To analyze the impact of systematic second-look surgery plus hyperthermic intraperitoneal chemotherapy (HIPEC) performed 1 year after resection of the primary tumor, in asymptomatic patients at high risk of developing peritoneal carcinomatosis (PC).
MethodsBetween 2012 and 2016, 33 patients without any sign of peritoneal recurrence on imaging studies were prospectively included in the study and underwent second-look surgery aimed at treating limited PC earlier and were prospectively recorded. They were selected based on 5 primary tumor-associated criteria: resected minimal synchronous macroscopic PC (n=10), synchronous ovarian metastases (n=2), positive peritoneal cytology (n=2), pT4 primary tumors (n=15) and perforation (n=4).
ResultsPC was found and treated by cytoreduction plus HIPEC in 10 of the 33 (30.3%) patients, although it was detected in only 2/15 patients of the pT4 subgroup (13.3%). The patients without PC underwent complete abdominal exploration plus HIPEC. Median follow-up was 14.5 months. One patient died postoperatively at day 55. Severe morbidity rate (Clavien–Dindo III–V) was low (15.2%). The 3-year overall survival rate was 93% and the 3-year disease-free survival rate was 33%. Peritoneal recurrences occurred in 4 patients (12.1%), 2 of whom had macroscopic PC discovered at the second-look (20%), while the other 2 patients had no macroscopic PC (8.7%) (P=.04).
ConclusionsThe second look+HIPEC strategy in our series of patients at high risk of developing PC, allows its early detection and its treatment in 30.3% of cases, with a very low rate of peritoneal recurrence. It is important to continue evaluating the results to increase the accuracy of the inclusion criteria, especially the pT4 criterion that in this series has a low predictive power for the occurrence of PC.
Analizar el impacto de la cirugía de second look (CSL) combinada con quimioterapia intraperitoneal hipertérmica (HIPEC) realizada un año después de la cirugía del tumor primario en pacientes asintomáticos con alto riesgo de desarrollar carcinomatosis peritoneal (CP) tras resección de cáncer colorrectal.
MétodosEntre febrero 2012 y febrero 2016, 33 pacientes con alto riesgo de recidiva peritoneal, sin signos de recurrencia en pruebas de imagen fueron prospectivamente incluidos en el estudio y sometidos a CSL con el objetivo de tratar posibles recidivas peritoneales precozmente. Los pacientes fueron seleccionados por 5 criterios: pT4 (n = 15), citología peritoneal positiva por cáncer (n = 2), tumor perforado (n = 4), enfermedad peritoneal sincrónica resecada (n = 10), metástasis ováricas sincrónicas resecadas (n = 2).
ResultadosSe detectó carcinomatosis peritoneal (CP) en 10 de los 33 pacientes (30,3%) (CP+), en los cuales se realizó citorreducción completa más HIPEC. En el subgrupo de los pacientes pT4 (n = 15) se detectó CP solo en 2 casos (13,3%). El resto de los pacientes (CP-) fueron sometidos a HIPEC profiláctica. La mediana de seguimiento después de CSL ha sido de 14,5 meses. La tasa de morbilidad postoperatoria grave (Clavien-Dindo III-V) fue del 15,2% (5/33) y la mortalidad del 3,0% (1 paciente al 55.° día postoperatorio). La supervivencia global a 3 años fue del 93% y la supervivencia libre de enfermedad del 33%. Tras CSL + HIPEC, 4/33 pacientes (12,1%) recidivaron en el peritoneo, 2 CP + (20%) y 2 CP - (8,7%) (p = 0,04).
ConclusionesLa realización de CSL + HIPEC en nuestra serie de pacientes con alto riesgo de desarrollar CP permite su detección temprana y su tratamiento en el 30,3% de los casos, con una tasa muy baja de recurrencia peritoneal posterior. Es importante continuar evaluando los resultados para aumentar la precisión de los criterios de inclusión, especialmente del criterio pT4, que en esta serie tiene un bajo poder predictivo para la aparición de CP.
