Brown recluse spider (Loxosceles reclusa) bites are uncommon in our setting, but it is important to consider the possibility of these lesions in the differential diagnosis of cellulitis because they can lead to fatal consequences. We present a case of avascular necrosis of a lower extremity secondary to a brown recluse spider bite in Spain.
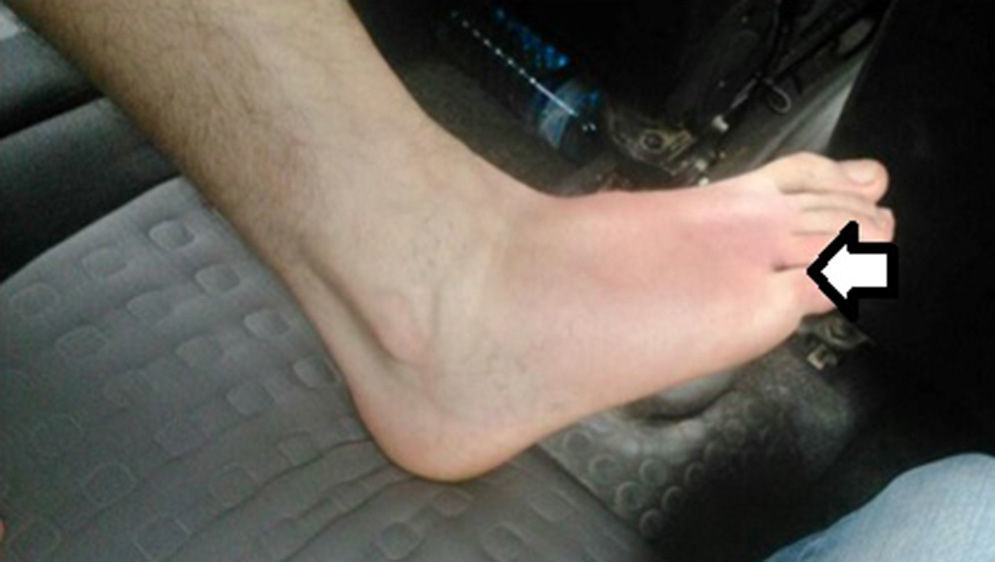
The patient is a 43-year-old male with no prior history of interest who came to our emergency room due to symptoms of pain in the right foot with Celsus signs after an arachnid bite within the previous 48h. Associated symptoms included fever, nausea, vomiting, arthralgia, headache and dark urine. Physical examination confirmed fever of 38°C, choluria, and normal remaining vitals. There was a papular lesion in the interdigital space between the 4th and 5th toes of the right foot associated with incipient cellulitis on the dorsal side of the foot (Fig. 1). Lab workup showed: 29720 leukocytes/μl (85% polymorphonuclear); PCR 14.7; lactate 1.1; CPK 138; urea 32; creatinine 0.98; other parameters were normal. Chest radiograph was normal. Doppler ultrasound of the lower extremities was normal.
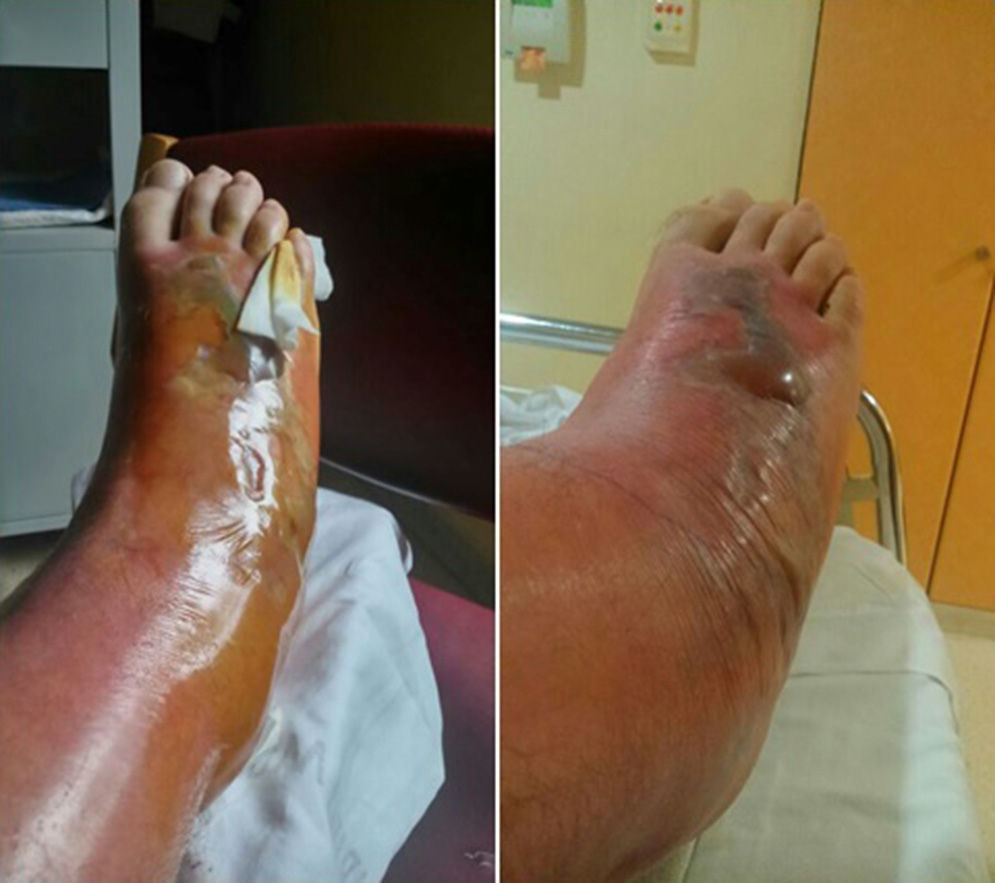
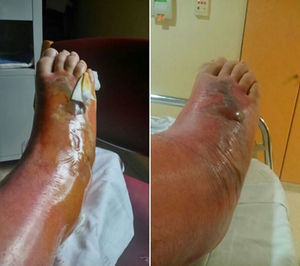
The patient was hospitalised for treatment with antibiotic therapy (amoxicillin–clavulanate) and local treatment (elevated limb, local cooling, compression). After 24h, the cellulitis progressed along the anterior side and completely surrounded the leg by the 4th day. Afterwards, a large blister appeared, measuring 15cm×5cm and ncompassing the dorsal side of the foot, along with necrosis of the skin and subcutaneous cell tissue, accompanied by lymphangitis (Fig. 2). Vancomycin was initiated to widen the spectrum of coverage, which led to analytical and clinical improvement that was slow but favourable; the cellulitis progressively diminished and the patient was discharged on the 12th day. In later follow-up office visits, the patient has been asymptomatic, with maintained strength and mobility.
Brown recluse spiders (L. reclusa) are poisonous spiders also known as “corner spiders” or “brown fiddlers” because they are photophobic and their head has the shape of a violin. Although this spider is originally from the United States,1,2 it seems to have become adapted to our environment, specifically in the region of Seville, where several cases of bites have been reported3 and where our patient had been working.
Symptoms range from asymptomatic patients, local signs around the bite area, to systemic repercussion with kidney failure.1,2 Pain presents within the first few hours and progressively increases in intensity, with associated pruritus and erythema. 24h after the bite, a dusky, erythematous, ring-shaped area appears around the bite,4 which becomes an ischaemic ulcer within 48–72h,2 as occurred in our patient. These ischaemic ulcers can cover a large area and there have been cases of their reaching 30cm in diameter; this situation usually requires surgical debridement and, occasionally, skin grafts.5 The severity of the cutaneous lesions, however, does not correlate with the development of systemic toxicity.
