The treatment of patients with non-functioning pancreatic neuroendocrine tumours (NFPNET) is resection in locally pancreatic disease, or with resectable liver metastases. There is controversy about unresectable liver disease.
MethodsWe analysed the perioperative data and survival outcome of 63 patients who underwent resection of NFPNET between 1993 and 2012. They were divided into 3 scenarios: A, pancreatic resection (44 patients); B, pancreatic and liver resection in synchronous resectable liver metastases (12 patients); and C, pancreatic resection in synchronous unresectable liver metastases (6 patients). The prognostic factors for survival and recurrence were studied.
ResultsDistal pancreatectomy (51%) and pancreaticoduodenectomy (38%) were more frequently performed. Associated surgery was required in 44% of patients, including synchronous liver resections in 9 patients. Two patients received a liver transplant during follow-up. According to the WHO classification they were distributed into G1: 10 (16%), G2: 45 (71%), and G3: 8 (13%). The median hospital stay was 11 days. Postoperative morbidity and mortality were 49% and 1.6%, respectively. At the closure of the study, 43 (68%) patients were still alive, with a mean actuarial survival of 9.6 years. The WHO classification and tumour recurrence were risk factors of mortality in the multivariate analysis. The median actuarial survival by scenarios was 131 months (A), 102 months (B), and 75 months (C) without statistically significant differences.
ConclusionsSurgical resection is the treatment for NFPNET without distant disease. Resectable liver metastases in well-differentiated tumours must be resected. The resection of the pancreatic tumour with unresectable synchronous liver metastasis must be considered in well-differentiated NFPNET. The WHO classification grade and recurrence are risk factors of long-term mortality.
El tratamiento de los tumores neuroendocrinos pancreáticos no funcionantes (TNEPNF) es la resección en caso de enfermedad localizada o metástasis hepáticas resecables. Existe controversia en metástasis hepáticas irresecables.
MétodosAnalizamos los datos perioperatorios y de supervivencia de 63 pacientes resecados por TNEPNF entre 1993 y 2012, dividiéndolos en 3 escenarios: A, resección pancreática (44pacientes); B, resección pancreática y hepática por metástasis hepáticas sincrónicas (12pacientes), y C, resección pancreática en presencia de metástasis hepáticas irresecables (6pacientes). Se estudiaron factores pronósticos de supervivencia y recidiva.
ResultadosLas cirugías más frecuentes fueron, pancreatectomía corporocaudal (51%) y duodenopancreatectomía cefálica (38%). El 44% de los pacientes requirieron una cirugía asociada, resecando sincrónicamente páncreas e hígado en 9. Dos pacientes recibieron un trasplante hepático durante el seguimiento. Según la clasificación de la OMS, se distribuyeron en G1: 10 (16%), G2: 45 (71%) y G3: 8 (13%). La morbimortalidad postoperatoria fue del 49 y del 1,6%, respectivamente. Al cierre del estudio, 43 (68%) seguían vivos, con una supervivencia actuarial media de 9,6años. La clasificación de la OMS y la recidiva fueron factores de riesgo de mortalidad en el estudio multivariante. La supervivencia actuarial mediana por escenarios fue de 131meses (A), 102meses (B) y 75meses (C), sin diferencias estadísticamente significativas.
ConclusionesEl tratamiento del TNEPNF sin enfermedad a distancia es la resección. Las metástasis hepáticas resecables en los tumores bien diferenciados deben resecarse. La resección del tumor pancreático con metástasis hepáticas sincrónicas irresecables debe considerarse en TNEPNF bien diferenciados. El grado de clasificación de la OMS y la recidiva son factores de riesgo de mortalidad a largo plazo.
Pancreatic neuroendocrine tumours (PNET) currently account for 2%–5% of pancreatic tumours.1–7 With the increased use of new imaging techniques, the diagnosis of non-functioning PNET (NF-PNET) has increased, currently representing 68%–80% of PNET.3,5–15 NF-PNET are not associated with specific clinical symptoms, as they do not produce an excess of active hormones. The diagnosis may be incidental, but they are often discovered at a very advanced local stage of the disease or even with liver metastases.3,6,7,9–13,16 Currently, the accepted treatment for these patients is surgical resection, as demonstrated by several studies that report improved survival after surgery.6,8–13,16–18 The aggressiveness of PNET has been defined by several criteria, including local invasion, histological evidence of lymphatic, vascular or perineural invasion, and distant metastases.1–3 In 2010, the World Health Organisation (WHO) classified PNET as neuroendocrine tumour (NET) grade 1, NET grade 2, and neuroendocrine carcinoma grade 3, based on histological differentiation and proliferative activity (Ki67).4,5,7,9,19–23 Several authors have already demonstrated the usefulness of this classification to show evidence of worse survival in high grades.8,10,24 The aim of this study was to review the short- and long-term results after surgical treatment of NF-PNET over the last 20 years, and to study the prognostic factors of survival and relapse after surgical resection.
MethodsWe have reviewed the hospital records of patients referred to our Hepato-biliary-pancreatic Surgery and Liver Transplantation Unit for NF-PNET treatment, who underwent surgery between 1993 and 2012. We have prospectively registered the demographic, anatomical-pathological and follow-up data of 63 patients, who underwent resection of the primary pancreatic tumour with curative or palliative intent. Curative intent was defined as resection of the primary tumour, along with complete resection or ablation of any hepatic metastases. Palliative resection was defined as resection of the primary tumour in the presence of unresectable liver metastases, with the aim to prevent the local progression of the primary tumour, which could reduce the patient's quality of life and shorten survival. All patients had a preoperative CT scan, and, given the timeframe of the series, the CT technique was modified. From 1991 to 1994, non-helical CT was used; from 1994 to 2004, coronal helical CT scans were used; and, since 2003, 4-, 16-, and 64-slice multidetector CT scans have been used.
