Surgery is the only potentially curative treatment for pancreatic adenocarcinoma. However, only 15%–20% of patients are apt for surgical treatment at the time of diagnosis. The results depend on several factors, including tumor size, lymphatic and/or vascular involvement, extrapancreatic disease and involvement of resection margins.
Vascular invasion, which is commonly found during the diagnosis of tumors in the head and body of the pancreas, is currently an indication for neoadjuvant treatment.1 Afterwards, surgery with vascular resection may also be indicated to obtain macroscopically negative margins.2
Occasionally, vascular reconstruction cannot be performed by primary anastomosis, which requires the use of a prosthetic or autologous graft. For autologous grafts, the left renal or jugular veins are the most suitable options available for the reconstruction of the mesenteric-portal axis, as they have a similar diameter.3 Likewise, the falciform ligament4 and peritoneum are suitable for partial venous resections, and the posterior sheath of the anterior rectus muscle of the abdomen is useful as a tubular graft after complete circumferential venous resections, where a long venous segment must be replaced.5 However, synthetic prostheses made of polytetrafluoroethylene (PTFE) and Dacron are the most commonly used today, with minimal risk of liver necrosis or graft infection.6–8
We present two clinical cases in which resection of the splenomesenteric-portal venous confluence and pancreatic neoplasm was performed, followed by a venous reconstruction using an autologous tubular graft of the peritoneum and posterior sheath of the rectus abdominis muscle.
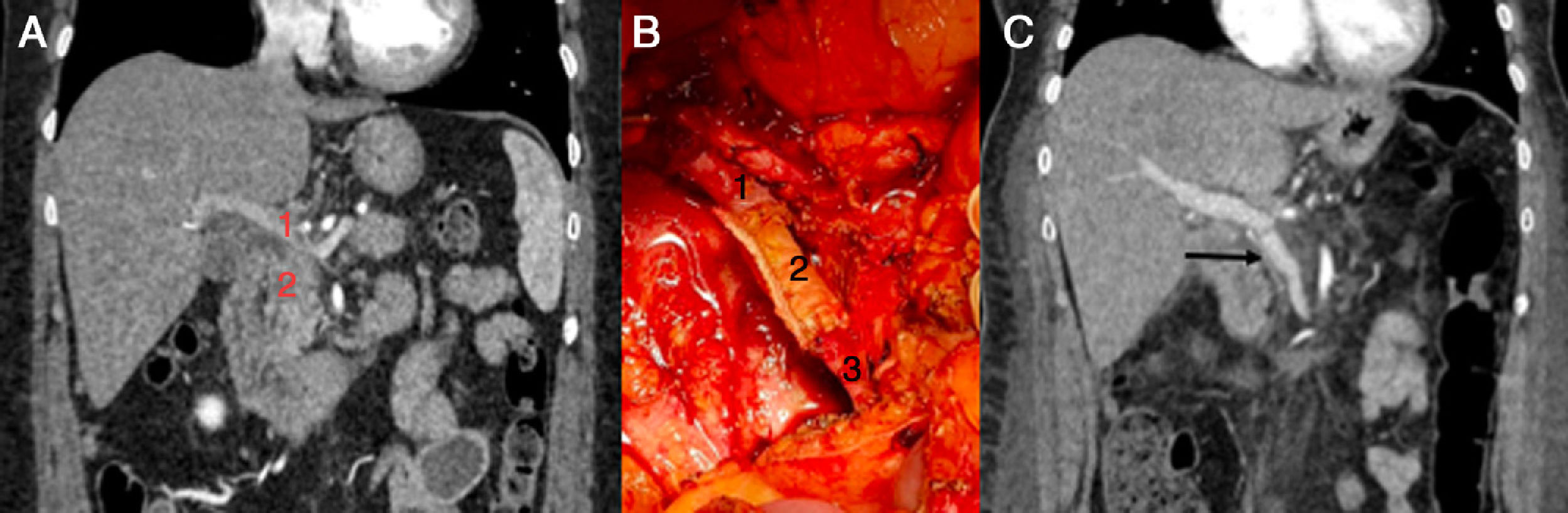
Case 1. (Fig. 1) A 51-year-old female patient diagnosed with a locally advanced head of the pancreas cancer by abdominal computed tomography (CT), measuring about 25×25mm, with invasion of the spleno-portal confluence and intrapancreatic bile duct. Endoscopic ultrasound was performed for a histological study, which was compatible with adenocarcinoma.
Initially, neoadjuvant treatment was performed by chemoradiotherapy. Follow-up CT scan to monitor the response showed that the disease remained stable, so surgical rescue was indicated. This was initiated with a pancreaticoduodenectomy. Then, as it was impossible to identify the splenic vein at its mesenteric-portal confluence due to tumor involvement, we decided to perform total pancreatectomy with splenectomy and complete circumferential vascular resection of the splenomesenteric-portal confluence. The pathology results reported a moderately-differentiated adenocarcinoma with discontinuous invasion of the entire pancreas, duodenal wall invasion, perineural invasion and focal involvement of the vascular margin; none of the 15 isolated lymph nodes were affected; pT3pN0.
