In pancreatic ductal adenocarcinoma (PDA), surgical resection is the only curative treatment, but due to its late clinical presentation only 15–25% patients are candidates for curative resection. The aim of this prospective, single-center study is to determine the diagnostic utility of preoperative PET-CT for early detection of PDA and early panIN lesions.
MethodsWe studied the histopathological features of PDA and different panIN lesions in 139 surgical samples from patients undergoing pancreatic resection (from 2010 to 2014), comparing these results with preoperative PET-CT and MDCT study. For tumor diagnosis in PET-CT maximum standard SUV 2.5 was used. Pancreatic baseline SUVmax is the maximum uptake of the radiotracer 18-2FDG on the ROI curve determined for the area of the normal pancreas after pathological reassessment with areas not affected by tumors or preneoplastic lesions. Tumor Uptake Index is the ratio between the tumor SUVmax and pancreatic baseline SUVmax.
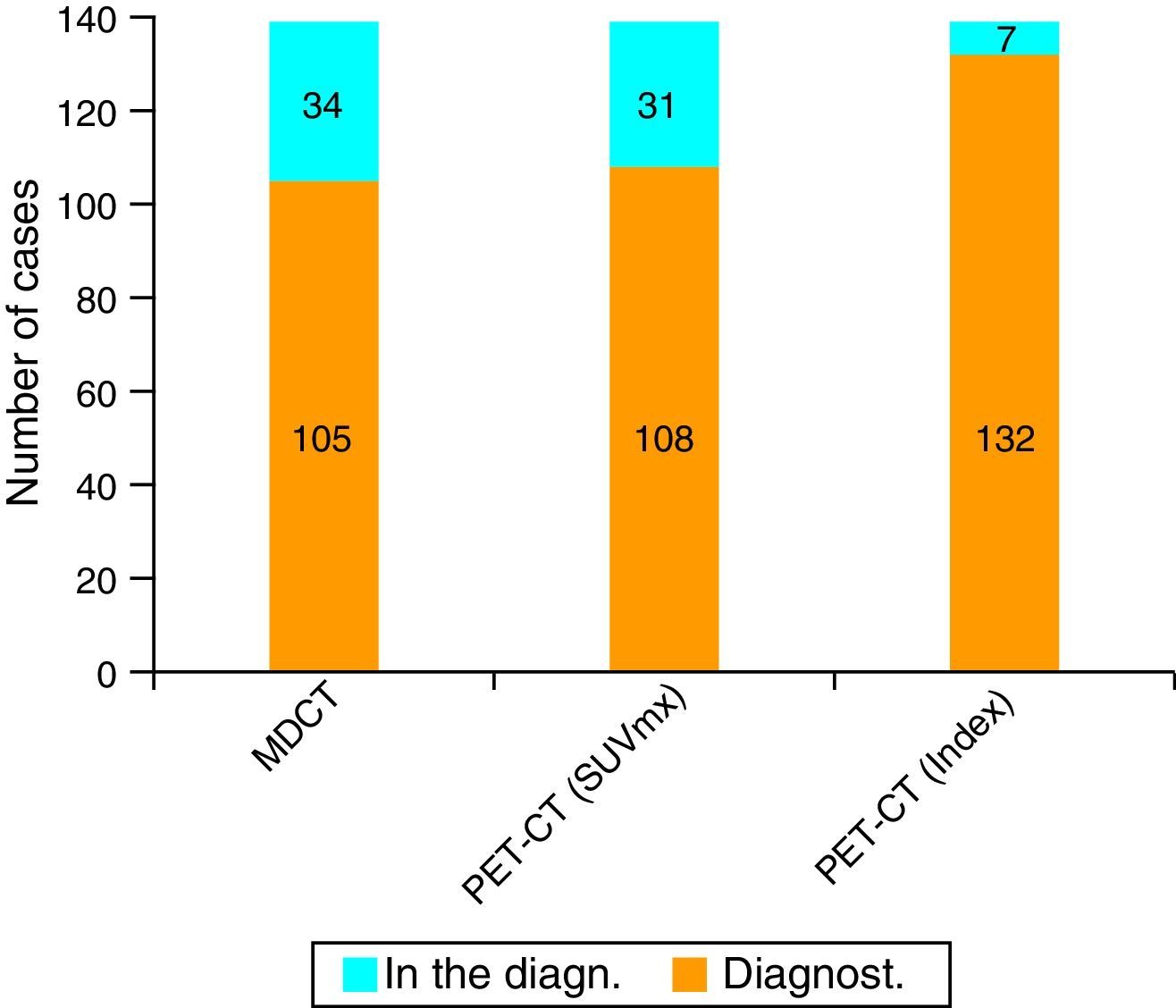
ResultsUsing an standard maximum SUV value of 2.5, PET-CT sensitivity was 77.7% (108 of the 139 cases) against 75.5% (105 of the 139 cases) of MDCT. But when we combined this value with maximum SUV of normal pancreatic tissue from each patient, PET-CT sensitivity improved its value to 94.9%.
