Lately there has been an increasing interest in identifying quality standards in different pathologies, among them colon cancer due to its great prevalence. The main goal of this study is to define the quality standards of colon cancer surgery based on a large prospective national study dataset.
MethodsData from the prospective national study ANACO were used. This study included a consecutive series of patients operated on for colon cancer in 52 Spanish hospitals (2011–2012). Centers with less than 30 patients were excluded. The present analysis finally included 42 centers (2975 patients). Based on the results obtained in 4 main indicators from each hospital (anastomotic leak, lymph-nodes found in the specimen, mortality and length of stay), a nomogram that allows the evaluation of the performance of each center was designed. Standard results for further 5 intraoperative and 5 postoperative quality indicators were also reported.
ResultsMedian of anastomotic leak and mortality rate was 8.5% (25th–75th percentiles 6.1%–12.4%) and 2.5% (25th–75th percentiles 0.6%–4.7%), respectively. Median number of nodes found in the surgical specimen was 15.1 (25th–75th percentiles 18–14 nodes). Median length of postoperative stay was 7.7 days (25th–75th percentiles 6.9–9.2 days).
Based on these data, a nomogram for hospital audit was created.
ConclusionsStandard surgical results after colon cancer surgery were defined, creating a tool for auto-evaluation and allowing each center to identify areas for improvement in the surgical treatment of colon cancer.
Existe un gran interés en los últimos años en identificar estándares de calidad en las distintas enfermedades, entre ellas, el cáncer de colon debido a su alta prevalencia. El objetivo del presente estudio es definir unos valores estándar de calidad en los resultados de la cirugía del cáncer de colon.
MétodosSe han utilizado los datos del estudio prospectivo multicéntrico nacional «ANACO», que incluye pacientes con cáncer de colon intervenidos en 52 hospitales españoles (2011-2012). Para el presente análisis se han excluido los centros con menos de 30 pacientes y han quedado finalmente 42 hospitales (2.975 pacientes). Se presentan los valores de 4 indicadores de calidad principales a partir de los cuales se ha creado un nomograma que permite definir unos resultados estándar de la cirugía del cáncer de colon. Además se proporcionan los resultados estándares de otros 10 indicadores de calidad secundarios (5 intraoperatorios y 5 postoperatorios).
ResultadosLa mediana de fuga anastomótica y de mortalidad de los 42 hospitales fue de 8,5% (percentiles 25-75: 6,1-12,4%) y de 2,5% (percentiles 25-75: 0,6-4,7%), respectivamente.
La mediana de ganglios aislados fue de 15,1 (percentiles 25-75: 18-14 ganglios). La mediana de estancia hospitalaria postoperatoria fue de 7,7 días (percentiles 25-75: 6,9-9,2 días).
Basándonos en estos resultados se ha construido un nomograma para la autoevaluación de los distintos hospitales.
ConclusionesEl presente análisis ha permitido definir unos resultados quirúrgicos estándar tras la resección del cáncer de colon y se ha creado un instrumento de autoevaluación para las distintas unidades, de tal forma que cada centro puede identificar posibles áreas de mejora en el tratamiento de esta enfermedad.
Colorectal cancer is diagnosed in more than one million people per year worldwide, and it is the most frequently diagnosed cancer in Spain when both sexes are considered.1,2 Surgery is the only curative treatment for colorectal cancer, although there has been great variability in the outcomes reported by different surgeons and hospitals. Increased knowledge and standardization of treatment, coupled with the prevalence of this disease, have led to greater interest in monitoring results.
There has recently been more research activity to identify quality indicators for different diseases. These indicators make it possible to measure the results of therapy for different diseases at different hospitals, including colon cancer, as reflected in recent publications.3–5 However, once the quality indicators for each disease have been identified, it is necessary to define specific standard values for these indicators, based on everyday clinical practice.
The aim of the present study is to define the standard surgical outcomes following oncologic resection of colon cancer, based on data from a large national multicenter prospective study. In addition, to facilitate the self-assessment of each unit, we intend to create a nomogram based on the main outcome variables.
MethodsData for the present analysis were used from the ANACO study, which is a prospective, observational, multicenter national study, whose main objective was to identify risk factors for anastomotic leakage after bowel resection for cancer.6
The inclusion of the different participating hospitals was voluntary, and there was no individual or institutional financial compensation for the study participants.
A total of 58 hospitals initiated the study, 6 of which were excluded because they did not include the patients consecutively, so the final total was 52 participating hospitals, representing 26.6% of the tertiary hospital beds in Spain. For the present analysis, another 10 hospitals were excluded because they included less than 30 patients (the results would be unreliable due to wide confidence intervals).