In colorectal cancer (CRC), peritoneal carcinomatosis (PC) is detected at the diagnosis of the primary tumor in 10% of patients; meanwhile, 40%–70% of treated patients present recurrence, located exclusively in the peritoneum in 10%–35%.1–5 These patients with PC have been classically considered incurable. Although the median survival has increased considerably with contemporary chemotherapy (CTx) (based on oxaliplatin or irinotecan), reaching 12.7 months,6 prolonged survival is anecdotal.7–10 However, the prognosis has improved with the use of cytoreduction surgery (CR) combined with hyperthermic intraperitoneal chemotherapy (HIPEC). Five-year survival in patients with PC is 30% and could exceed 40% in selected patients.11,12 The best results are obtained in patients with low tumor load (Peritoneal Cancer Index, or PCI). PCI is the most important prognostic factor,8,13,14 with a 5-year survival rate of 49% in patients with PCI <7 compared to survival rates <10% with PCI ≥20.13 Patients with extensive peritoneal involvement (PCI >21) do not benefit from this technique.
All our efforts should be aimed at identifying patients with peritoneal carcinomatosis at the earliest possible stage. Early diagnosis of PC is difficult, due to the absence of symptoms at early stages and the poor sensitivity of current imaging tests to detect low-volume peritoneal disease.15 The Elias group has proposed systematically performing second-look surgery (SLS)+HIPEC in patients undergoing surgery with curative intent for CRC with no evidence of peritoneal disease in the follow-up but who present factors that increase the risk of recurrence; they have detected PC in 56% of the cases treated with this strategy.16 The selection criteria of the French group included patients who had tumor perforation at diagnosis or those who underwent resection for limited PC or ovarian metastasis simultaneously and radically with surgery for the primary tumor. However, there are other factors that clearly correlate with increased risk for peritoneal recurrence: fundamentally, whether the primary tumor is pT417–20 or positive cytology in the primary surgery.21,22
The objective of our study was to analyze the impact of SLS+HIPEC in a group of patients who had been previously operated on for CRC, with no evidence of peritoneal recurrence during follow-up but with several high-risk criteria to develop it, in an attempt to diagnose and treat PC at an early stage.
MethodsThis prospective study was approved by the hospital's Ethics Committee. All patients were informed of the study objectives and gave their signed consent specifically for inclusion.
The following inclusion criteria were applied:
- 1.
Patients with CRC who had been treated surgically with curative intent and had a high risk for developing PC (pT4, positive cytology, perforation of the primary tumor, synchronous peritoneal disease resected with the primary tumor [RSPD] or resection of synchronous ovarian metastases [RSOM]).
- 2.
Absence of any radiological signs of recurrence (CT of thorax–abdomen–pelvis always, PET-CT selectively in cases of doubt), no symptoms or signs in lab work (tumor markers) some 4–6 months after the end of adjuvant CTx, with the only exception of the existence of limited liver metastasis deemed resectable in the same procedure.
- 3.
Patients with a good general status (WHO performance status <2).
The study was carried out from February 2012 to February 2016, selecting those patients who met the inclusion criteria of the Tumor Committee, when there were no signs of peritoneal recurrence in the follow-up conducted 4–6 months after the end of adjuvant CTx (according to protocol, 12 cycles of FOLFOX, 6 months). Patients underwent SLS by laparotomy or laparoscopy when feasible. In patients with PC, CR+HIPEC was performed. In those who did not present PC, only HIPEC was performed. We also performed omentectomy, appendectomy and adnexectomy (postmenopausal) if these had not been done during the primary tumor surgery, in addition to the resection of limited hepatic metastases in the few patients who presented them.
PCI was calculated, although it was only considered positive after definitive histological confirmation (gold standard). HIPEC was performed with oxaliplatin at a dose of 460mg/m2, mean temperature of 42°C (41–43°C) for 30min, after 5-fluoracil (400mg/m2) and leucovorin (20mg/m2) in intravenous perfusion 30min beforehand.23–25
Postoperative hospital stays and major complications were recorded (Dindo–Clavien classification III–V).26,27 Patients were followed according to the oncological protocol of the University Hospital of Fuenlabrada (Madrid, Spain): thoracoabdominal–pelvic CT and determination of tumor markers every 3 months during the first 2 years and every 6 months in the 3 subsequent years. Patients with PC+ and those with simultaneous resection of liver metastases received complementary CTx.
Statistical AnalysisThe data were analyzed in February 2016. The qualitative variables were reported with their distribution frequencies and compared with Pearson's χ2 test or Fisher's exact test. The quantitative variables were expressed with the mean (standard deviation [SD]) or median (interquartile range) if they did not have a normal distribution, and were compared using the Student's t test or the Mann–Whitney U test. Survival rates were analyzed with the Kaplan–Meier method and compared using the log-rank test. Clinically or statistically significant variables were adjusted using a multivariate Cox regression model. All statistical analyses were performed using SPSS v19.0. A P value <.05 was considered significant.
