Transanal endoscopic microsurgery (TEM) was developed as a less aggressive alternative treatment for rectal lesions (mainly adenomas and adenocarcinomas). However, its use for other rectal lesions has become more frequent, trying to reduce the morbidity associated with more invasive techniques. The aim of this study is to describe our experience in the use of TEM in other rectal lesions.
MethodsRetrospective and descriptive study including patients operated with TEM (from June 2008 to December 2016) for the treatment of rectal lesions different from adenomas or adenocarcinomas.
ResultsAmong the 138 patients treated by TEM in our department, 10 patients were operated on for rectal lesions other than adenomas or adenocarcinomas. Rectal lesions were 3 neuroendocrine tumors, a neuroendocrine tumor metastasis, a rectal stenosis, a cloacogenic polyp, an endometrioma, a retrorrectal tumor, a presacral abscess and a lesion in the rectovaginal septum. Mean operative time was 72min and postoperative stay was 4.2 days. Only one patient needed a reoperation, due to rectal bleeding.
ConclusionsTEM could be a useful tool for the treatment of rectal lesions different from adenomas or adenocarcinomas, potentially decreasing the morbidity associated with more aggressive surgical techniques.
La microcirugía endoscópica transanal (TEM) se diseña como una alternativa menos agresiva en el tratamiento de lesiones rectales (principalmente adenomas y adenocarcinomas). Sin embargo, su uso se ha ampliado a otras lesiones rectales para intentar disminuir la morbilidad añadida a técnicas más invasivas. El objetivo de este estudio es mostrar nuestra experiencia en el uso de la TEM en el tratamiento de otras lesiones rectales, diferentes de adenomas y adenocarcinomas.
MétodosEstudio retrospectivo descriptivo en el que se incluyen pacientes intervenidos mediante TEM para el tratamiento de lesiones rectales (diferentes a adenomas o adenocarcinomas) desde junio de 2008 hasta diciembre de 2016.
ResultadosEntre los 138 pacientes operados mediante TEM en nuestro servicio, 10 fueron tratados por lesiones diferentes a adenomas o adenocarcinomas. Las lesiones rectales fueron 3 tumores neuroendocrinos primarios, una metástasis de tumor neuroendocrino, una estenosis rectal, un pólipo cloacogénico, un endometrioma, un tumor retrorrectal, un absceso presacro y una lesión sin filiar en tabique rectovaginal. El tiempo operatorio medio fue de 72min y la estancia postoperatoria de 4,2 días. Solo un paciente necesitó reintervención por rectorragia.
ConclusionesLa aplicación del TEM para el tratamiento de lesiones rectales diferentes a adenomas o adenocarcinomas puede ser una herramienta útil que potencialmente ayude a disminuir la morbilidad asociada a otros tipos de técnicas quirúrgicas más invasivas.
In the 1980s, Buess et al.1 described transanal endoscopic microsurgery (TEM) as an alternative in the treatment of certain rectal lesions. This technique enables rectal lesions to be resected up to 18–20cm from the anal margin. This is the maximum range of a specifically designed rectoscope that incorporates stereoscopic optics for three-dimensional viewing and a CO2 insufflation–aspiration system that maintains stable pneumorectum, providing optimal visualization of the rectal ampulla. Thus, TEM is a safe technique from an oncological and surgical standpoint, with limited morbidity (4%–24%) that is irrelevant in most cases.2
The key to TEM is correct patient selection. Presently, the indications of this technique are not limited to benign and early malignant rectal tumors susceptible to local treatment. The range of indications has been expanding beyond rectal tumors,3,4 and the technique has been used in the treatment of other types of lesions.
The objective of our study is to demonstrate our experience with TEM in the treatment of atypical rectal lesions (no rectal carcinomas or adenomas).