The study included patients with colon cancer (located more than 15cm from the anal verge, measured with rigid rectoscopy) treated with scheduled or emergency surgery, primary anastomosis without a protective stoma and local curative intent. Patients were included consecutively from a one-year period: September 2011 to September 2012.
Exclusion criteria were: patients younger than 18 years of age, local R2 resection, patients included in other clinical trials, and patients who lacked relevant basic information.
The data were entered prospectively by the different researchers through a web page created for this purpose and an online registry with personalized password access for each researcher.
Random data inclusion quality controls were conducted at the participating hospitals during the study period. Forty-two pre- and intra-operative variables were collected, in addition to the postoperative results during the first 60 days after surgery and pathology data.
For the present analysis, based on the literature,7–15 the following variables were considered quality indicators for colon cancer surgery:
- 1.
Main quality indicators: percentage of anastomotic leakage, postoperative mortality, postoperative hospital stay and number of lymph nodes isolated in the resected specimen.
- 2.
Secondary quality indicators:
Intraoperative variables: percentage of perioperative transfusion, surgeries initiated by laparoscopy, conversion to open surgery, surgeries completed by laparoscopy, intraoperative complications.
Postoperative and pathological variables: percentage of R0 resections, postoperative complications, surgical wound infection, reoperations and mortality due to anastomotic leakage (failure to rescue).7
Anastomotic leakage was considered during the first 60 days post-op. The term “anastomotic leakage” was used according to the definition proposed by The United Kingdom Surgical Infection Study Group in 1991, which is the escape of luminal contents from the surgical union between 2 hollow organs.16 The diagnosis of anastomotic leakage was done: (1) radiologically, by computed tomography with a water soluble contrast enema and presence of intra-abdominal collection adjacent to the anastomosis; (2) clinically, with evidence of extravasation of the luminal content or gas through the wound or drain; (3) endoscopically; or (4) intraoperatively. No diagnostic tests were performed in asymptomatic patients to rule out anastomotic leakage.
The follow-up of postoperative complications in the first 60 days after the surgical intervention was carried out with periodic visits in the surgery outpatient consultation. The presence of complications was defined by previously established criteria for each complication.
The “intraoperative complication” variable was defined as any unexpected intraoperative event requiring a deviation from standard surgical technique.
The study protocol was approved by the Ethics Committees of the participating hospitals, and patients signed informed consent forms.
Statistical AnalysisIn the statistical analysis, each hospital was considered an individual unit, regardless of the number of patients included. For each hospital, we have calculated the rate of each of the categorical outcome variables and the median of the continuous variables (number of nodes and postoperative stay).
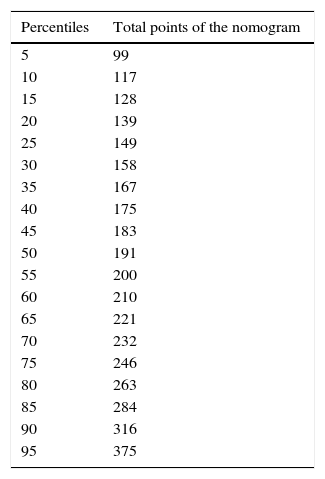
For each variable, percentiles were estimated with the Harrell-Davis technique17 represented in nomograms. For the self-evaluation nomogram, we used the results of the quality indicators that had previously been defined as “main”.
To analyze the correlation between the outcome variables expressed in percentiles and hospital characteristics, the non-parametric Mann–Whitney U test (qualitative characteristics) and a beta regression model (number of beds) were used.
For the statistical analysis, IBM® SPSS® Statistics version 21 and R (version 3.3.1) software were used. A P<0.05 was considered statistically significant.
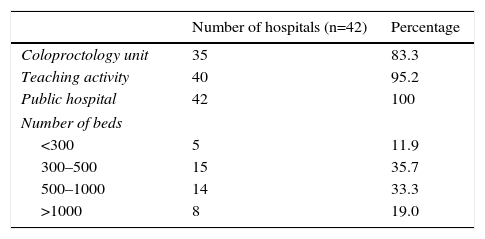
ResultsIncluded in the analysis were 42 hospitals, with a total of 2975 patients. Out of the total, 35 hospitals (83.3%) had a specific coloproctology unit, 40 (95.2%) were teaching centers and all 42 belonged to the Spanish national healthcare system. As for the number of beds per institution, the majority of the hospitals included had between 300 and 500 beds (35.7%) or between 500 and 1000 beds (33.3%). The median number of patients included per hospital was 62, ranging from 30 to 153 patients (Table 1).