We analysed clinical and pathological characteristics, surgical treatment, postoperative morbidity and mortality according to the Clavien-Dindo classification,25,26 and long-term and disease-free survival rates. Postoperative mortality was defined as patient death within the first 30 days after surgery, or during hospitalisation. The follow-up dates were obtained from the follow-up office visits recorded in the medical file of each patient, and the follow-up time was calculated starting at the time of surgery. The anatomical–pathological parameters analysed were maximum tumour diameter, degree of histological differentiation based on the proliferation rate (mitotic index and Ki67), presence of angiolymphatic or perineural invasion, and lymph node or distant metastases. The tumour proliferation index was retrospectively analysed, and the remaining data were collected prospectively. Histological sections were obtained from the paraffin block archives and were evaluated by an expert pathologist. Tumours were classified according to the WHO 2010 PNET classification as either TNE grade 1 or grade 2 or neuroendocrine carcinoma grade 3.19–22 The classification was based on the proliferation index (mitotic index and Ki67). Thus, grade 1 tumours were considered to have a mitotic index <2 and Ki67 <3%; grade 2 showed a mitotic index of 2–20 and Ki67 between 3 and 20%; meanwhile, grade 3 carcinomas had a mitotic index >20 and Ki67 >20%.
Last of all, the series was divided into 3 clinical scenarios for better statistical analysis. Scenario A included patients with pancreatic resection due to localised or locally advanced disease in the pancreas, in the absence of distant disease. Scenario B included patients who had undergone pancreatic and hepatic resection due to the presence of resectable, synchronous liver metastases. Scenario C included patients who had undergone pancreatic resection in the presence of unresectable synchronous liver metastases. Long-term patient survival rates were compared according to these clinical scenarios.
Statistical AnalysisAs for the statistical study, in the first phase a descriptive analysis was conducted for each of the continuous variables with the calculation of measures of central tendency (mean or median) and dispersion (standard deviation and range), and the qualitative variables according to their percentage. We then analysed the long-term risk factors for mortality and relapse, and a univariate and multivariate Cox regression study was carried out. The actuarial survival curves were calculated and compared using the Kaplan–Meier method and the log-rank test, respectively. A bivariate study of the series was conducted according to stages of the WHO classification and tumour size, in order to show evidence of their association with other variables. For the analysis of the quantitative variables of independent data, the non-parametric Mann–Whitney U test was used. For the analysis of the qualitative variables, the chi-squared test or Fisher's exact test were used according to their normal distribution. Significant associations were those with a significance level of P≤.05. The statistical package used was SPSS v. 20.
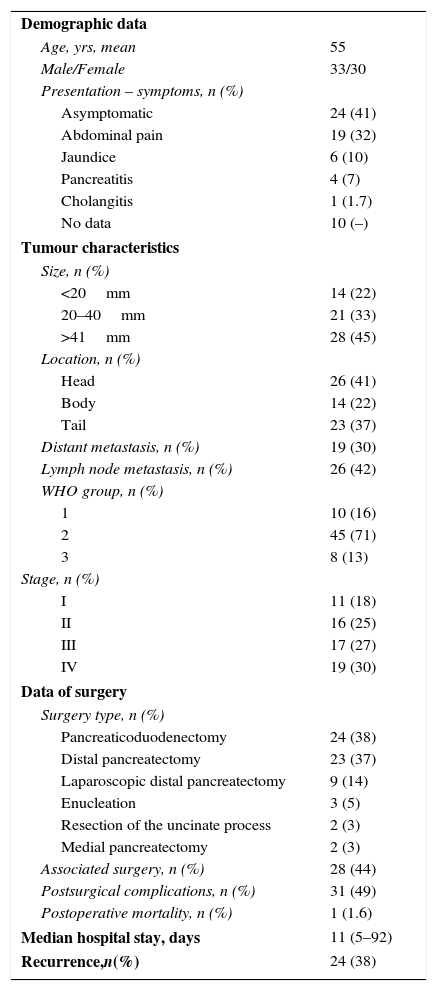
ResultsDescriptive StatisticsIn our study, the 63 resected NF-PNET occurred more frequently in men, with a mean age of 55 (26–77) years. The most frequent symptoms were abdominal pain in 32% and obstructive jaundice in 10%. Interestingly, 41% of patients were asymptomatic at the time of diagnosis and treatment (Table 1). The lesions were most commonly located in the head of the pancreas. In the histological study, 55% of cases presented angioinvasion and 49% perineural invasion. Mean tumour size was 50 (9–200) mm; 14 (22%) patients had tumours ≤2cm, 21 (33%) had tumours between 2 and 4cm, and 28 (44%) had tumours >4cm. In 19 patients, evidence of extrapancreatic disease was detected: 18 (28%) patients had synchronous liver metastases and one patient had metastasis in the adrenal gland. The most frequent surgery was distal pancreatectomy in 51% of the cases, 28% of which were performed by laparoscopy (Table 1). Pancreaticoduodenectomy was performed in 38% of the cases. Associated surgery was required in 44% of the patients (Figs. 1 and 2): in 2 patients, the vena cava was resected and a cadaveric graft was implanted; in 2 patients, the celiac trunk was resected; in 11, liver metastases were resected; in 11, splenectomy, 4 nephrectomy, 3 colectomy, 3 gastrectomy, and 2 adrenalectomy.