MethodsThis is a retrospective, descriptive study including a consecutive series of patients undergoing TEM for the treatment of rectal lesions from June 2008 to December 2016. The study was approved by the hospital Ethics Committee. The inclusion criteria for our study were atypical rectal lesions that were treatable by TEM. Excluded from the study were those lesions that are frequently treated with this technique (adenomas not treatable by colonoscopic resection, early neoplastic rectal lesions—T1N0M0—with good prognostic factors, neoplastic lesions at more advanced stages in selected patients—high surgical risk, refusal of radical surgery or stoma, and palliative intent).
Patients were studied in the coloproctology outpatient clinic using a complete patient medical history, rectal examination in the Sims position, rigid rectoscopy, colonoscopy, endoanal ultrasound, pelvic magnetic resonance or thoracoabdominal CT scan, depending on each case.
The procedures were carried out using TEM equipment (Wolf GmbH, Knittlingen, Germany). All patients underwent rectoscopy in the operating room prior to surgery and were placed so that the rectal lesion was located in the lower part of the rectoscope (when the lesion was posterior, the patient was placed in the lithotomy position; if it was on the anterior side of the rectum, the patient was placed in prone decubitus; and if lateral, in the corresponding lateral decubitus). Mechanical preparation was systematically performed in all patients, as well as the usual antibiotic and antithrombotic prophylaxis for colorectal surgery.
In the case of rectal tumors, the dissection was begun by marking with an electric scalpel resection margins ranging from 5 to 10mm, depending on the characteristics of the lesion. The resection was later conducted with an ultrasonic scalpel. Once the lesion had been resected, the area was irrigated with 1% povidone–iodine solution. The defect was closed (whenever possible) with a continuous suture fixed with silver clips. In case of surgical resection, the pathologist was systematically called in for intraoperative evaluation.