When we analyzed the main quality indicators, we observed that the median rate of anastomotic leaks in the 42 hospitals was 8.5%, with the 25th and 75th percentiles situated at 6.1% and 12.4%, respectively. The mean number of lymph nodes extracted with each surgical specimen was 15.1 (range 7–36), with the 25th and 75th percentiles situated at 18 and 14 lymph nodes, respectively; the total was more than 12 at 90% of the hospitals. Median 60-day mortality was 2.5%, with the 25th and 75th percentiles at 0.6% and 4.7%, respectively. Lastly, median postoperative hospital stay was 7.7 days, with a range between 5 and 11.5 days (25th and 75th percentiles: 6.9 and 9.2 days).
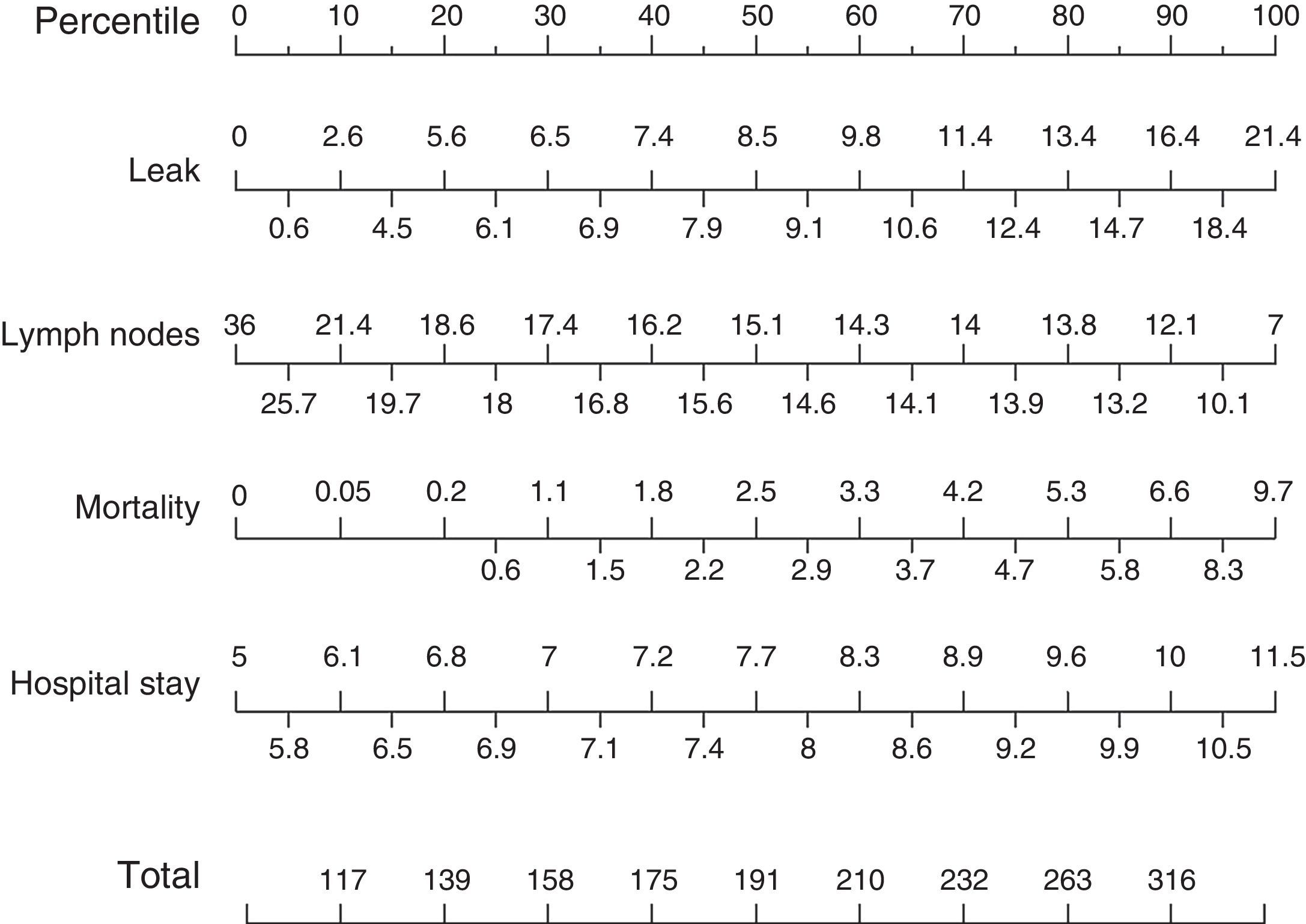
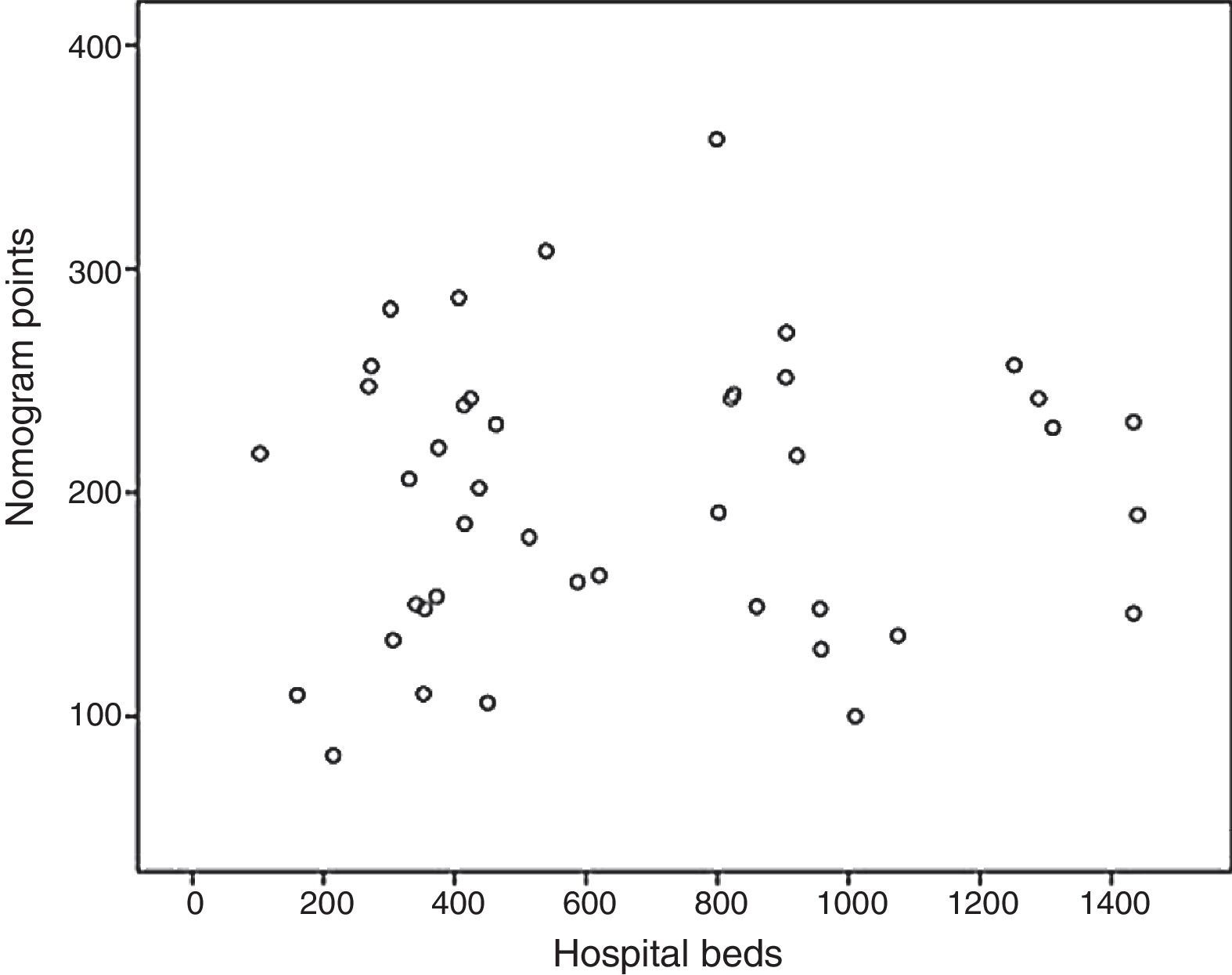
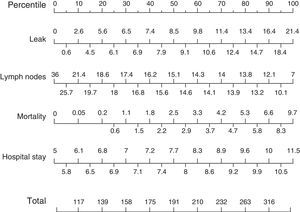
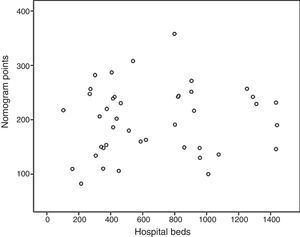
Based on these results a nomogram was constructed (Fig. 1). Each unit, aware of their own results, can obtain a score for each of the variables (the score is expressed on the upper bar of the nomogram, corresponding with the percentile). The overall result is calculated for each unit by adding up the scores of the 4 variables, which allows for comparisons with the results of the other hospitals included in the analysis (lower bar of the nomogram). Table 2 provides more details about the global results of the nomogram, expressed in percentiles. The quality of the surgical results obtained (overall score on the nomogram) was not associated with the characteristics of the unit (minimum P=0.16) or with the number of beds in the hospital (P=0.56) (Fig. 2).