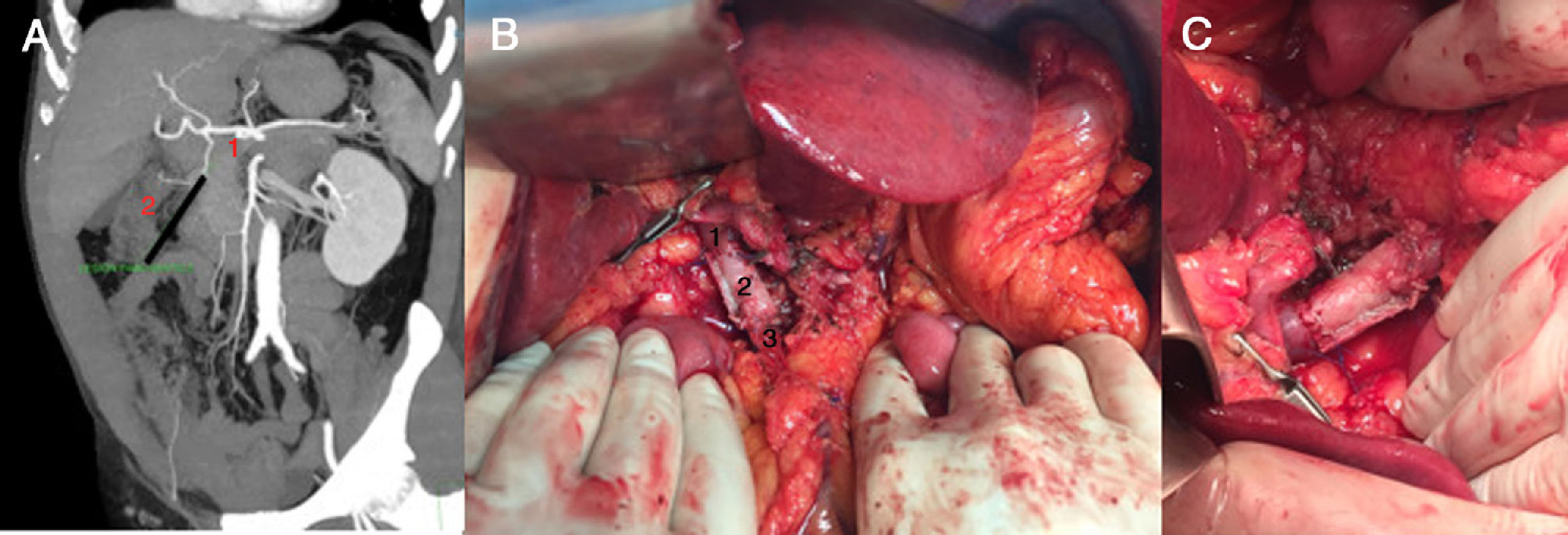
Case 2. (Fig. 2) Following an episode of acute prostatitis, a 63-year-old male patient was diagnosed with pancreatic cancer, which was initially identified by ultrasound as a hypodense image, defined mL, 18×14mm. The study was completed with a CT angiogram and pancreatic magnetic resonance imaging, which reported a cystic tumor in the head of the pancreas, causing pancreatic duct ectasia in the body and tail, with multiple lymphadenopathies. The lesion was in close contact with the first part of the duodenum, with no other observed lesions suggestive of metastasis. After assessment by the multidisciplinary committee, the patient was considered a candidate for surgery with curative intent. During the operation, a locally advanced mass was observed with a lymphadenopathy conglomerate that invaded the portal vein and splenomesenteric confluence. Given these findings, we decided to perform total pancreatectomy with cholecystectomy, splenectomy and portal resection. The definitive pathology results identified a moderately differentiated intestinal adenocarcinoma, with extramural venous invasion and extensive perineural permeation; pT3N1.
Images of Case 2: A) abdominal CT angiography prior to surgery; coronal view in which the pancreatic lesion is observed: mesenteric-portal confluence (1), pancreatic tumor (2); B) vascular graft already positioned before unclamping: portal vein (1), tubular graft of abdominal rectum (2), superior mesenteric vein (3); C) final graft.
In both cases, vascular reconstruction was carried out with en bloc resection of the peritoneum and posterior sheath of the rectus abdominis muscle, in accordance with the technique described by Elias et al.9 The peritoneal side was marked so that it remained oriented towards the inside of the graft, then the graft was introduced in a 2.5% glutaraldehyde solution for 5min to solidify the piece, followed by rinsing in saline twice for 2min. This procedure facilitates handling and reduces the risk of collapse once the graft is in place. To make the tubular graft, it was placed around a rectal probe (CH 32) of a diameter similar to that of the vessel to be reconstructed, to be then sutured laterally with a linear stapler and a vascular cartridge. Placement was performed using two continuous semicircular sutures of Prolene 4/0 and growth factor on the vessel wall, both at the proximal and distal ends.
Both patients presented correct postoperative recovery and were discharged with only low-molecular-weight heparin at prophylactic doses (which they had also received during hospitalization) during the first postoperative month (subcutaneous enoxaparin, 40mg every 24h). In both cases, a follow-up CT was performed one month after surgery, which showed correct graft function.
Autologous tubular graft of the peritoneum and posterior sheath of the abdominal rectus muscle is a feasible, safe and accessible alternative in cases requiring resection and vascular reconstruction in pancreatic surgery. This is especially true in unplanned cases, as in Case 2, in which the vascular involvement was not evident in the preoperative study and was an intraoperative finding.
The advantages of the technique are that it is available, is easy to obtain without the need to sacrifice an autologous jugular or renal vein, has a low risk of infection, requires no long-term anticoagulant treatment and is low in cost.
Please cite this article as: Galofré Recasens M, Sentí Farrarons S, García Domingo MI, Espin Álvarez F, Cugat Andorrà E. Injerto autólogo de vaina posterior de músculo recto del abdomen para la reconstrucción vascular del confluente espleno-mesentérico-portal en cirugía pancreática. Cir Esp. 2020;98:52–54.