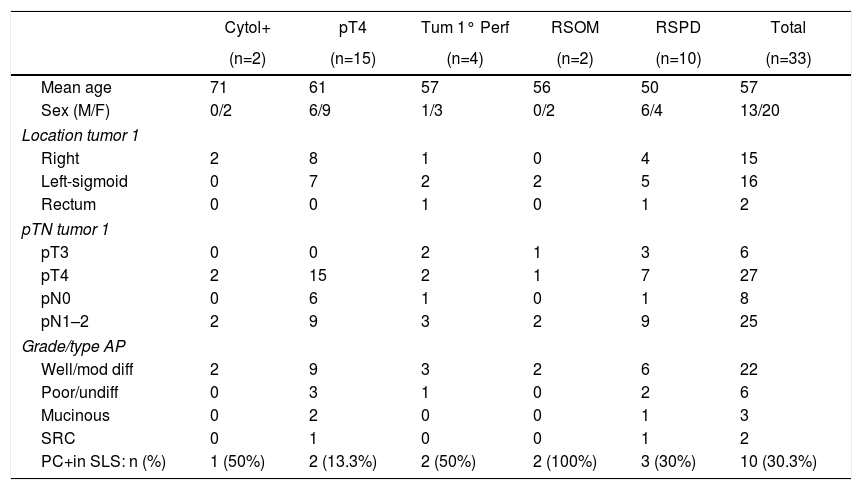
ResultsSLS+HIPEC was performed in 33 patients. We found macroscopic PC (PC+) in 10 out of the 33 patients (30.3%). The overall incidence of PC and the incidence by subgroups (according to each risk factor of PC analyzed) are shown in Table 1. In three patients, minor hepatic resections were also performed due to the coexistence of metastases.
Patient Characteristics and Incidence of PC by Subgroups (Inclusion Criterion).
| Cytol+ | pT4 | Tum 1° Perf | RSOM | RSPD | Total | |
|---|---|---|---|---|---|---|
| (n=2) | (n=15) | (n=4) | (n=2) | (n=10) | (n=33) | |
| Mean age | 71 | 61 | 57 | 56 | 50 | 57 |
| Sex (M/F) | 0/2 | 6/9 | 1/3 | 0/2 | 6/4 | 13/20 |
| Location tumor 1 | ||||||
| Right | 2 | 8 | 1 | 0 | 4 | 15 |
| Left-sigmoid | 0 | 7 | 2 | 2 | 5 | 16 |
| Rectum | 0 | 0 | 1 | 0 | 1 | 2 |
| pTN tumor 1 | ||||||
| pT3 | 0 | 0 | 2 | 1 | 3 | 6 |
| pT4 | 2 | 15 | 2 | 1 | 7 | 27 |
| pN0 | 0 | 6 | 1 | 0 | 1 | 8 |
| pN1–2 | 2 | 9 | 3 | 2 | 9 | 25 |
| Grade/type AP | ||||||
| Well/mod diff | 2 | 9 | 3 | 2 | 6 | 22 |
| Poor/undiff | 0 | 3 | 1 | 0 | 2 | 6 |
| Mucinous | 0 | 2 | 0 | 0 | 1 | 3 |
| SRC | 0 | 1 | 0 | 0 | 1 | 2 |
| PC+in SLS: n (%) | 1 (50%) | 2 (13.3%) | 2 (50%) | 2 (100%) | 3 (30%) | 10 (30.3%) |
SRC: signet ring cells; Cytol+: positive cytology; PC: peritoneal carcinomatosis; SLS: second-look surgery; RSPD: resected synchronous peritoneal disease; RSOM: resected synchronous ovarian metastases; Tum 1 perf: primary tumor perforation.
The mean time between surgery of the primary tumor and SLS was 10.8 months (range 8–18). In the PC+ group, the mean (SD) was 11.9 (8.2) months. In the group without findings (PC−), the interval was 10.3 (3.8) months (P>.05).
The approach was by laparotomy in 25 patients and by laparoscopy in 8 (2 conversions). All laparoscopies were performed according to the pT4 criterion (primary surgery had been performed by laparoscopy). Only minimal PC+ (PCI 3) was discovered in one of the 6 pure laparoscopic approaches, which was the only one that presented recurrence.
Only one of the three patients who underwent simultaneous resection of liver metastases presented PC+.