Nomogram of standard colon cancer surgery results; values are expressed as rate or number of lymph nodes. To calculate the quality of the results in a surgical unit, we first calculate the points of each of the 4 main variables using the percentile bar (upper line) and, by adding the 4 values, we obtain an overall score that we will compare with the standard results (lower line).
Nomogram Results: Overall Results for Colon Cancer Treatment Obtained From the Nomogram, Expressed in Percentiles; the Lower the Percentile, the Better the Results.
| Percentiles | Total points of the nomogram |
|---|---|
| 5 | 99 |
| 10 | 117 |
| 15 | 128 |
| 20 | 139 |
| 25 | 149 |
| 30 | 158 |
| 35 | 167 |
| 40 | 175 |
| 45 | 183 |
| 50 | 191 |
| 55 | 200 |
| 60 | 210 |
| 65 | 221 |
| 70 | 232 |
| 75 | 246 |
| 80 | 263 |
| 85 | 284 |
| 90 | 316 |
| 95 | 375 |
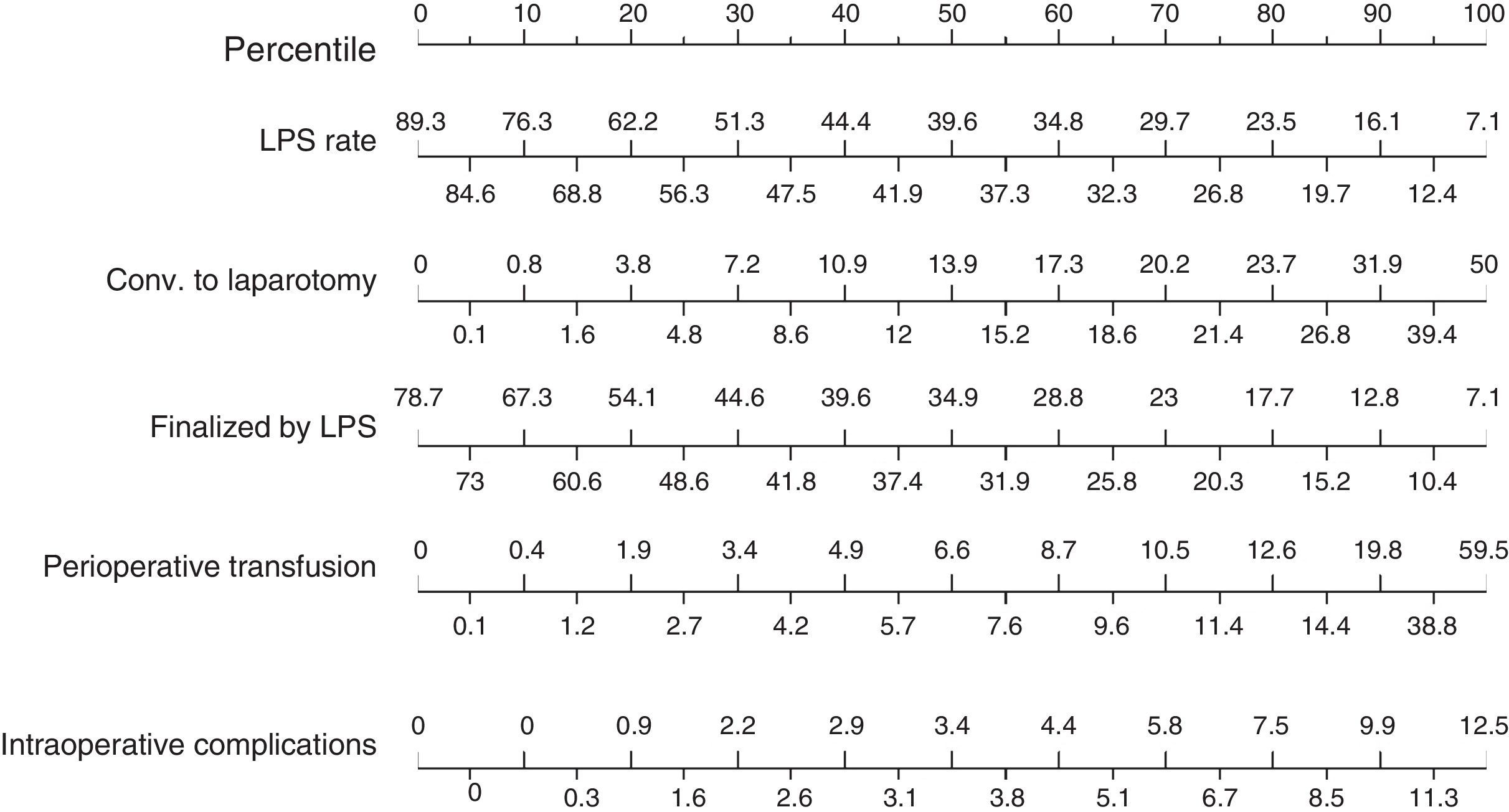
As for the results of the secondary intraoperative variables (Fig. 3), the rate of surgeries initiated laparoscopically presented a median of 39.6% (25th and 75th percentiles: 56.2% and 27.1%), with a conversion rate to laparotomy (50th percentile) of 13.9% (median rate of surgeries completed by laparoscopy: 34.9%). The perioperative transfusion rate was 6.6%, and the median intraoperative complication rate was 3.4%.