Patients initiated oral intake of fluids on the day of the intervention. The complications present in the postoperative period have been reported in accordance with the Clavien–Dindo classification.5
Statistical AnalysisFor the statistical analysis, SPSS software (version 20) was used. A descriptive analysis was completed of the study variables. For the qualitative variables, frequencies were calculated; for the quantitative variables, medians and ranges were calculated.
ResultsBetween June 2008 and December 2016, a total of 138 patients were treated with TEM, 47 of which had been referred from different hospitals in our autonomous community of Spain. The treated lesions included 48 (34.8%) adenocarcinomas and 80 (58%) rectal adenomas. Ten patients (7.2%) had a diagnosis of atypical rectal lesions (not carcinomas or adenomas) and were therefore included in the present analysis. Fifty percent were women, and mean age was 47 years (27–81).
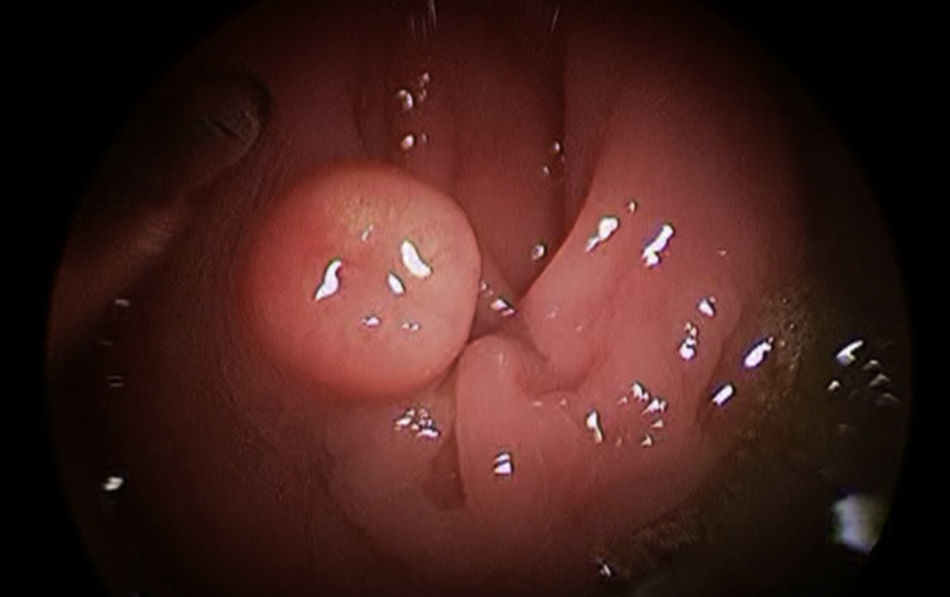
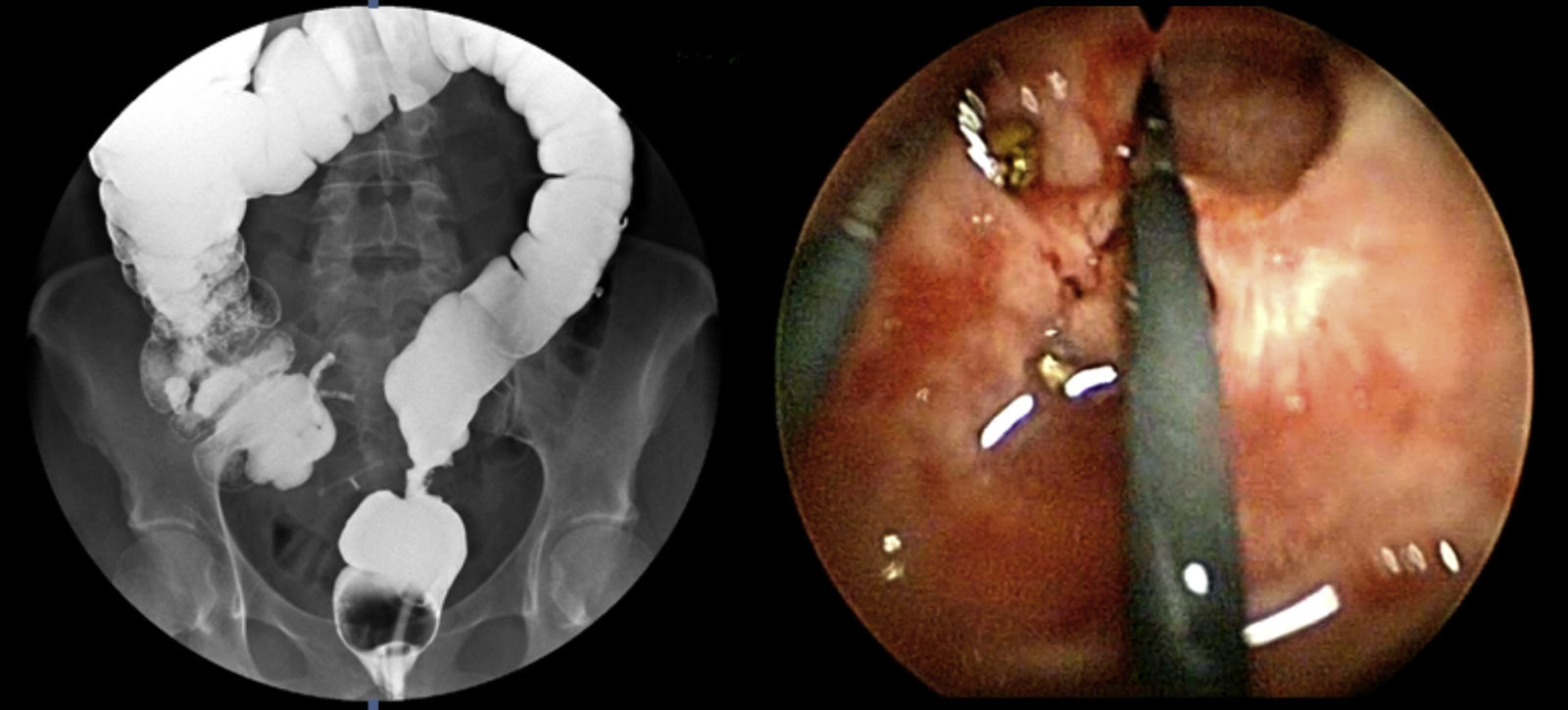
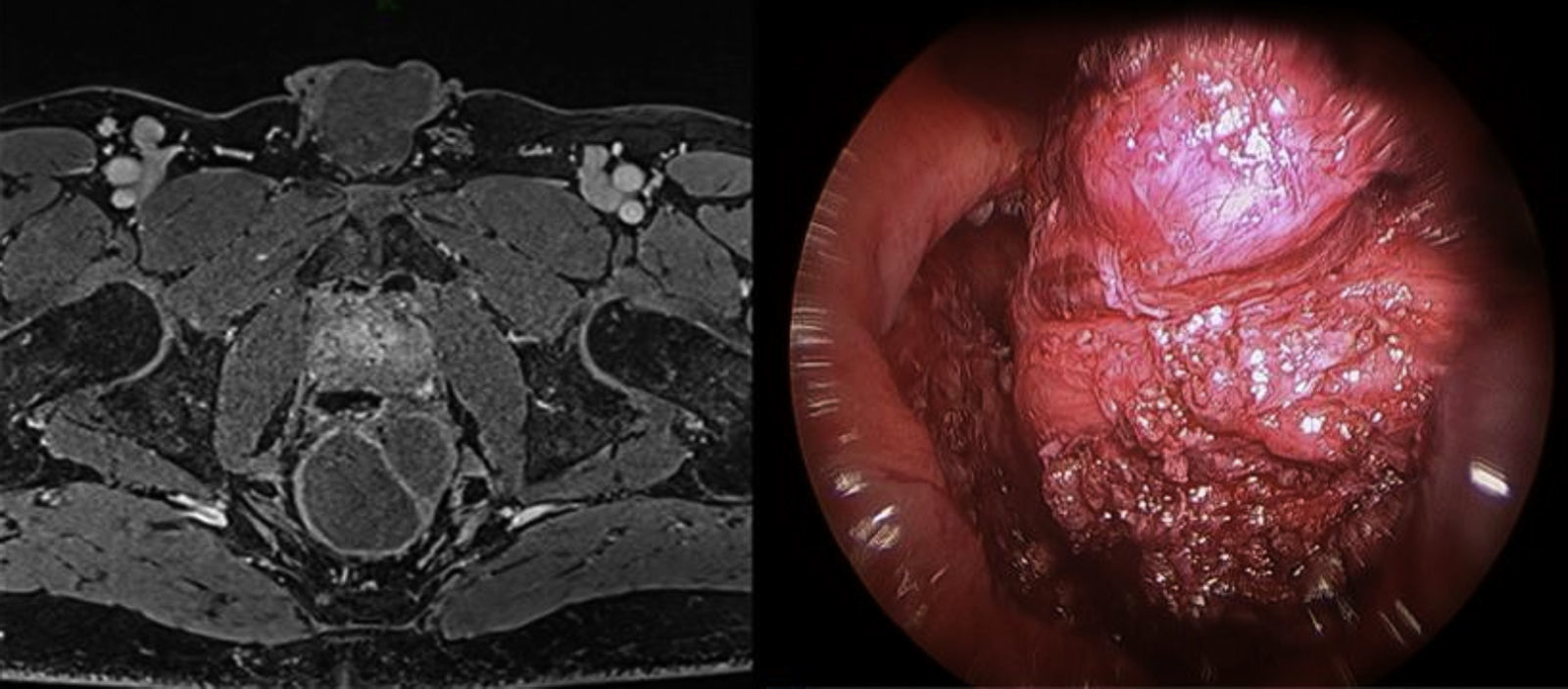