Descriptive Analysis of the Patients After Surgical Resection for Non- functioning Pancreatic Neuroendocrine Tumour; Experience With 63 Cases, Hospital Universitari de Bellvitge, 1993–2012.
| Demographic data | |
| Age, yrs, mean | 55 |
| Male/Female | 33/30 |
| Presentation – symptoms, n (%) | |
| Asymptomatic | 24 (41) |
| Abdominal pain | 19 (32) |
| Jaundice | 6 (10) |
| Pancreatitis | 4 (7) |
| Cholangitis | 1 (1.7) |
| No data | 10 (–) |
| Tumour characteristics | |
| Size, n (%) | |
| <20mm | 14 (22) |
| 20–40mm | 21 (33) |
| >41mm | 28 (45) |
| Location, n (%) | |
| Head | 26 (41) |
| Body | 14 (22) |
| Tail | 23 (37) |
| Distant metastasis, n (%) | 19 (30) |
| Lymph node metastasis, n (%) | 26 (42) |
| WHO group, n (%) | |
| 1 | 10 (16) |
| 2 | 45 (71) |
| 3 | 8 (13) |
| Stage, n (%) | |
| I | 11 (18) |
| II | 16 (25) |
| III | 17 (27) |
| IV | 19 (30) |
| Data of surgery | |
| Surgery type, n (%) | |
| Pancreaticoduodenectomy | 24 (38) |
| Distal pancreatectomy | 23 (37) |
| Laparoscopic distal pancreatectomy | 9 (14) |
| Enucleation | 3 (5) |
| Resection of the uncinate process | 2 (3) |
| Medial pancreatectomy | 2 (3) |
| Associated surgery, n (%) | 28 (44) |
| Postsurgical complications, n (%) | 31 (49) |
| Postoperative mortality, n (%) | 1 (1.6) |
| Median hospital stay, days | 11 (5–92) |
| Recurrence,n(%) | 24 (38) |
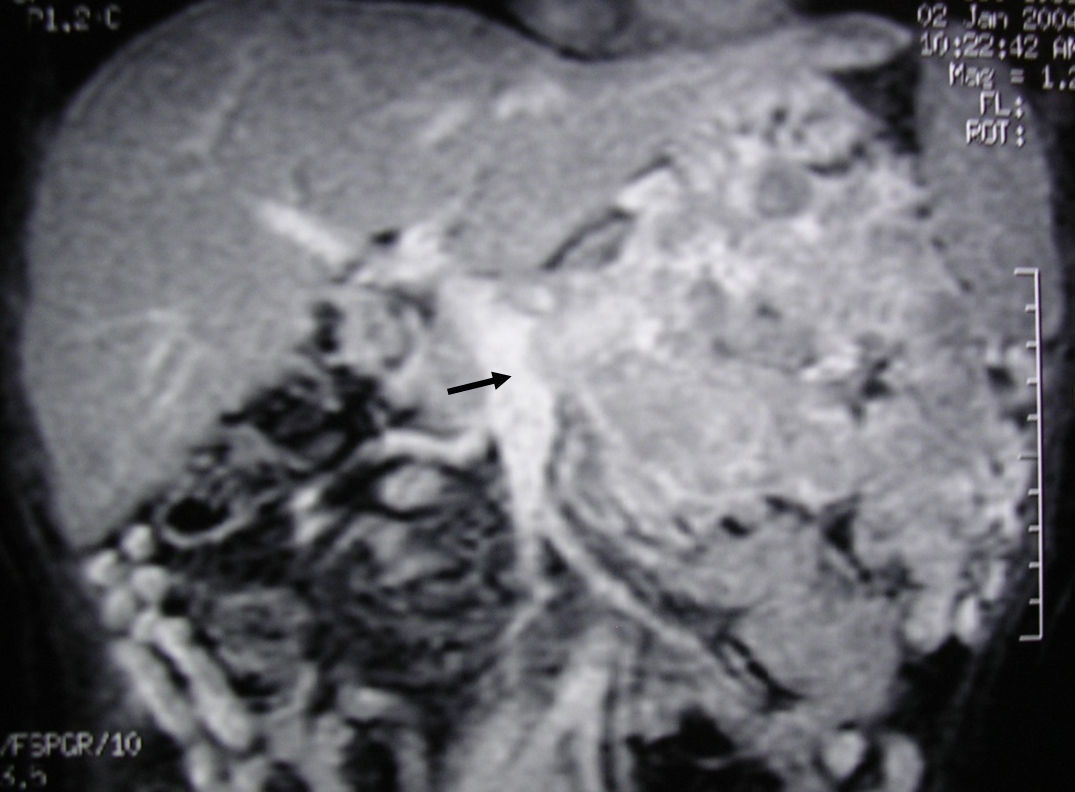
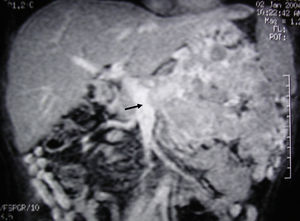
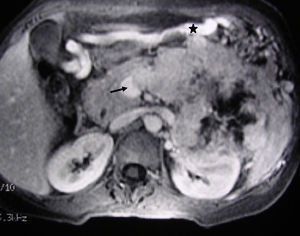
MRI image of liver: 3D T1 sequence coronal reconstruction with fat suppression and intravenous contrast in the portal phase demonstrating a voluminous pancreatic tumour lesion compatible with a neuroendocrine tumour with splenic and renal infiltration. The tumour thrombus in the splenic vein is observed (arrow) to be protruding towards the lumen of the superior mesenteric vein. At the time of diagnosis (January 2004), the patient was 39 years old; surgical treatment included distal pancreatectomy with resection of the portomesenteric venous confluence, splenectomy, left hemicolectomy and left nephrectomy. Currently (May 2016), the patient is alive and without relapse after 12 years of follow-up.
