of the digestive tract.27,29–31
The technique involves marking the limits of the lesion with electrocoagulation and submucosal injection of a saline solution containing epinephrine and methylene blue or indigo carmine. A circumferential incision is made in the mucosa and the submucosal layer is dissected until complete excision of the lesion.
The location in the EGJ is a major technical challenge, even in expert groups. There is a higher rate of complications, as well as a longer duration of the procedure and a lower proportion of successful resections compared to other locations of the gastrointestinal tract.
ESD is a safe technique for the treatment of early EGJ cancer. The majority of the results come from the Japanese literature, with reported complete resection rates of 87%, curative resection of 75%7 and a 5-year survival rate comparable to that obtained with surgery (93.9% vs 97.3%), with reduction of complications.32
The main complications described after the performance of ESD include hemorrhage (3.4%), perforation (2.5%) and stenosis (6.9%),7 most of which can be controlled endoscopically.
However, the guidelines of the European Society of Gastrointestinal Endoscopy recommend EMR compared to ESD in most cases of early EGJ cancer in BE.24 This is because EMR has shown an incidence of complete cancer remission comparable to ESD with a lower risk of complications.27
Histopathological evaluation of the resected tissue will define the tumor size, state of the lateral and deep margins, presence of ulceration, degree of differentiation, and vascular and lymphatic invasion. In those specimens with positive deep margins, deep submucosal involvement or lymphovascular invasion, surgery to achieve curative treatment is recommended.
On the other hand, we must consider lesions classified as Siewert III as well as lesions whose origin is between 2 and 5cm below the cardia. These tumors are treated as gastric and, according to the guidelines of the NCCN,33 endoscopic treatment (EMR or ESD) is recommended in early gastric cancer for lesions less than or equal to 2cm in diameter, when histologically they are either well or moderately differentiated tumors, do not penetrate beyond the submucosa (SM2), have no lymphovascular invasion, and negative lateral and deep resection margins are obtained. While in these cases endoscopy is the treatment of choice, the guidelines do not specify under what circumstances the different techniques should be indicated.
Endoscopic Ablation Techniques: Radiofrequency AblationEndoscopic ablation techniques destroy the tissue by thermal damage either in the form of heat (RFA) or cold (cryotherapy). We will refer to the RFA, as it is the most effective and most frequently used in our setting.
RFA is an endoscopic treatment method that destroys the esophageal mucosa by means of thermal damage generated by radiofrequency. It is able to eradicate BE, low-grade dysplasia and early EGJ cancer in BE.34 In the case of dysplasia or early cancer in BE, eradication of BE is necessary to prevent the development of metachronous neoplasms that may occur in 15% of cases with incomplete eradication of BE. RFA treats the entire BE segment in one session, including the treatment of larger areas, so the combined EMR + RFA treatment offers a lower recurrence rate than isolated EMR and fewer complications.35
There are 2 esophageal RFA methods:
- -
Circumferential RFA34: uses a balloon with bipolar electrodes on the surface, whose activation causes the release of energy (12J/cm2) and burning of the target Barrett esophagus. Its main indication is the treatment of circumferential BE with a diameter greater than or equal to 3cm. Subsequently, after removing the sloughed mucosa, a second session on the BE is repeated.
After 2 months, the regeneration of the esophageal epithelium is reviewed, which should be scaly, and if there is persistence of BE another RFA session (usually focal) is performed. The average number of sessions required to achieve complete ablation is usually 2 or 3.36
- -
Focal RFA: uses a bipolar electrode mounted on the distal end of the endoscope on an articulated platform (Fig. 2). The device rests on the target tissue and energy is released.37 It is used in the circular treatment of the Z line and in cases of BE with small islets or tongues, with a circumferential extension of less than 2cm or in the residual BE after circumferential ablation.
RFA should be performed after EMR when the BE is associated with nodular lesions containing HGD and/or intramucosal carcinoma.38 In BE with flat HGD, the RFA is performed directly since the eradication of dysplastic BE prevents the development of cancer. It is important to perform RFA on a flat mucosa with no nodules to ensure that the RFA reaches the muscularis mucosae.