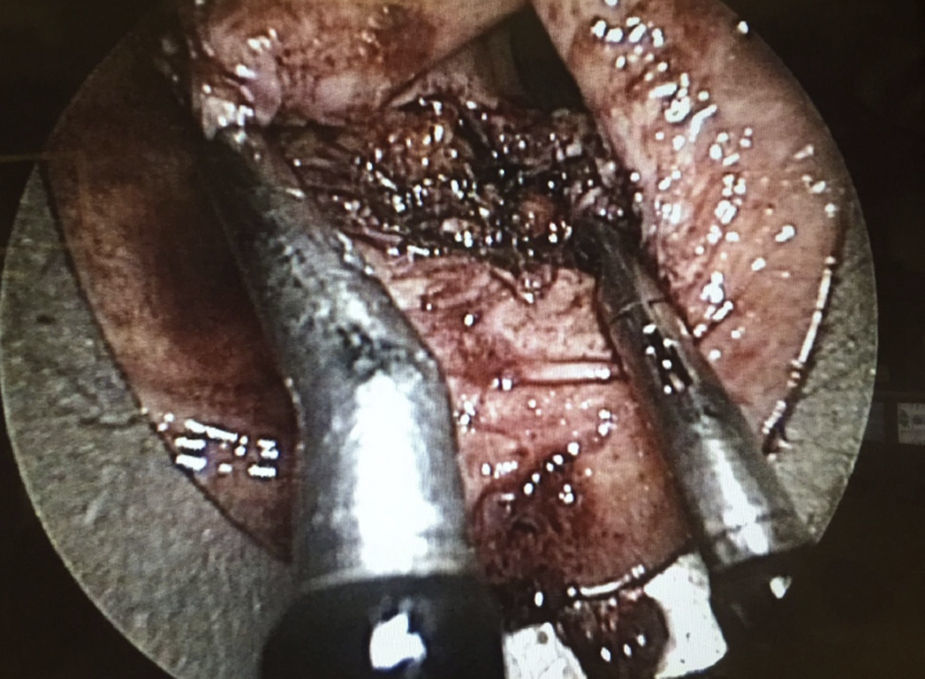
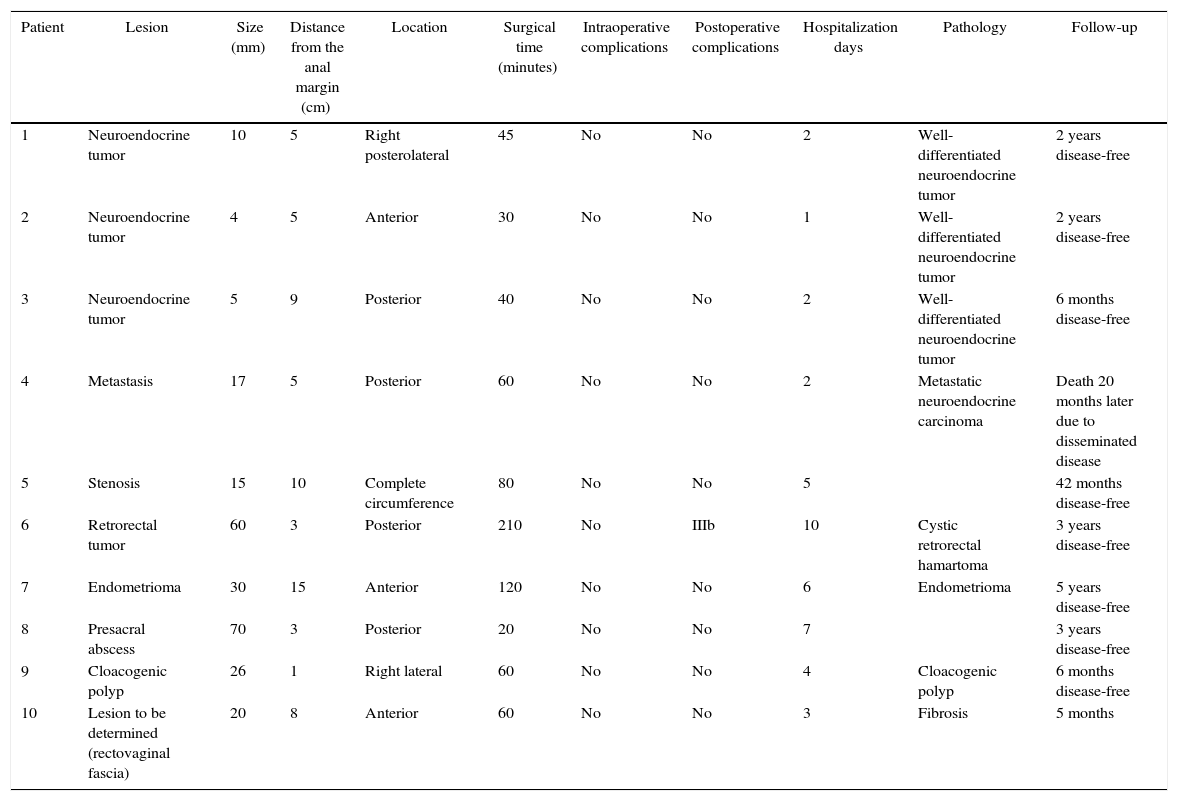
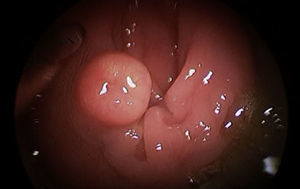
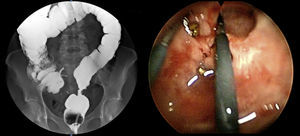
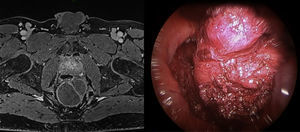
The preoperative diagnoses of these lesions included: 3 primary neuroendocrine tumors (Fig. 1), one neuroendocrine tumor retrorectal metastasis, one anastomotic stenosis (Fig. 2), one retrorectal tumor (Fig. 3), one endometrioma, one presacral abscess, one cloacogenic polyp, and one unidentified lesion in the rectovaginal septum (Fig. 4). The characteristics are shown in Table 1.
Intra- and Postoperative Characteristics of the Cases.
| Patient | Lesion | Size (mm) | Distance from the anal margin (cm) | Location | Surgical time (minutes) | Intraoperative complications | Postoperative complications | Hospitalization days | Pathology | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Neuroendocrine tumor | 10 | 5 | Right posterolateral | 45 | No | No | 2 | Well-differentiated neuroendocrine tumor | 2 years disease-free |