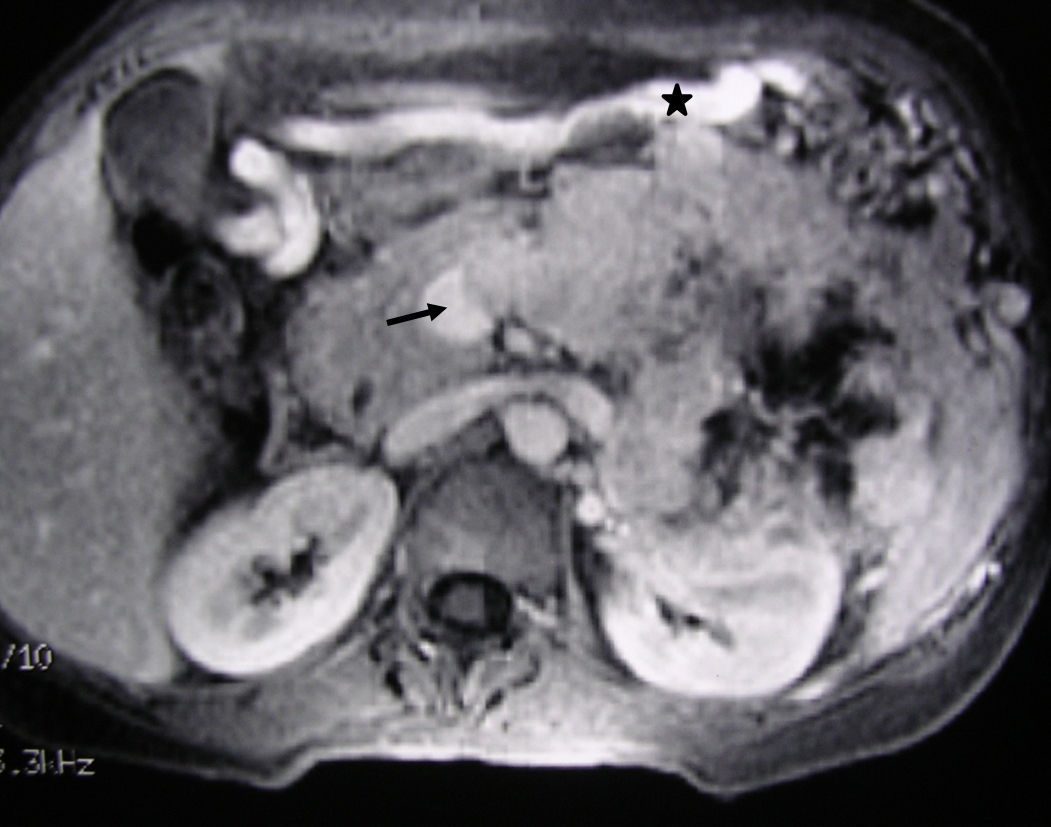
MRI image of the liver (same clinical case as Fig. 1): 3D T1 axial image with fat suppression and intravenous contrast that shows the neuroendocrine tumour of the body-tail of the pancreas and the tumour thrombosis of the splenic vein (arrow). The voluminous gastroepiploic vein (asterisk) translates segmental portal hypertension.
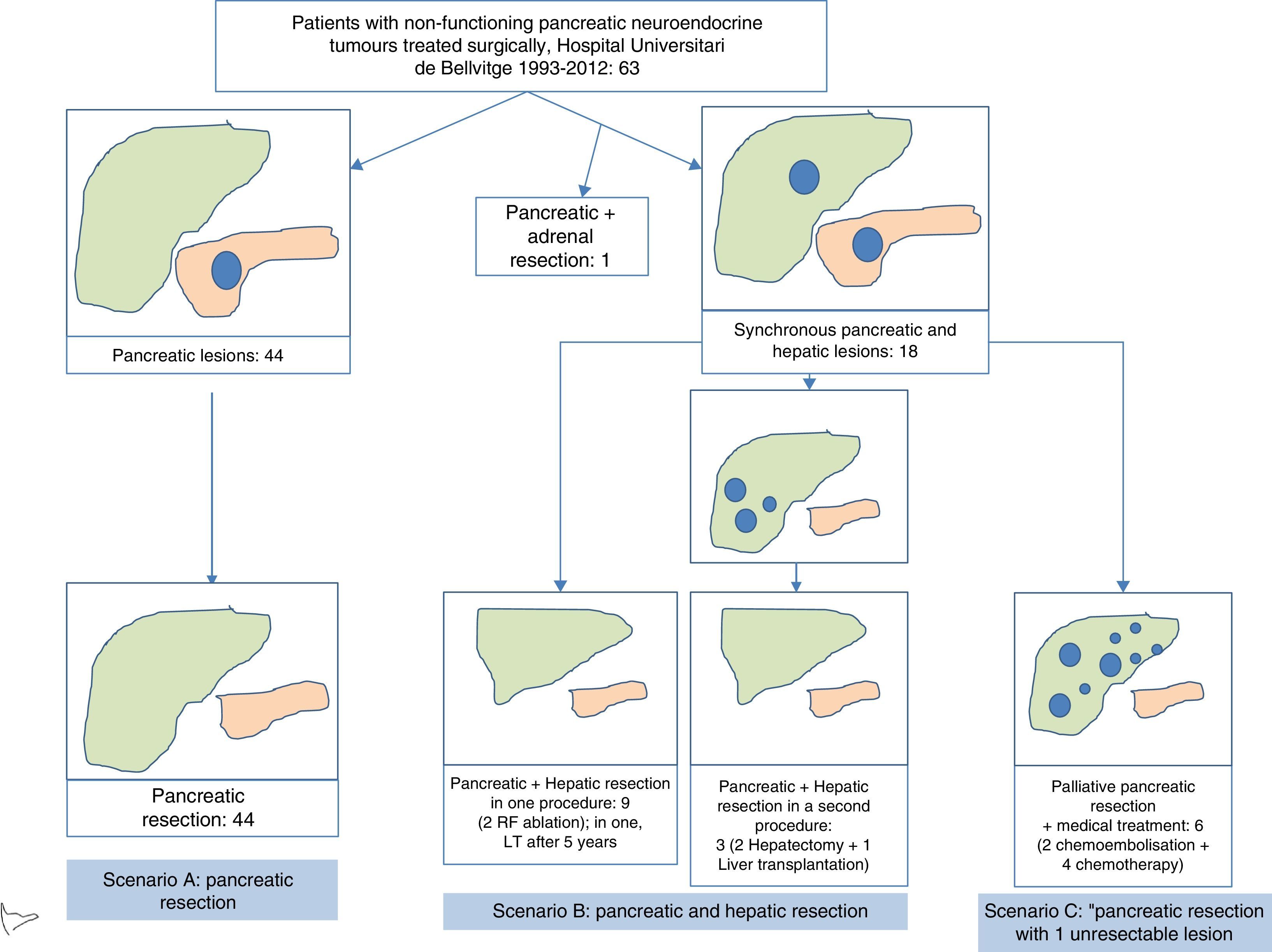
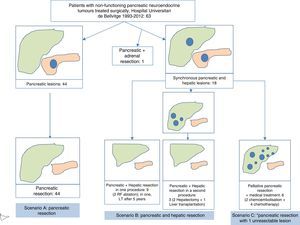
In terms of the defined scenarios, 44 patients belonged to scenario A, 12 scenario B, and 6 scenario C (Fig. 3). One patient with suprarenal metastasis was not included in any of the proposed scenarios. Out of the 18 patients with liver metastases, 9 were treated during the surgery for primary resection: in 7, liver resection was done, and in 2 liver resection and radiofrequency ablation. One of these patients received a liver transplant 5 years later due to hepatic recurrence. In 3 patients, the liver metastasis were treated in a second operation, in 2 patients liver resection was conducted 2 months after resecting the primary tumour, and in one patient liver transplantation was performed. Out of the 6 patients who underwent palliative pancreatic resection, liver chemoembolization was indicated in 2, and 4 patients were treated with adjuvant chemotherapy. Postoperative morbidity was 49% (31/63). According to the Clavien-Dindo classification, 85% were type I–II–IIIA and 15% type IIIB–V. The most frequent complications were intra-abdominal collections in 12 patients and pancreatic leakage in 15. Postoperative mortality was 1.6% (1/63), and this patient died after massive hemoperitoneum in the postoperative period. Mean postoperative hospital stay was 11 (5–92) days (Table 1).