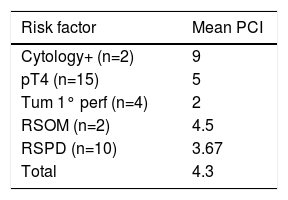
The mean (SD) PCI in the PC+ cases was 4.3 (2.9). The PCI by subgroups of the cases with PC+ is specified in Table 2.
PCI by Subgroups (Inclusion Criterion).
| Risk factor | Mean PCI |
|---|---|
| Cytology+ (n=2) | 9 |
| pT4 (n=15) | 5 |
| Tum 1° perf (n=4) | 2 |
| RSOM (n=2) | 4.5 |
| RSPD (n=10) | 3.67 |
| Total | 4.3 |
RSPD: resected synchronous peritoneal disease; RSOM: resected synchronous ovarian metastases; PCI: peritoneal cancer index; Tum 1 perf: primary tumor perforation.
In the univariate analysis, none of the factors (sex, location, risk subgroups, T–N stages of the primary tumor) was a predictor for macroscopic PC.
At the end of the surgery, HIPEC was performed in all patients, PC+ (n=10) and PC− (n=23). The mean duration (SD) of the surgery was greater in patients with PC+ (349 [101]min) than in the PC− group (276 [82]min) (P<.05).
Morbidity and mortality: the postoperative period transpired with no major complications in 28 out of the 33 patients (84.9%). Severe morbidity (Dindo–Clavien classification grades 3–5) was 15.2% (5/33), including postoperative death. In the 23 PC− patients, the rate of major complications was 4.3% (1/23), while in the 10 PC+ patients it was 40% (4/10) (P=.03). One patient (RSPD subgroup) (3.0%) died due to postoperative multiple organ failure. The estimated PCI during SLS of this patient was 2; the lesions were resected, intestinal transit reconstructed with closure of a protective ileostomy performed in the first intervention (right hemicolectomy and rectosigmoid resection) and HIPEC applied. The patient was re-operated twice: hemoperitoneum and dehiscence of the ileal anastomosis and biliary peritonitis secondary to jejunal perforation. After multiple complications (pneumonia, DVT, septic shock), the patient died due to multiple organ failure after 55 days in the Intensive Care Unit.
The median hospital stay was 8 (15) days; in PC+ it was 12 (38) days, and in PC− 7 (11) days (P=.12).
Follow-up and survival: the median follow-up after SLS was 14.5 months. No patients were lost to follow-up.
Median survival has still not been reached.
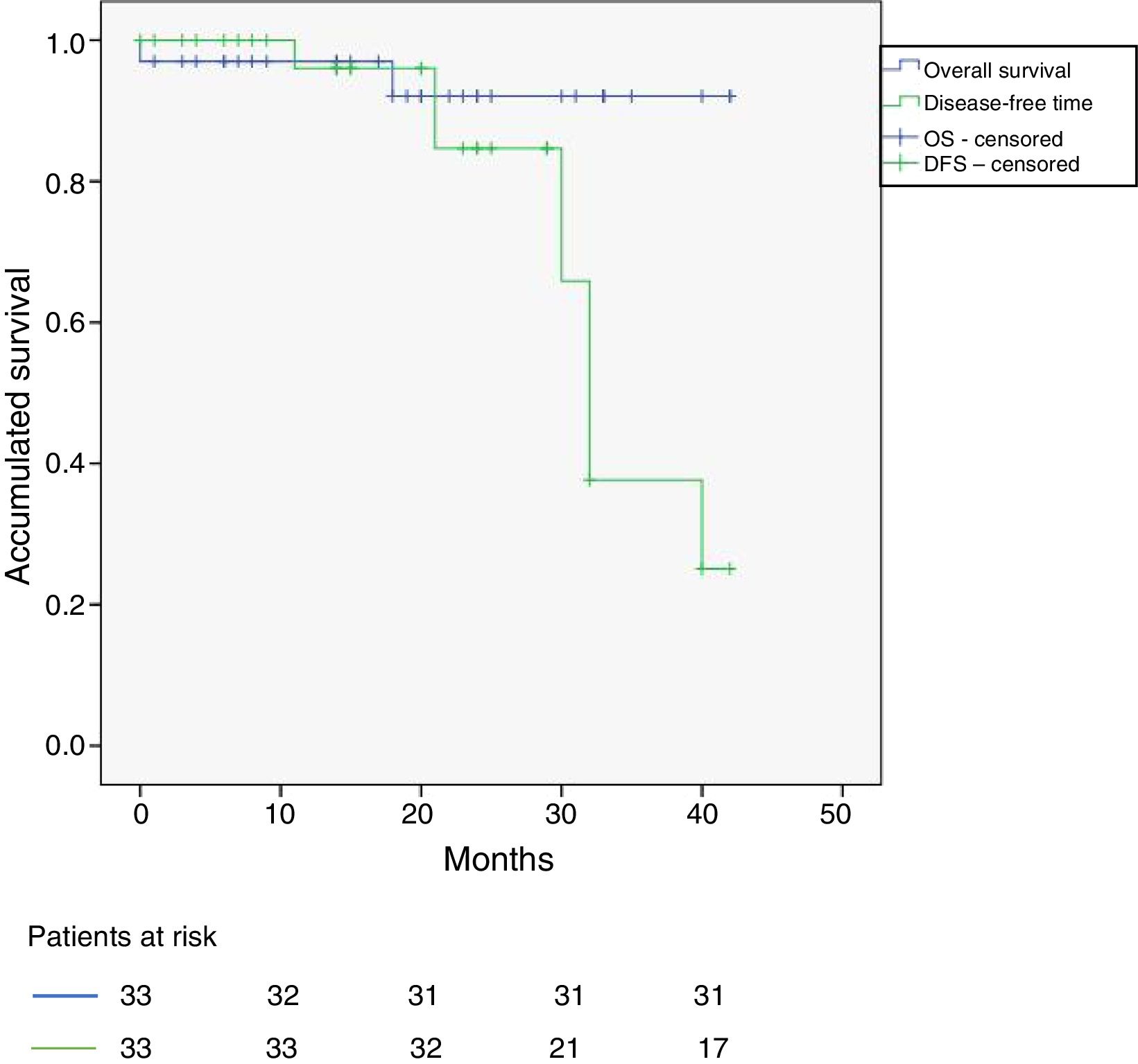
Overall survival (OS) and disease-free survival (DFS) of the 33 patients appear in Fig. 1. Three-year OS and DFS were 93% and 33%, respectively.