ConclusionA combination of studies of PET-CT in tumor and non-tumor tissue of each patient might be a very useful diagnostic tool not only for preoperative diagnosis of PDA, but also for early panIN lesions.
El único tratamiento curativo del cáncer de páncreas (CP) es la exéresis quirúrgica, pero debido a su presentación clínica tardía solo el 15-25% de los pacientes son candidatos a resección curativa. El objetivo de este trabajo, prospectivo y unicéntrico, es determinar la utilidad de la PET-TC preoperatoria en el diagnóstico precoz del CP, en su estadificación y en la detección de estadios precursores de la enfermedad en una serie de 139 pacientes sometidos a intervención quirúrgica con «intención curativa» y con el diagnóstico histológico de adenocarcinoma ductal.
MétodosHemos estudiado las características histopatológicas del CP y de las diferentes lesiones panIN en las piezas quirúrgicas de 139 pacientes sometidos a resección pancreática durante el periodo 2010-2014, comparando estos resultados con los datos preoperatorios de una tomografía computarizada multidetector con contraste trifásico (TCMD) y una PET-TC en la que la captación de glucosa fue determinada por el SUV, considerando malignidad por encima de 2,5.
ResultadosEn nuestra serie, la sensibilidad de la PET-TC para el diagnóstico tumoral fue del 77,7% (108 de los 139 casos) versus el 75,5% (105 de los 139 casos) para la TCMD. Cuando combinamos este valor máximo del SUV tumoral con el SUV máximo de tejido pancreático normal de cada paciente, la sensibilidad diagnóstica de la PET-TC para el CP asciende al 94,9% (132 de los 139 casos).
ConclusiónUna combinación de los estudios del PET-TC en el tejido tumoral y no tumoral de cada paciente puede ser una herramienta diagnóstica muy útil no solo para el diagnóstico preoperatorio del CP, sino también para las lesiones panIN.
The only curative treatment for pancreatic cancer (PC) is surgical removal.1–7 The 5-year survival rate is less than 5%, although it reaches 30% in patients who undergo surgery with curative intent associated with adjuvant radiotherapy and chemotherapy.8–10
Today, PC survival rates may be able to be improved if early diagnosis is reached with precise tumor staging, in addition to the detection of precursor lesions of PC, known as pancreatic intraepithelial neoplasms (panIN).11–15 Some authors16–22 suggest that 18-fluorodeoxyglucose in positron emission tomography-computed tomography (PET-CT) could provide benefits in this area. Furthermore, in experimental models it has been observed that PET may be useful for the detection of precursor lesions of PC, although its utility has never been evaluated in humans.23
The objective of our study is to determine the utility of preoperative PET-CT in the early diagnosis of PC, its staging and the detection of precursor stages of the disease in a series of 139 patients who underwent surgery with curative intent and had a histological diagnosis of ductal adenocarcinoma.
MethodsPatientsAt our hospital, 139 prospective patients diagnosed with PC underwent pancreatic resection with curative intent in the study period from 2010 to 2014. Mean age was 60.6±12.9years (range: 37–79 years); 88 patients (63.3%) were male and the remaining 51 (36.7%) were female. The variables studied included: tumor location and tumor size, histologic grade, TNM stage, lymphovascular invasion, perineural invasion, lymph node metastases, presence of panIN lesions, maximum tumor SUV, maximum SUV for panIN and maximum baseline SUV of the pancreas.