Flowchart of patients with non-functioning pancreatic neuroendocrine tumours treated at the Hospital Universitari de Bellvitge from 1993 to 2012 (n=63). Scenario A included patients with pancreatic resection due to disease located in the pancreas or locally advanced disease, in the absence of distant disease. Scenario B includes patients who underwent pancreatic and hepatic resection, due to the presence of resectable synchronous liver metastases. Scenario C includes patients who underwent pancreatic resection in the presence of unresectable synchronous liver metastases. Long-term patient survival was compared by clinical scenario, with no statistically significant differences being observed.
According to the WHO classification, the patients were distributed as follows: 10 G1 (16%); 45 G2 (71%), and 8 G3 (13%). According to the TNM classification, the patient distribution was: 11 stage I (18%); 16 stage II (25%); 17 stage III (27%); and 19 stage IV (30%). 42% of patients had lymph node metastases. Regarding the current state of the patients and with a mean follow-up of 4.6 years, 43 (68%) were still alive. Twenty-four patients (38%) had recurrence (Table 1).
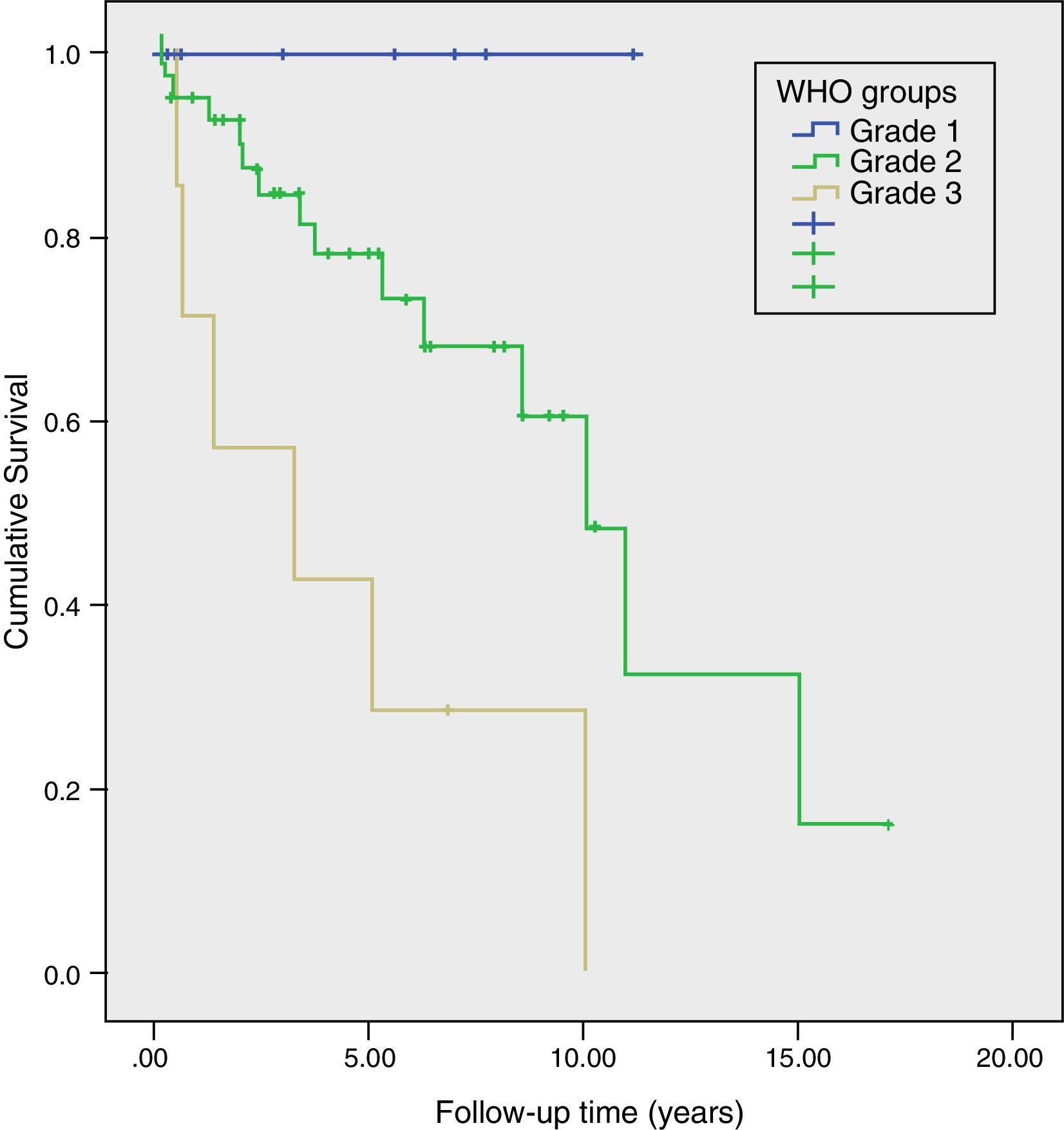
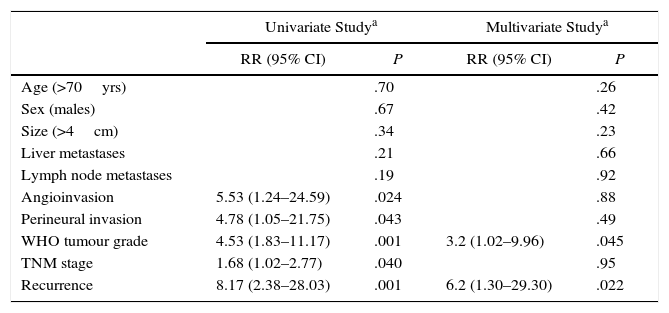
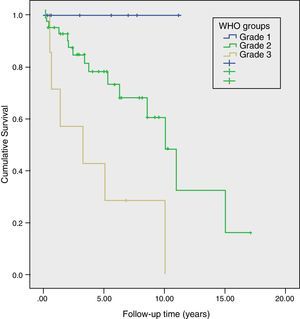
Long-Term Survival and Risk Factors for MortalityMean actuarial survival was 9.6 years, with 5- and 10-year actuarial survival rates of 73% and 51%, respectively. The univariate study showed that angioinvasion, perineural invasion, relapse during follow-up, TNM stage and WHO classification grade were risk factors for mortality. In the multivariate analysis, only the WHO classification grade (HR=3.2, 95% CI: 1.02–9.96) and tumour recurrence (HR=6.2; 95% CI: 1.30–29.30) were long-term risk factors for mortality (Table 2). Likewise, a Kaplan–Meier study was conducted according to the WHO groups, which showed a 5-year survival rate in G1, G2, and G3 of 100%, 73%, and 29%, respectively (P=.002) (Fig. 4). During follow-up, 32 patients in scenario A are still alive and 13 have died, with a median actuarial survival of 131 months (±10); 7 from scenario B are still alive and 5 have died, with a median actuarial survival of 102 months (±41); and, 4 from scenario C are still alive and 2 have died, with a median actuarial survival of 75 months (±0). In the Kaplan–Meier analysis, the distribution of patients according to the clinical scenario did not detect statistically significant differences.