| 2 | Neuroendocrine tumor | 4 | 5 | Anterior | 30 | No | No | 1 | Well-differentiated neuroendocrine tumor | 2 years disease-free |
| 3 | Neuroendocrine tumor | 5 | 9 | Posterior | 40 | No | No | 2 | Well-differentiated neuroendocrine tumor | 6 months disease-free |
| 4 | Metastasis | 17 | 5 | Posterior | 60 | No | No | 2 | Metastatic neuroendocrine carcinoma | Death 20 months later due to disseminated disease |
| 5 | Stenosis | 15 | 10 | Complete circumference | 80 | No | No | 5 | 42 months disease-free | |
| 6 | Retrorectal tumor | 60 | 3 | Posterior | 210 | No | IIIb | 10 | Cystic retrorectal hamartoma | 3 years disease-free |
| 7 | Endometrioma | 30 | 15 | Anterior | 120 | No | No | 6 | Endometrioma | 5 years disease-free |
| 8 | Presacral abscess | 70 | 3 | Posterior | 20 | No | No | 7 | 3 years disease-free | |
| 9 | Cloacogenic polyp | 26 | 1 | Right lateral | 60 | No | No | 4 | Cloacogenic polyp | 6 months disease-free |
| 10 | Lesion to be determined (rectovaginal fascia) | 20 | 8 | Anterior | 60 | No | No | 3 | Fibrosis | 5 months |
aClassified according to Clavien–Dindo.