MethodsResectability and preoperative staging were evaluated by three-phase, contrast-enhanced, multidetector CT (MDCT) and a PET-CT in which the glucose uptake was determined by SUV, considering malignancy above 2.5. In our series, in 103 cases (74.1%) pancreaticoduodenectomy was performed; in 26 cases (18.7%), distal pancreatectomy was done, while the remaining 10 patients underwent total pancreaticoduodenectomy.
The precision for staging PC of both PET-CT and MDCT was calculated based on intraoperative findings and pathology reports for each patient. All the surgical specimens were studied by 2 pathologists specialized in pancreatic pathologies. In all cases, the histologic type was ductal adenocarcinoma. Staging was done in accordance with the 7th Edition of the TNM Classification of the AJCC/UICC.24,25
Definition of Concepts- •
SUV: standard uptake value (SUV) is the most extensively used semiquantitative index that measures the uptake of FDG by the lesion (MBq/cc) according to the injected dose corrected for the weight of the patient (MBq/kg).
- •
Maximum tumor SUV is the maximum uptake of 18-2FDG (2-deoxy-2-(18F) fluoro-d-glucose) radioactive tracer in the ROI curve determined by the tumor area being studied. A maximum SUV≥2.5 is considered pathologic.
- •
Maximum SUV of panIN is the maximum uptake of radioactive tracer 18-2FDG in the ROI curve determined for the area with histopathological lesions compatible with panIN lesions reevaluated after the histopathological analysis of the surgical specimen.
- •
Maximum baseline SUV of the pancreas is the maximum uptake of radioactive tracer 18-2FDG in the ROI curve determined for the area of the normal pancreas after being reevaluated pathologically with areas unaffected by the tumor or by preneoplastic lesions.
- •
Tumor uptake rate (TUR) is the quotient between maximum tumor SUV and the maximum baseline SUV of the pancreas.
The sensitivity of CT for the detection of PC was calculated according to the histological results of the resected specimen. The McNemar test was used to compare the detection sensitivity of PET-CT vs MDCT. The statistical analysis was calculated with SPSS software version 20.0 (SPSS Inc., Chicago, IL), Fisher's exact test and Spearman's correlation coefficient. A P value <.05 was considered statistically significant.
ResultsHistopathological Characteristics of our SeriesIn the histology study (AP) of the surgical specimens, the most frequent tumor location was the head of the pancreas (113 out of 139, which is 81.3% of cases), while the remaining 26 cases were located in the body and tail of the pancreas (18.7% of cases). The head of the pancreas was also the most frequently reported location with the other techniques, with percentages in CT and in PET-CT of 75.5% (105 out of 139 patients) and 77.7% (108 of 139 patients), respectively. Analyzing these results, there is a statistically significant (SS) correlation between tumor location in the AP with the tumor location on CT (P=.01) and PET-CT (P=.02).
In the AP, mean tumor size was 2.7±0.75cm (range: 1.5–4cm). Mean tumor size on CT was 2.5±1.25cm and on PET-CT 2.6±0.87cm. When these results are analyzed, there is a SS correlation between the tumor size in the AP with the tumor size on CT (P=.01) and PET-CT (P=.02).
With regards to histological grade, in 29 patients a well-differentiated tumor was observed (20.9%); in 103 patients (74.1%), the tumor was moderately differentiated; and in the 7 remaining patients (5.1%), the tumor was poorly differentiated. In our series, in 54 out of 139 cases (38.8%) and in 76 out of 139 cases (54.6%), there were lymphovascular and perineural invasions, respectively.
As for TNM classification, 24.4% (34 patients) were T1, 36% (50 cases) were T2 and the remaining 39.6% (55 cases) were T3. Also, 21.6% of the patients had no lymph node metastatic invasion (30 cases), while the remaining 78.4% (109 cases) were N1. As for stage, 37.4% (52 patients) were classified as a stage less than or equal to IIA and the remaining 62.6% (87 patients) were stage IIB.