Analysis of the Risk Factors for Long-Term Mortality After Surgical Resection Due to Non-Functioning Pancreatic Neuroendocrine Tumour; Experience With 63 Cases, Hospital Universitari de Bellvitge, 1993–2012.
| Univariate Studya | Multivariate Studya | |||
|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | |
| Age (>70yrs) | .70 | .26 | ||
| Sex (males) | .67 | .42 | ||
| Size (>4cm) | .34 | .23 | ||
| Liver metastases | .21 | .66 | ||
| Lymph node metastases | .19 | .92 | ||
| Angioinvasion | 5.53 (1.24–24.59) | .024 | .88 | |
| Perineural invasion | 4.78 (1.05–21.75) | .043 | .49 | |
| WHO tumour grade | 4.53 (1.83–11.17) | .001 | 3.2 (1.02–9.96) | .045 |
| TNM stage | 1.68 (1.02–2.77) | .040 | .95 | |
| Recurrence | 8.17 (2.38–28.03) | .001 | 6.2 (1.30–29.30) | .022 |
95% CI, 95% confidence interval; n.s., not significant.
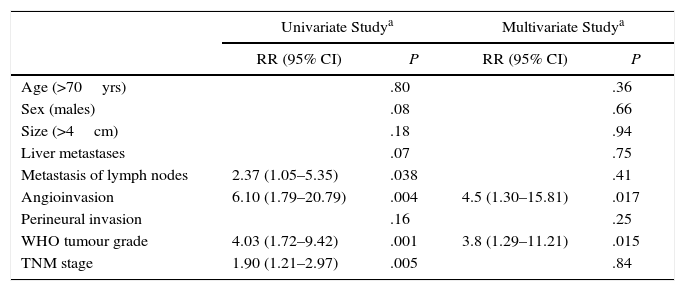
Mean disease-free actuarial survival was 8.9 years and 55% and 37% after 5 and 10 years. The univariate Cox model demonstrated that angioinvasion, the presence of pathological lymphadenopathies, TNM stage, and the WHO classification grade were risk factors for relapse. In the multivariate analysis, it was observed that only the WHO classification grade (HR=3.8, 95% CI: 1.29–11.21) and angioinvasion (HR=4.5; 95% CI: 1.30–15.82) were risk factors for relapse (Table 3). In terms of the clinical scenario, the relapse was identified in 14 patients from scenario A (11 of whom died), 9 patients in scenario B (5 of whom died) and one patient in scenario C (who died during follow-up).
Analysis of Risk Factors for Long-Term Recurrence After Surgical Resection for Non-Functioning Pancreatic Neuroendocrine Tumour; Experience With 63 Cases, Hospital Universitari de Bellvitge, 1993–2012.
| Univariate Studya | Multivariate Studya | |||
|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | |
| Age (>70yrs) | .80 | .36 | ||
| Sex (males) | .08 | .66 | ||
| Size (>4cm) | .18 | .94 | ||
| Liver metastases | .07 | .75 | ||
| Metastasis of lymph nodes | 2.37 (1.05–5.35) | .038 | .41 | |
| Angioinvasion | 6.10 (1.79–20.79) | .004 | 4.5 (1.30–15.81) | .017 |
| Perineural invasion | .16 | .25 | ||
| WHO tumour grade | 4.03 (1.72–9.42) | .001 | 3.8 (1.29–11.21) | .015 |
| TNM stage | 1.90 (1.21–2.97) | .005 | .84 | |
95% CI, 95% confidence interval; n.s., not significant.