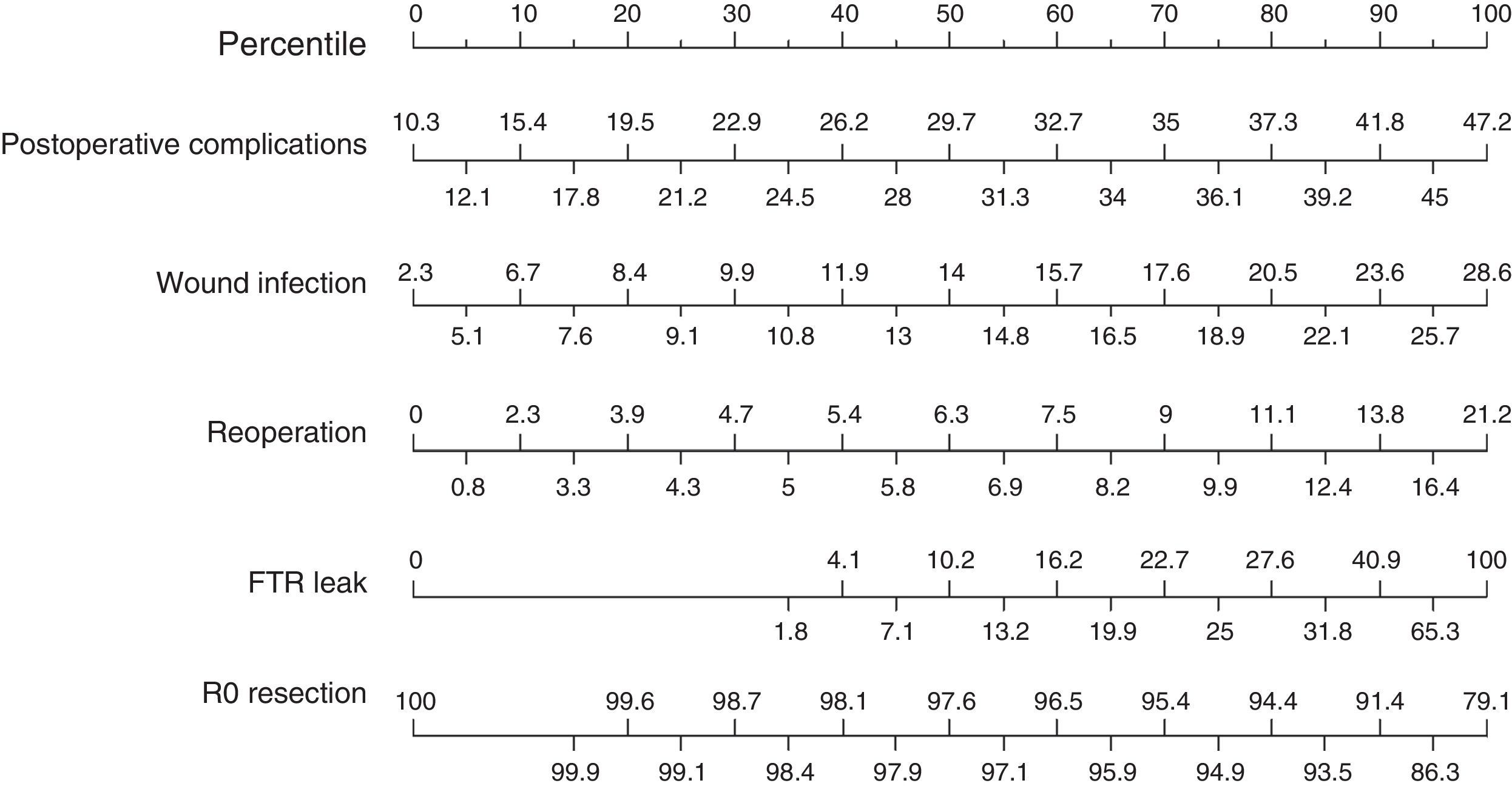
Finally, we analyzed the results of the secondary postoperative variables (Fig. 4) and obtained a median rate of postoperative complications of 29.7%, with the 25th and 75th percentiles situated at 21.2% and 36.1%, respectively. Mean surgical wound infection rate was 14%, and the reoperation rate was 6.3%. Failure to rescue after anastomotic leakage, which expresses the mortality rate due to anastomotic leakage, was 10.2%. Finally, the percentage of R0 resections presented a median of 97.6%.