Lastly, in 109 of the 139 patients (78.4%), metastatic lymphadenopathies were observed in the surgical specimen. Nonetheless, preoperatively only 75 patients (53.9%) had suspected lymph node metastasis on MDCT, and only 10 patients (7.2%) on PET-CT, which demonstrated the limited sensitivity of PET-CT. In our series, there was no SS correlation between the presence or absence of lymph node metastases and their detection on MDCT or PET-CT.
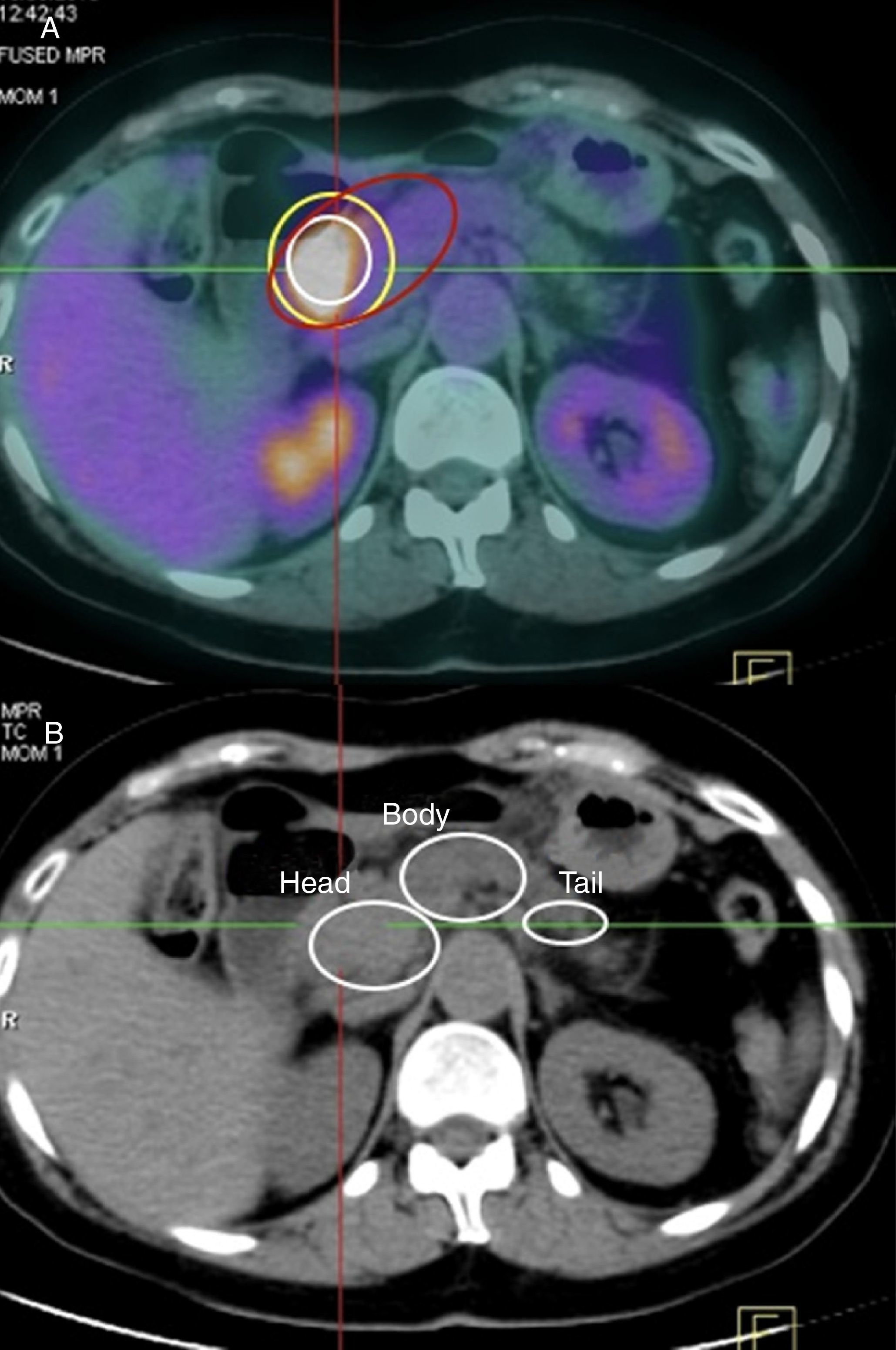
Maximum Standardized Uptake Value of the Normal Pancreas, Pancreatic Tumor and Intraepithelial Pancreatic NeoplasmsMost authors consider pathological a maximum SUV≥2.5. Nonetheless, as there is great interindividual uptake variability in healthy pancreatic tissue on PET-CT, our series has measured maximum baseline SUV of the pancreas for each of the patients, with a median value of 1.28. In addition, for each patient the PET-CT findings correlated pathologically with areas that were unaffected by the tumor or by preneoplastic lesions. In our series, the median maximum tumor SUV was 3.29.