NF-PNET are growing in prevalence, as demonstrated by recent publications by the National Cancer Institute SEER programme.8,27 The incidence of NET between 1973 and 1977 was reported to be 1.7 per million inhabitants, while between 2003 and 2007 it rose to 4.3 per million inhabitants. When analysing the tumours smaller than 2cm, the current incidence has doubled compared to the incidence of 22 years ago.27 Improvements in diagnostic tests and the increase in life expectancy have favoured an increase in the diagnosis of these lesions, which are mostly asymptomatic. As is known, the difficult management of these patients basically stems from the diverse forms of presentation, and therefore the different treatments indicated. Thus, the clinical spectrum at the time of diagnosis ranges from patients with a millimetric indolent lesion to large tumour masses with venous infiltration and synchronous liver metastases. Logically, treatments should be individualised depending on the extent of the lesion. In the current study, we have analysed our experience in the surgical treatment of NF-PNET, with emphasis on long-term prognostic factors, including pathological and clinical factors.
Pathological FactorsDuring the last decade, the clinical–pathological aspects of PNET have been extensively defined. The recent consensus documents of the European and American societies (ENETS and NANETS), as well as the World Health Organisation (WHO, 2010), have redefined the nomenclature and the different classification stages for PNET. Years ago, some authors defended the European or American classifications (ENETS-pTNM, AJCC-pTNM)1,28 for the study and comparison of surgical series, to include details such as tumour size or lymph node involvement. Our study demonstrates the prognostic validity of the 2010 WHO classification, being one of the variables that influence the survival and recurrence rates of the patients studied. Thus, a high grade in the 2010 WHO classification implies an increased risk of death and long-term relapse among patients treated at our centre, in line with other authors.6,10,12,13 Several pathological tumour factors, such as the Ki-67 proliferation index, angioinvasion, perineural invasion, number of mitoses, peripancreatic infiltration and nodal involvement, have been defined as variables that influence the prognosis of patients with NF-PNET.7,10–12,18,19,24,28–30 In our study, we have included several variables regarding the resected tumour, such as angioinvasion and perineural invasion. We have shown that tumour angioinvasion implies a long-term elevated risk of recurrence in the univariate and multivariate studies: the univariate study of risk factors for death showed that angioinvasion was a risk factor, although the multivariate study did not confirm this. As we can see, angioinvasion is one of the factors that influences the poor progress of surgically treated patients, as previously demonstrated by other authors.18,29
Surgical TreatmentSurgical resection is the treatment of choice in this type of tumour and is associated with improved survival.6,8–14,16–19,24,30 In small differentiated lesions (G1–G2), depending on the location in the pancreatic gland, minimal pancreatic surgery can be considered, minimising the impact on long-term exocrine and endocrine function.9,14,27 However, the aggressiveness of G3 NF-PNET would indicate regional lymphadenectomy to complement cancer surgery, since it is a neuroendocrine carcinoma. In fact, the presence of lymph node involvement at the time of surgery has been demonstrated by some series as a factor for worse prognosis.7,10,18,24,29,30 In our experience, and similar to other authors,2,8,11 we have not found an independent relationship between lymph node involvement and worse survival. Pancreatic resection of NF-PNET sometimes requires the resection of neighbouring organs or structures due to their large volume or vascular infiltration.9,31 In our series, we have associated resection of other organs in 28 cases, obtaining a survival rate similar to cases without associated resection.
Treatment ScenariosIn 2001, a group from MD Anderson in Texas published an extensive study about 163 NF-PNET interventions16 that inspired numerous articles on the subject. It described up to 7 different scenarios for the treatment of NF-PNET. Scenarios 1 through 3 referred to resected pancreatic lesions, depending on the involvement of the surgical margins, while scenario 4 was for unresected pancreatic lesions. Scenarios 5 through 7 referred to pancreatic lesions with synchronous liver metastases, either with surgery or without surgery. The patient referred to the surgical service for evaluation must be correctly staged to plan the best treatment. In our opinion, the best scheme to plan treatment is to divide patients with NF-PNET into 3 basic scenarios, already discussed previously. We will now comment on the results of the study based on these.
Scenario A. Pancreatic Resection With No Distant DiseaseThe benefit of surgery in NF-PNET without hepatic involvement on patient survival is well documented.8,9,11,13,16,17 In our experience, the 32 patients in this situation are still alive, with a median actuarial survival of 131 months. However, the large volume of some lesions involves a high incidence of vascular resections31 or resections of neighbouring organs, as we have done in 44% of our cases. In 2011, the Massachusetts11 and Verona19 groups published their experiences with resected pancreatic lesions. These articles demonstrated that the resected NF-PNET had different prognoses depending on the size of the resected tumour, which we were not able to reproduce in our study. These articles raise a controversy regarding the management of small NF-PNET. While Haynes et al.11 advocated the resection of all NF-PNET despite being small (<2cm) or incidental because of their possibility of malignancy, Bettini et al.19 advocated follow-up in incidental lesions <2cm, as only 6% of their series showed tumour aggressiveness that justified pancreatic surgery. The high morbidity of pancreatic surgery, together with a low percentage of G3 in small tumours, were the arguments for medical management. Finally, to support nonsurgical management, in 2012 the Mayo Clinic group4 published a series of 77 consecutive cases without surgery, with no progression of the disease during follow-up. Recently, non-surgical treatment of small NF-PNET has already been collected in several international clinical guidelines.32 In our centre, we have monitored 29 patients with incidental lesions smaller than 3cm from 2007 to 2015, with no evidence of tumour progression or extension of the disease in any of the cases,33 with a mean follow-up of 39 months.