There were 6 recurrences at the time of the review (February 2016), 4 of them around 30 months of follow-up. Out of the 6 cases treated by laparoscopy, only one had presented peritoneal recurrence (the only PC+). None of the three patients with simultaneous resection of liver metastases had peritoneal or hepatic recurrence. The pT4 criterion was statistically significant (P=.037) as a predictor of post-SLS recurrence in the univariate study, but it lost significance in the Cox regression multivariate analysis (P=.068). In the PC+ group, 4 patients (40%) relapsed and one of them died due to the disease. The recurrence was in the peritoneum (2), locoregional/anastomosis (1) and liver (1). In the PC−, 2 patients (8.7%) relapsed and none died during follow-up. Recurrence was: peritoneum and locoregional/anastomosis (1) and peritoneum and uterus (1).
In short, 4 patients (12.1%) presented recurrence in the peritoneum: 2 in the PC+ group (20%) and 2 in the PC− group (8.7%) (P=.04).
DiscussionIn our series, SLS diagnosed PC in 30.3% of the patients with CRC with high risk with no previous clinical–radiological findings. The Elias12 group found PC findings in up to 56% of patients with more restrictive selection criteria, as they considered that there is not enough information to include pT4 or positive cytology. Other risk factors have been described for carcinomatosis.28,29 There is relevant information on the increased risk of peritoneal recurrence in tumors with positive cytology21,22 and in pT4,17–20 which is why they have been included in our study. There are several trials underway that explore the possibility of initially administering HIPEC in tumors staged preoperatively as cT4.
According to our results, the inclusion criteria have been selected successfully, since more than 30% of patients presented PC during SLS. But PC has only been detected in 13.3% of pT4; if we excluded them,16 the PC+ group would be 44.4%. The consideration of pT4 should still be quarantined.
In our study, patients with RSPD had a PC+ rate of 30% (60% in the Elias article16). We consider that this criterion should be incorporated as an inclusion criterion for SLS. The previous resection of ovarian metastases, which we considered separately but could be considered together with the RSPD subgroup, turned out to be the most reliable criterion (the two patients had undetected peritoneal relapse). These last two subgroups would be candidates for simultaneous HIPEC in the first surgery, provided that the peritoneal disease had been diagnosed preoperatively and in the context of a hospital with adequate infrastructure (with a HIPEC program).