The mean size of the lesions described was 23.1mm (4–70mm), with a mean distance from the anal margin of 6.1cm (1–15cm). The locations of the lesions were as follows: anterior (3 cases), posterolateral (one case), lateral (one case), posterior (4 cases) and complete circumferential (one case).
Mean surgical time was 72min (30–210), and there were no intraoperative complications. During the dissection of a rectal endometrioma involving complete excision of the wall lesion, the peritoneal cavity was accessed in a controlled manner, and the defect was sutured.
In the postoperative period, one patient had rectal bleeding that required revision surgery (grade IIIc complication according to Clavien–Dindo classification) and hemostasis. The patient had undergone surgery for a retrorectal cyst with an inverted T transmural posterior longitudinal incision in the distal third of the rectum, with subsequent partial continuous suturing of the incision and placement of a drain tube in the cavity.
Mean hospital stay was 4.2 days (1–10). No late postoperative complications have been observed. Mean patient follow-up was 26 months (5–60): all patients were disease-free, except for one patient who died 20 months after the intervention due to disseminated cancer (stage IV).
DiscussionTEM is a minimally invasive alternative in the treatment of rectal tumors. Traditionally, these tumors have required an abdominal approach using sphincter-preserving surgery (low or ultra-low anterior resection) or abdominoperineal resection. These radical interventions, in addition to occasionally requiring a stoma (permanent or temporary), entailed significant morbidity/mortality and a considerable rate of complications, including genitourinary alterations, sexual dysfunction and defecatory disorders.6–8
The indications for TEM for the treatment of rectal lesions include: (a) surgical treatment of benign rectal tumors not treatable with endoscopic resection; (b) early-stage malignant tumors (T1N0) with criteria for a good prognosis; (c) after neoadjuvant therapy in highly selected cases or in the context of a controlled clinical trial; and (d) palliative treatment in patients at more advanced stages, with high surgical risk, or who refuse radical surgery.9–11 In addition to these indications, in recent years different authors have advocated TEM in the treatment of other rectal lesions, which has extended the indications for its use to other types of atypical or rare diseases.12
Treatment of benign stenosis may involve different procedures: endoscopic dilations, stent placement, surgical resection, transanal strictureplasty, etc. This diversity of procedures indicates that there is no ideal method for all stenoses. Repeated endoscopic dilatations are non-invasive but often ineffective, especially due to recurrences.13 An effective alternative to abdominal resection of the anastomotic area is the use of TEM14 by means of the longitudinal incision of the fibrotic tissue. This technique is a less invasive method which, furthermore, may be of choice in especially low anastomotic stenoses. Thus, Baatrup et al.14 presented satisfactory results in 5 out of 6 TEM-operated patients. Serra-Aracil et al.12 demonstrated 2 cases with significant improvements after the endoanal approach. This type of technique usually requires sutures, given the high possibility of perforation of the cavity.
The appearance of carcinoid tumors in the gastrointestinal tract is uncommon; the rectum is its third most frequent location, with a less aggressive nature. As a consequence of screening methods, the early detection rate has increased significantly in recent years, with an incidence between 2% and 4.5%.15,16 Therapeutic recommendations remain controversial, although local excision is advocated in lesions smaller than 1cm and from 1 to 2cm without atypical histopathological characteristics (muscularis propia, lymphatic, vascular or perineural invasion; anaplasia; presence of multiple mitoses; cellular pleomorphism; or mucin production).17 The largest series published in the United States (24 patients treated by TEM) concludes that it is a safe and effective procedure as long as the resection margins are negative.18 Kinoshita et al.19 included 27 patients who presented no recurrences for 70 months. Ortenzi et al.20 describe a series of 21 cases with good results and advocate its use over other endoscopic techniques. Careful pathology examination of the resected specimen is required to evaluate the size, depth of invasion, status of the margins and presence of risk factors for metastasis. Hence, our insistence on joint intraoperative evaluation between pathologist and surgeon once the surgical specimen has been resected. In our opinion and that of other authors, tumors with muscularis propia, lymphatic or vascular invasion, or an increased rate of cell proliferation (such as Ki-67), should be treated by rescue surgical resection, although other publications advocate very close monitoring.21
Retrorectal tumors are a rare entity whose origin in two-thirds of cases is congenital. Most cases are asymptomatic, especially if benign, and are discovered incidentally during physical examination. The use of diagnostic biopsy is contraindicated. The most frequently used imaging technique is pelvic MRI, which indicates the location of the lesion and its relation to adjacent structures. Treatment is complete surgical excision in order to avoid the risk of relapse, infection and the rare—but possible—malignant transformation. Traditionally, access to the retrorectal space has been accomplished through an abdominal or posterior approach, or a combination of both. Different authors22,3 have defended the use of TEM in the treatment of retrorectal tumors as a safe and effective option, which avoids more aggressive procedures such as the Kraske technique. In this context, Zoller et al.22 described a series of 3 patients with caudal cysts that showed good results after transanal excision.