Scenario B. Pancreatic Resection in the Presence of Resectable Synchronous Liver MetastasesSynchronous liver metastases are a frequent finding in NF-PNET and a risk factor for long-term mortality and recurrence that has been widely demonstrated,6,8,10,12,16,18,25,28 although we have not been able to reproduce this finding. Between the end of the 1990s and the first decade of 2000, some groups published experiences favourable to liver resection of NF-PNET metastases, arguing improved patient survival and quality of life. Five-year survival rates of between 70% and 82% were also observed.34–37 Despite this, long-term relapse is the norm.6,36–39 In an interesting paper, Elias et al.39 demonstrated that current imaging techniques underestimate the presence of liver metastases in more than 50% of patients. In 2010, 2 studies with large numbers of patients were published, which identified prognostic factors.8,40 Mayo et al.40 published the experience of 8 European and American hospitals, with 339 PNET; the multivariate analysis identified synchronous metastases, non-functioning tumours, and presentation with extrahepatic disease as poor prognostic factors. That same year, Franko et al.8 published one of the series with 2158 cases analysed retrospectively, demonstrating that resection of the primary tumour and/or metastasis was associated with a better prognosis in the subgroup of patients with liver metastases. However, so far there are few experiences in the literature comparing patients who had been treated surgically with those who had not. In 2006, Osborne et al.41 studied 61 patients who were operated on versus 59 embolised patients, demonstrating longer survival in the surgically treated patients. This study was criticised, since the 2 groups of patients were not comparable. Later, in 2011, Mayo et al.42 published an analysis that added greater rigour to the comparison. It analysed the experience of 9 medical centres, comparing the resection of hepatic metastases versus intra-arterial treatment and performing a “propensity score” analysis to minimise selection bias. They demonstrated that the survival of surgical patients was superior to that of the non-surgical patients. Finally, a recent meta-analysis43 compared 161 resected versus 374 unresected cases. Significantly longer survival was found in patients treated with resection of liver metastases versus those treated with embolisation. Thus, it seems clear that liver resection in patients with resectable lesions is warranted. As we have mentioned, the 12 patients of our series who underwent pancreatic and hepatic resection had a mean actuarial survival of 102 months, a fact that does not differ from the survival of resected patients without hepatic metastases.
Scenario C. Pancreatic Resection in the Presence of Unresectable Synchronous Liver MetastasesThe type of liver resection used will depend on the number of metastases, the site, and the postoperative hepatic functional reserve. In patients with bilobar liver metastases, ablative or chemoembolisation techniques are alternatives that provide for radical resection. Recently, other strategies have been proposed in cases of disseminated disease to allow for surgery. In this context, the Beaujon Hospital group44 described a 2-step approach for primary tumour resection and synchronous and bilobar liver metastases. This procedure allowed for complete resection of the disease with no mortality, an acceptable morbidity (some 20% in each procedure), and a long survival in selected patients with multilobar liver metastases. However, in some cases patients may not be able to undergo liver resection due to the large amount of hepatic involvement, and only the primary pancreatic tumour is resected. This scenario raises doubts about the benefit of pancreatic surgery in this context, and several series before our own have analysed it. Nguyen et al.45 and Solorzano et al.16 demonstrated superior survival in resected patients (60% and 49% after 5 years, respectively) compared to the unresected patients (30% and 16% after 5 years, respectively) thanks to this strategy. Bettini et al.46 reported only a better quality of life, despite showing no differences in survival. Bertani et al.47 and Keutgen et al.48 have demonstrated, in unique multivariate studies, that resection of the primary tumour in the presence of unresectable liver metastases is associated with better long-term survival. In our experience, 4 out of the 6 patients included in this scenario are still alive at the end of the study, with a survival similar to the rest. In our opinion, and in agreement with other authors,9,47,48 patients with pancreatic involvement and unresectable synchronous hepatic metastases should be studied by percutaneous needle aspiration to assess the degree of tumour differentiation. In cases with well-differentiated tumours (G1–G2), the primary lesion would be resected, whereas poorly differentiated tumours (G3) that progress rapidly do not benefit from resection.
Finally, in patients under 50 years of age with a previously resected pancreatic tumour and symptomatic unresectable hepatic involvement with no other therapeutic possibilities, liver transplantation should be evaluated within the multidisciplinary approach.48–51 Le Treut et al.49 have recently published a European multicentre study with 35 hospitals. They demonstrated that, with correct patient selection, 5-year survival and disease-free survival rates of 80% and 57%, respectively, can be achieved. Lastly, current European and American clinical guidelines (ENETS9 and NANETS52) include the option of transplantation in highly selected cases.
In conclusion, the treatment of choice for NF-PNET without distant disease is radical surgical resection. In well-differentiated small lesions, depending on their location in the pancreatic gland, conservative surgery may be indicated. It must be taken into account that the role of surgery is controversial in asymptomatic lesions smaller than 2cm. In our experience, follow-up is a valid option in incidental small-sized lesions (<2cm), especially in elderly patients. Resectable, synchronous hepatic metastases in well-differentiated tumours should be resected together with the primary tumour, either simultaneously or sequentially. Thus, in our opinion, surgery should be considered as the first option in this scenario. Resection of the pancreatic primary tumour with unresectable synchronous hepatic metastases should be considered in the therapeutic strategy of patients with well-differentiated NF-PNET. The surgical indication in this scenario should be thoroughly analysed, excluding cases with generalised liver disease, or in the presence of extra-abdominal disease. Lastly, in our experience, the WHO classification grade and tumour recurrence are risk factors for long-term mortality after NF-PNET resection.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Busquets J, Ramírez-Maldonado E, Serrano T, Peláez N, Secanella L, Ruiz-Osuna S, et al. Tratamiento quirúrgico de los tumores neuroendocrinos no funcionantes de páncreas basado en 3 escenarios clínicos. Cir Esp. 2016;94:578–587.