Studies suggest that this ablation technique is highly effective in eliminating Barrett's mucosa and the dysplasia associated with time that minimizes the disadvantages of photodynamic therapy and argon plasma coagulation (esophageal stenosis, subsquamous foci of BE). In a meta-analysis that included 3802 patients, Phoa et al.39 found a complete dysplasia eradication rate of 91% per year. The eradication rate of BE stands at 85%–90% after 4–5 years. The recurrence rate ranges from 13% to 33% and is more frequent in the Z-line and the distal esophagus,40 so periodic checks are required.
Regarding the possible adverse effects, stenosis develops in 5% of patients, followed by chest pain in 3% and hemorrhage in 1%.41
After endoscopic treatment (EMR, ESD or RFA), patients should be treated with full-dose proton pump inhibitors to promote mucosa healing. The treatment should be continued for 4 to 6 weeks, depending on the size of the mucosa lesion, since it is the period of mucosa regeneration.42
Likewise, given the risk of recurrence, patients treated endoscopically require regular endoscopic monitoring. In most studies, patients are evaluated endoscopically every three months for the first year, after which time the follow-up studies are done anually.43
Surgical Treatment: When and How?As previously mentioned, deep tumor invasion of the esophageal wall increases the risk of lymph node involvement. The risk is low when tumor involvement is limited to the mucosa, but this risk increases when there is invasion of the submucosa, reaching 20% in sm2 tumors.44 For this reason, in the presence of suspected submucosal neoplastic invasion or the presence of pathological factors with a poor prognosis, the therapeutic guidelines33 indicate radical oncological surgery as a curative treatment. To this end, the correct localization of the lesion will be crucial since this can determine the surgical procedure to follow. In other words, for tumors identified as Siewert category III, which are considered subcardial neoplasms that invade the esophagogastric junction, treatment with gastrectomy is established. This is unlike Siewert I and II tumors, for which esophagectomy is proposed.
Thus, after initial endoscopic resection, surgery should be considered in the presence of the following findings:
- •
Vascular or lymphatic infiltration
- •
Poorly differentiated tumor (Grade≥3)
- •
Infiltration of the submucosa≥500μm
- •
Presence of residual tumor in the resection margin (R1)
- •
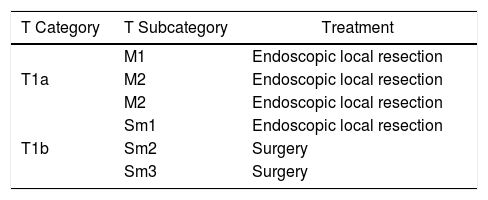
Endoscopic resection is technically impossible (Table 1, treatment algorithm)
Another indication for surgical treatment after EMR/ESD is the management of complications after this technique. As mentioned in the previous section, the complication rates after local endoscopic resection are low, reported at somewhere between 3.5% and 5.2% in the series with the highest number of cases.45 Surgical management is reserved for cases that have not been able to be treated by endoscopic techniques or in cases of large perforation, diffuse peritonitis or hemodynamic instability.
ConclusionGastrointestinal endoscopy is one of the pillars of the diagnostic and therapeutic management of early EGJ cancer. New imaging techniques may provide a better diagnosis, which can be difficult with conventional endoscopy. The combination of endoscopic resection and ablation techniques has achieved a high cure rate, with a good safety profile.
On the other hand, the suspicion of deep invasion of the submucosa, presence of unfavorable pathological characteristics or inability to perform endoscopic resection require surgeons to opt for surgical resection to achieve curative treatment.
Lastly, a multidisciplinary approach with the participation of oncologists, endoscopists, surgeons, radiologists and pathologists is decisive to determine the best therapeutic strategy for each patient. The decision-making process should contemplate factors such as comorbidities and patients should be involved, especially in borderline cases.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Junquera F, Fernández-Ananín S, Balagué C. Opciones terapéuticas en el tratamiento del cáncer precoz de la unión esofagogástrica. Cir Esp. 2019;97:438–444.