The rectum may sometimes be affected by extra-pelvic endometriosis, which accounts for 15% of intestinal presentations.23 As a benign entity, intensive treatment and its consequent complications are not systematically accepted in young women. There are two possible approaches: radical surgery with colorectal resection, or a less aggressive treatment based on local resection.23–25 Ortenzi et al.20 reported 6 patients treated surgically by TEM, with one patient who presented involvement of the surgical margins, and no postoperative recurrences.
Drainage of pelvic or perirectal abscesses above the levator ani may be another therapeutic option for TEM, described by Serra-Aracil et al., when the transanal approach or interventional radiology is not possible.12
In addition to the cases presented in our study, other atypical indications for the treatment of rectal lesions have been described in the literature, widening the therapeutic spectrum. Sharma et al.26 presented the first reported case of rectal amyloidoma with obstruction, treated by TEM with satisfactory results. Excision of condylomata in the anal canal extending into the rectal ampulla has been described by different authors.12,20 Between 5% and 10% of GIST tumors are located in the rectum and show 2 characteristics that make them suitable lesions for treatment by TEM: the low percentage of metastases in locoregional lymph nodes and their tendency to protrude into the lumen. Lesions smaller than 2cm with a low mitotic index are considered to have a good prognosis and, therefore, local resection is the treatment of choice.12,20 Several cases of rectourethral fistulas repaired by TEM have been reported with satisfactory results.12,27 Sporadically, other indications have been described, including rectal prolapses, rectal melanomas, rectovaginal fistulas, rectal duplications, as well as the removal of fecalomas and foreign bodies.12,4,28
The main limitation of our study is based on the limited number of patients included in the series with atypical indications. It is therefore difficult to obtain solid conclusions.
In short, the application of TEM for the treatment of atypical rectal lesions can be a useful tool that could potentially help reduce the morbidity associated with other more radical types of surgery. In any event, the success of this technique is based on proper patient selection through detailed study, thereby facilitating suitable therapeutic indications and treatment by experienced surgeons.
Authorship/CollaborationsManuel Ferrer-Márquez: study design, analysis and interpretation of the results, article composition, critical review and approval of the final version.
Francisco Rubio-Gil: data collection, analysis and interpretation of the results, critical review.
Sofía Ortega Ruiz: data collection, analysis and interpretation of the results, critical review.
Antonio Álvarez-García: critical review and approval of the final version.
Jaime Jorge-Cerrudo: critical review and approval of the final version.
Elisabet Vidaña-Márquez: critical review and approval of the final version.
Ricardo Belda-Lozano: critical review and approval of the final version.
Ángel Reina Duarte: article composition and critical review.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Ferrer-Márquez M, Rubio-Gil F, Ortega-Ruiz S, Blesa-Sierra I, Álvarez-García A, Jorge-Cerrudo J, et al. Microcirugía endoscópica transanal en el tratamiento de lesiones rectales atípicas. Cir Esp. 2017;95:335–341.