The anaphylactic condition caused by the poison is known as loxoscelism, which can cause systemic failure if not controlled (14% of cases).6 The most frequent systemic symptoms are: shivering, pruritus, general malaise, fever and nausea, accompanied by leukocytosis and occasionally haemolysis.7 Less frequent are: jaundice, kidney failure, convulsions, haematuria, disseminated intravascular coagulation, septic shock and, rarely, death, which is more frequent in paediatric patients.7
Diagnosis is purely clinical and based on the combination of signs, symptoms and proper patient anamnesis, as there is no laboratory test to identify the condition.
Treatment of the wound involves local cleansing, maintained limb elevation, analgesia, antihistamines, tetanus vaccine if not correctly vaccinated, and antibiotic treatment.1 The use of corticosteroids is controversial: while there are groups that indicate it, others believe that it can favour the progression or development of necrosis. In case of systemic toxicity, they have been used to prevent kidney failure8 and haemolysis. In our case, we did not use corticosteroids due to the patient's clinical stability.9–11 Surgical treatment of necrotic ulcers is done in the stable phase, without cellulitis, and never in initial stages because early surgical debridement of necrotic ulcers can be associated with their worsened condition and functional limitation of the affected extremity.12
Other local measures include: the use of hyperbaric oxygen therapy,9,13–15 nitroglycerin patches16 and negative pressure therapy,5 because these have been seen to reduce the size of the necrotic ulcers while increasing the formation of collagen and fibroblasts in the ulcer.
As for the treatment of systemic symptoms, close patient monitoring is recommended in an intensive care unit with scheduled analyses due to the risk for thrombocytopenia, haemolysis, leukocytosis and haemoglobinuria. If any of these conditions developed, intensive volume repletion would be indicated to avoid kidney failure. Molecular biology treatments are still in experimental phase,17 but we cannot rule out that an antidote for L. reclusa may be obtained in the future.
We conclude that brown recluse spider bites should be suspected in a patient with cellulitis after a bite. Anamnesis is of vital importance for diagnosis, as loxoscelism can be fatal as there is no specific treatment.
Conflict of InterestsWe declare that there was no conflict of interests.
Please cite this article as: Guillén-Paredes MP, Martínez-Fernández J, Morales-González Á, Pardo-García JL. Necrosis séptica de miembro inferior secundaria a picadura de araña reclusa parda. Cir Esp. 2016;94:e13–e15.