DiscussionWe can define “quality of care” as the degree to which medical services achieve the expected health outcomes in individuals and in the population, using existing scientific knowledge.18 To measure this quality, several indicators have emerged, which have been defined in multiple ways. Basically, they are quantitative measurements that determine the quality of medical care and can be used to evaluate, monitor and improve the quality of patient and population healthcare, as well as other services involved that influence outcomes.19
There are several publications that have proposed different variables as indicators of quality care in colorectal surgery. Gagliardi et al. have identified 15 indicators obtained from bibliographic reviews that have already been validated by individual institutions.20,21 Ludt et al. have also validated 52 quality indicators for the treatment of colorectal cancer.12 In the United States, several agencies have identified quality indicators for the treatment of colorectal cancer, including the American Society of Clinical Oncologist Recommendations,9 the National Comprehensive Cancer Network11 and the American Society of Colon and Rectal Surgeons.14 After a review of the existing literature, the National Cancer Institute established 92 quality indicators for the treatment of colorectal cancer.8 More than a decade ago, the Asociación Española de Cirujanos (Spanish Association of Surgeons) took a step further and developed a project that sought to establish standard values in the management of colorectal cancer. In spite of its novel approach, the study sample was relatively small (417 patients) and intermixed patients who had undergone surgery for both colon and rectal cancer.15
In the present study, we have defined a series of quality indicators in colon cancer resection. Based on the results of the hospitals included, we have calculated quality standards for each of the selected indicators, with the subsequent construction of a nomogram using them.
The anastomotic leakage rate in our study was 8.5%, which is higher than previous publications, whose reported figures range from 3%22 to 7.5%.23 This is probably because our study presents a less restrictive definition of anastomotic leakage, together with a longer follow-up time (60 days). The postoperative mortality rate in the first 60 days after surgery was 2.5%, which is lower than reports from previous studies.23 It should be mentioned that more than 90% of the hospitals included in the study obtained more than 12 lymph nodes in the surgical specimen, a limit established as an quality indicator for correct oncologic resection.
When we compared the current surgical results presented in this study with those from a few years ago at the same national level, there has been a clear evolution in the treatment of colon cancer. Previously, hospital stays were more than 16 days (doubling current figures), surgical wound infection rates were almost 20% (compared to the current 14%), and fewer lymph nodes were isolated in the surgical specimen.15
There is great variability in morbidity and mortality rates after colon cancer surgery between different surgery units. Nonetheless, these differences may be due to diverse factors unrelated to the quality of the surgical treatment. Hence, the concept of “failure to rescue” has emerged,7,24 which is defined as the mortality rate of patients who present postoperative complications. It is an important quality indicator when evaluating the management of this group of high-risk patients. In the present study, “failure to rescue” related to anastomotic leakage was 10.2%, which is lower than previous reports (11.1%–16.8%).25
After the identification of quality indicators and the implementation of programs based on them, a significant improvement in the treatment of colorectal cancer has been demonstrated, both in results and costs.26,27 The advantage of quality indicators is that they can be quantified, which enables us to measure the quality of the medical care we are providing the population. The problem that arises is that, in order to evaluate the quality of care of a particular surgical service or unit, it is necessary to have references or quality standards with which to compare. Hence the interest of the present analysis, where we present limits for quality care in colon cancer surgery based on the results from a large number of Spanish public hospitals. This provides for comparison among units in order to identify areas of potential improvement. It also enables the evolution of the results to be assessed over time and evaluates the effectiveness of different measures implemented in the treatment of colon cancer.
To our knowledge, the present study is the first that tries to define specific standard results after oncologic resection for colon cancer. It should be noted that the results obtained are based on the most extensive prospective study of colon cancer published to date.
A limitation of this study is that the results presented have been obtained in the national setting of Spain, and it would therefore be necessary to validate them in other countries.
In conclusion, the present analysis defines standard surgical results after resection of colon cancer in Spain and creates a self-assessment instrument for surgical units, so that each center can identify potential areas of improvement in the treatment of this disease.
FundingThis study was supported by the Asociación Española de Cirujanos (Spanish Association of Surgeons) and sponsored by Takeda, with no interference in data collection, analysis or conclusions of the study.
Authorship/CollaboratorsContributions of each of the authors:
- -
Study design: JS, MF, BF-L, JLR, EGG.
- -
Data collection: MF, JLR, MR, AM, MS, JV, UN, EGG, ANACO Study Group.