In the AP study, 68.3% of the patients (95 out of 139 cases) presented preneoplastic lesions, and 30 cases (21.6%) had grade 3 panIN lesions. The most frequent location was the head of the pancreas (53 cases), which represents 38.1% of the cases studied. Furthermore we found that 45 cases (32.6%) presented with multicenter panIN lesions in the surgical specimen. The median maximum SUV reached in the grade 3 panIN lesions was 2.8; in grade 2 panIN, it was 1.95; and in grade 1 panIN, 1.76.
Because there is much interindividual baseline and tumor uptake, in our series we have calculated an index between maximum tumor SUV and maximum baseline SUV, which enables us to establish as the lower limit for suspicion of a pancreatic cancer a value of 1.33. Meanwhile, in the study of the panIN lesions, the index or coefficient for maximum SUV of panIN versus the maximum baseline SUV of the pancreas was 1.03. Upon analyzing our series, 95% of the patients (132 cases) presented a maximum tumor SUV/maximum baseline SUV index greater than 1.33.
Validity of MDCT and PET-CT for the Diagnosis and Staging of Pancreatic CancerThe sensitivity of MDCT for the diagnosis of PC was 75.5% (105 out of 139 cases). In turn, the sensitivity of PET-CT was 77.7% (108 cases), using as a criterion for suspecting malignancy a maximum tumor SUV of 2.5. However, when we used the maximum tumor SUV/baseline SUV (uptake rate) coefficient with a cut-off point of 1.33, the sensitivity rose to 94.9% (132 out of 139 patients) (Figs. 1 and 2).
In spite of surgery with curative intent, many patients with PC develop tumor recurrence within 6–15months after surgery, which is related with the high biological aggressiveness of the tumor and extra-pancreatic tumor extension that is not detected during surgery.9 Moreover, in the natural history of ductal adenocarcinoma, thanks to molecular biology, there have been reports of precursor lesions, known as panIN,,11–14 in which the normal ductal epithelium would evolve toward an epithelium with metaplasia (panIN grades 1A and 1B), afterwards with mild or low-grade dysplasia (grade 2 panIN) and, progressively, to severe or high-grade dysplasia (grade 3 panIN) and, lastly, to invasive carcinoma with simultaneous accumulation of successive genetic mutations. For these reasons, the expectation is that the results of PC treatment can be improved with early diagnosis and precise tumor staging, in addition to the detection of panIN lesions.15 This is usually insufficiently assessed with conventional imaging techniques and, therefore, our intention is to analyze the usefulness of PET-CT in the early diagnosis of PC.
In our study, distribution by sex, mean age, tumor size, most frequent tumor location and mean maximum SUV coincide with the descriptions found in the bibliographic references we consulted.26–28
Currently, the utility of PET-CT is still not clear in the diagnosis and staging of PC.28,29 Its sensitivity for the initial diagnosis ranges from 73 to 94%, while its specificity drops to 60%–89%, which is related with the possible appearance of false positives that occur in inflammatory processes like pancreatitis, infected pseudocysts and prosthetic materials, etc.