Two of the four patients with a perforated tumor had PC in the SLS. The perforation in both cases was iatrogenic in the tumor area due to the placement of a stent, not due to colonic distension at a distance from the primary tumor. These latter patients should not be included for SLS.
Regarding positive cytology, one of our two patients had PC. Cytology is performed very infrequently, but it is an inexpensive, simple maneuver whose use should be generalized.
Although our intention was to perform the SLS+HIPEC around 4–6 months after the end of the adjuvant CTx in the absence of evidence of recurrence, patients were selected when presented in committee. The mean time transpired between surgery of the primary tumor and SLS in our series was 10.82 months, but some patients underwent surgery a little later (range 8–18), as long as they continued to meet the inclusion criteria. The approach was laparotomy in 25 patients and laparoscopy in 8 (the surgery of the primary tumor had also been laparoscopic and the peritoneal exploration during the SLS was satisfactory in 6, opting for conversion in the other two). It is accepted that CR+HIPEC by laparoscopy is possible and safe in strictly selected patients with limited peritoneal disease, being able to reduce postoperative complications. Although laparoscopy may underestimate the volume of peritoneal disease, this is less concerning in an abdomen without previous open surgery, this being our philosophy when selecting patients for laparoscopy and converting to open surgery in cases of minimal doubt.
The morbidity and mortality after CR+HIPEC correlates with the extension of the PC as well as the extension and complexity of the surgery. SLS is conducted in patients with no evidence of PC in the preoperative tests and, therefore, if PC is found, it is usually very limited (mean PCI 4.3 in our series) and requires limited resections with little morbidity. However, many patients require extensive adhesiolysis and closure of possible stomata that increase surgical risk. In no case have we found extensive disease. Our rate of major complications in PC− patients was very low (4.3%), while in PC+ patients it was similar to that of patients operated on for PC (40%). We consider both rates acceptable, although the existence of mortality forces us to try to fine-tune the indications.
Supported by the Elias study,30 in which patients with PC− who did not receive HIPEC had a peritoneal recurrence of 42%, we performed systematic HIPEC in all patients. Although the follow-up time was short (median 14.5 months), we obtained peritoneal recurrence rates of 8.69% in the PC− group and 12.12% in the overall series (data comparable with the Elias series, 6% and 17%, respectively).16 And while the 3-year OS of 93% is promising, our study provides a relatively low probability of DFS at 3 years (33%). Only 6 patients relapsed in the overall series, but 4 did so around the 3-year mark, which causes the DFS to fall after that point. The Elias16 series presents a high rate of extraperitoneal recurrence, with a 5-year DFS of 44%. Our relapses were mostly intra-abdominal (peritoneal, locoregional).
To ensure the benefit of this strategy, it is essential to carry out studies comparing it with the standard strategy (primary surgery+adjuvant CTx+follow-up). It will be interesting to see the results of the French multicenter study Prophylochip, which is currently recruiting patients.
In conclusion, SLS+HIPEC in our patient series provided early detection of PC and its treatment in 30.3% of cases, with a very low rate of peritoneal recurrence. However, this procedure entails an important morbidity rate, and even mortality. It is important to continue evaluating the results to increase the accuracy of the inclusion criteria, especially the pT4 criterion, which had a low predictive power for the appearance of PC in this series. This should only be done in perfectly controlled clinical studies in high-volume peritoneal carcinomatosis units to avoid the effects of the learning curve, especially in these indications that have not yet been established.
Authorship/CollaboratorsÁngel Serrano del Moral: design, analysis and interpretation, composition and critical review.
Estibalitz Pérez Viejo: data collection, study design.
Israel Manzanedo Romero: data collection, study design.
Gil Rodríguez Caravaca: interpretation of the results, critical review.
Fernando Pereira Pérez: interpretation of the results, critical review, approval of the final version.
Conflict of InterestThe authors have no conflict of interests to declare.
Please cite this article as: Serrano del Moral Á, Pérez Viejo E, Manzanedo Romero I, Rodríguez Caravaca G, Pereira Pérez F. Cirugía de second look más HIPEC en pacientes sin evidencia de recidiva con alto riesgo de desarrollar carcinomatosis tras resección de cáncer colorrectal. Cir Esp. 2018;96:96–101.
Part of the information provided in this manuscript was presented to the European Society of Coloproctology in Milan, September 2016, and the CNC Madrid, November 2016.