- -
Analysis and interpretation of the results: JS, MF, DH.
- -
Composition of the article: JS, MF.
- -
Critical review and approval of the final version: JS, MF, DH, BF-L, JLR, MR, AM, MS, JV, UN, EGG.
The authors have no conflicts of interest to declare.
The following collaborators of the ANACO Project (Spanish study on ANAstomotic leak after COlon resection for cancer) have participated in this present study: Miguel Ángel Álvarez Rico, Hospital Universitario de Burgos, Burgos; María Jesús García Brao, Complejo Hospitalario Universitario de A Coruña, La Coruña; Juan Manuel Sánchez González, Hospital Universitario Nuestra Señora de Candelaria, Tenerife; Mariela Braithwaite, Complejo Hospitalario Universitario Insular Gran Canaria, Las Palmas de Gran Canaria; Eva Martí Martínez, Hospital Dr. Peset, Valencia; José Antonio Álvarez Pérez, Hospital Universitario Central de Asturias, Oviedo; Alejandro Espí, Hospital Clínico Universitario de Valencia, Valencia; Marta Trallero Anoro, Hospital Universitario y Politécnico La Fe, Valencia; Mónica Millán Scheiding, Hospital Universitario Bellvitge, Barcelona; Olga Maseda Díaz, Hospital Universitario Lucus Augusti, Lugo; Paula Dujovne Lindenbaum, Hospital Universitario Fundación Alcorcón, Alcorcón; Andrés Monzón Abad, Hospital Universitario Miguel Servet, Zaragoza; Isabel Blesa Sierra, Complejo Hospitalario Torrecárdenas, Almería; Francesc Feliú Villaró, Hospital Universitario Joan XXIII, Tarragona; Javier Aguiló Lucía, Hospital Lluis Alcanyis, Játiva; José Bargallo Berzosa, Hospital de Terrassa, Terrassa; Natalia Alonso Hernández, Hospital Universitario Son Espases, Palma de Mallorca; Francisco Javier Labrador Vallverdú, Hospital Universitario de Guadalajara, Guadalajara; Pedro Antonio Parra Baños, Hospital General Universitario Reina Sofía, Murcia; Guillermo Ais Conde, Hospital General de Segovia, Segovia; Antonio Codina Cazador, Hospital Universitario Josep Trueta, Gerona; Juan Hernandis Villalba, Hospital General de Elda, Elda; Carlos Álvarez Laso, Hospital de Cabueñes, Gijón; Sonia Martínez Alcaide, Hospital de La Ribera, Alcira; María Nieves Cáceres Alvarado, Hospital do Meixoeiro, Vigo; Ignacio Rey Simó, Complejo Hospitalario Universitario de A Coruña, La Coruña; Josep Montero García, Hospital General de Granollers, Granollers; Alfonso García Fadrique, Fundación Instituto Valenciano de Oncología, Valencia; Vicente Aguilella Diago, Hospital Clínico Universitario Lozano Blesa, Zaragoza; Javier García Septiem, Hospital Universitario de Getafe, Getafe; Jacinto García García, Hospital Universitario de Salamanca, Salamanca; Luca Ponchietti, Hospital de Torrevieja, Torrevieja; María Soledad Carceller Navarro, Hospital de Manises, Valencia; María Ramos Fernández, Hospital Costa del Sol, Marbella; Raquel Conde Muiño, Hospital Universitario Virgen de las Nieves, Granada; Daniel Huerga Álvarez, Hospital Universitario de Fuenlabrada, Fuenlabrada; Pablo Menéndez Sánchez, Hospital Gutiérrez Ortega, Valdepeñas; Carlos Maristany Bienert, Hospital Universitario Mutua de Terrassa, Terrassa; María Teresa García Martínez, Complejo Hospitalario Universitario de Vigo, Vigo; Celia Moreno Muzas, Hospital Obisco Polanco, Teruel; Carlos Pastor Idoate, Fundación Jiménez-Díaz, Madrid; Alejandro Andicoechea, Hospital de Jove, Gijón; Adolfo Pedro Alonso Casado, Hospital Universitario La Princesa, Madrid; José Vicente Roig Vila, Consorcio Hospital General Universitario de Valencia, Valencia; Ignacio Goded Broto, Hospital San Jorge, Huesca; Pablo Collera, Complejo Asistencial y Universitario de Manresa, Manresa; and Antonio Arroyo Sebastián, Hospital General de Elche, Elche.
Please cite this article as: Sancho-Muriel J, Frasson M, Hervás D, Flor-Lorente B, Ramos Rodriguez JL, Romero Simó M, et al. Resultados quirúrgicos estándar tras resección oncológica de colon. Creación de un nomograma para la autoevaluación. Cir Esp. 2017;95:30–37.