Moreover, several studies30–32 have compared the results of PET with those of CT and MRI, publishing contradictory results as to whether the former provides additional information over other techniques. Other authors33,34 consider that the association of PET with MDCT is able to achieve better spatial-anatomic resolution, although their use with intravenous contrast is not widespread, thereby losing sensitivity for the diagnosis of small tumors and metastasis.35
In 2011, Fendrich et al.23 published a study in which they had conducted an experimental model for detecting precursor lesions of PC in mutated K-RAS rats, which developed panIN lesions that evolved into invasive PC. This study demonstrated that there was overexpression of GLUT-2 in panIN lesions, starting with type 1B and in more advanced stages, which was associated with an increase in the transport and intracellular accumulation of FDG, observed on PET-CT. Supported by the results of these authors,23 we performed this study on patients with suspected PC, and for that reason preoperative MDCT and PET-CT were ordered in all cases. In our study, the sensitivity of MDCT for the diagnosis of PC was 75.5%, while for PET-CT it was 77.7%. Likewise, no SS differences were obtained in TNM classification. In our series the median maximum SUV was 3.29; the mean was not used as a positioning measurement because the series presented quite disparate values ranging from 1.18 to 12.73. In our study, we observed that if we used a PET-CT cut-off point to suspect malignancy at a maximum SUV of 2.5 (as used by several authors),18–21 we lost 23.8% of the patients with PC during the preoperative diagnosis. To resolve this problem, based on the large interindividual uptake variability of FDG in healthy pancreatic tissue (baseline) and in the presence of false positives and negatives depending on instrumentation bias, for each individual we measured the tumor uptake rate (TUR). Using this index, in our series we can confirm that 95% of the patients with PC presented a TUR higher than 1.33. The sensitivity of PET-CT in the diagnosis of TUR obtained with this new index was 94.9%, compared to 76.2% that was obtained when using maximum SUV greater than or equal to 2.5 as a diagnostic parameter.
In our series, 68.3% of the patients presented panIN lesions. We retrospectively evaluated the translation of the anatomical areas affected by these lesions in terms of the uptake of baseline FDG of the pancreas on PET-CT, obtaining statistically significant differences in the uptake of FDG versus healthy pancreatic tissue for panIN grade 1, grade 2 and grade 3, respectively. In our series, the TUR utilized for the diagnosis of the panIN lesions was 1.03, so we were able to diagnose 95% of the patients affected by said lesions. Perhaps with the utilization of TUR we can detect multicentric panIN grade 2 and grade 3 lesions in the body and tail of the pancreas, which would require total pancreatectomies. Likewise, it could be used in patient groups at high risk for developing PC, such as hereditary chronic pancreatitis, Gardner syndrome, Peutz-Jeghers syndrome, etc.
To conclude, in our experience PET-CT is a valuable tool for the preoperative diagnosis of PC and preneoplastic panIN lesions, although the analysis should be individualized and very meticulous in each patient studied. The use of TUR could be useful for diagnosis in patients with suspected malignancy and unclear imaging studies (MDCT).
AuthorshipStudy design: Francisco Sánchez Bueno, Rocío García-Pérez, María Antonia Claver Valderas and Pascual Parrilla Paricio.
Data collection: Francisco Sánchez-Bueno, Rocío García-Pérez, Laura Frutos Esteban and Matilde Fuster Quiñonero.
Analysis and interpretation of the results: Francisco Sánchez Bueno, Rocío García-Pérez, María Antonia Claver Valderas, Jesús de la Peña Moral and Matilde Fuster Quiñonero.
Article composition: Francisco Sánchez Bueno, Rocío García-Pérez, Eduardo Ortiz Ruiz and Laura Frutos Esteban.
Critical review and approval of the final version: Francisco Sánchez-Bueno, Rocío García-Pérez and Pascual Parrilla Paricio.
Conflict of InterestsThe authors have no conflicts of interest to declare.
Please cite this article as: Sánchez-Bueno F, García-Pérez R, Claver Valderas MA, de la Peña Moral J, Frutos Esteban L, Ortiz Ruiz E, et al. Utilidad de la 18-fluorodeoxiglucosa en la tomografía por emisión de positrones-tomografía computarizada (PET-TC) preoperatoria en el diagnóstico precoz del cáncer de páncreas exocrino: estudio en 139 casos resecados. Cir Esp. 2016;94:511–517.