The use of statins and medicated stents (MS) is the best available therapy for the treatment of severe coronary disease in selected cases. However, the vascular effects of the simultaneous use of both therapies are unknown.
Materials and methodsAn experimental study was carried out on 60 NZ rabbits with advanced atherosclerosis, distributed in four groups of 15 animals each. Group 1: Control. Group 2: paclitaxel-eluting stent (PES) in the thoracic aorta. Group 3: Atorvastatin 2.5mg/day po+PES implant, and Group 4: Atorvastatin 2.5mg/day po. They were followed up at 30, 60 and 90 days. Histo-morphometric analyses were carried out.
ResultsA total of 60 PES were successfully implanted. One animal from Group 3 died due to respiratory infection. PES increased the lumen diameter and area, as well as the vessel area; atorvastatin induced a potent plaque regression. In the PES group, the lumen diameter was 4.25±0.0mm, lumen area was 14.2±0.4mm2, vessel area was 16.7±0.0mm2, and plaque/media area ratio was 0.1±0.0. In the PES+atorvastatin group the measurements were 4.9±0.1mm (p<0.001), 18.6±0.8mm2 (p=0.005), 21.6±0.9mm2 (p=0.007) and 0.8±0.08 (p=0.032), respectively.
ConclusionsOur results confirm the potent synergistic mechanical effect of the PES and plaque regression of the statins in an animal model with advanced atherosclerosis.
El uso de estatinas y stents farmaco-activos (SF) son la mejor terapia disponible para el tratamiento de la enfermedad arterial coronaria severa en casos seleccionados. Sin embargo, desconocemos los efectos sobre la pared vascular de ambas terapias con el uso simultáneo.
Materiales y métodosEstudio experimental en 60 conejos NZ con ateroesclerosis avanzada, distribuidos en cuatro grupos de 15 animales cada uno. Grupo 1: Control. Grupo 2: stent farmacoactivo con paclitaxel (SFP) en aorta torácica. Grupo 3: Atorvastatina 2.5mg/día VO + implante de SFP, y Grupo 4: Atorvastatina 2.5mg/día VO. Seguimiento a 30, 60 y 90 días. Se realizaron análisis histomorfométricos.
Resultados60 SFP fueron implantados exitosamente. Un animal del grupo 3, falleció por infección respiratoria. SFP incrementaron el diámetro y área del lumen, y el área del vaso; y la atorvastatina indujo una potente regresión de placa. En el grupo con SFP el diámetro del lumen fue 4.25±0.0mm, área del lumen 14.2±0.4 mm2, área del vaso 16.7± 0.0 mm2, e índice placa/área de la media 0.1±0.0. En el grupo con SFP+atorvastatina las mediciones fueron 4.9±0.1mm (p<0.001), 18.6±0.8 mm2 (p=0.005), 21.6±0.9 mm2 (p=0.007) y 0.8±0.08 (p=0.032) respectivamente.
ConclusionesNuestros resultados confirman el potente efecto sinérgico mecánico de los SFP y de regresión de placa de la atorvastatina en un modelo animal con aterosclerosis avanzada, explicando en parte los resultados en estudios clínicos.
Atherosclerotic disease continues to be the main cause of death in the world and its incidence appears to still be increasing. It is estimated that 1 in 3 American adults has one or more types of cardiovascular disease.1 The growing prevalence has generated the development of new diagnostic and therapeutic interventions for coronary disease, improving the final outcome.
The use of statins and coronary stents has helped in the management of obstructive coronary disease with beneficial results described in multicentric clinical studies. Optimal medical treatment of coronary disease is fundamental and the achievement of objectives in the control of risk factors has shown benefits. The statins are the most commonly prescribed agents for dyslipidemia control. A 40% reduction in LDL-c levels along with a 30% increase in HDL-c may result in an up to 70% reduction in cardiovascular risk.2 All the currently available statins have the ability to reduce LDL-c by 20–35%. High doses of simvastatin, atorvastatin and rosuvastatin reduce LDL-c by 42%, 55%, and 58% respectively.3 A meta-analysis showed that statins reduce LDL-c, coronary events and stroke by 21%, with a reduction in all causes of mortality.4 The benefits appear to be sustained over time,5 with a reduction in the incidence of cardiovascular events in primary and secondary prevention studies,6 including special populations.7,8 In addition, statins have been shown to stabilize9 and favor the regression10 of atherosclerotic plaque through their potent hypolipemic and pleiotropic effects.
Coronary artery stents have significantly improved the security and efficacy of percutaneous coronary interventions.11 Drug eluting stents (DES) have also shown a beneficial effect in the treatment of severe coronary disease by significantly reducing the appearance of restenosis, which implies a reduction in the need for new revascularization of the treated lesion (TLR) of up to 50–70% compared to bare metal stents (BMS)12; they also favor a reduction in major adverse cardiovascular events (MACE).13–16 In spite of the fact that in pathologic studies of human coronary arteries Joner et al.,17 comparing DES with BMS did not find differences in the treated atherosclerotic plaque area one month after implant, in experimental studies in atherosclerotic rabbits, we have previously described that DES with everolimus and beta-estradiol have a potent effect on the reduction of atherosclerotic plaque volume in the treated segment.18
Current guidelines and daily clinical practice recommend the simultaneous use of statins and stents for the treatment of severe obstructive coronary disease.19 Recent studies have shown the early clinical benefit of high doses of atorvastatin in patients undergoing coronary intervention.20 We do not know if there are synergistic biologic vascular effects between both therapies on the arterial wall. The present study on an atherosclerotic animal model, intends to quantify the biological effect on the arterial wall of the simultaneous use of statin and DES in atherosclerotic plaque.
Materials and methodsAn experimental study was carried out to evaluate the effect of atorvastatin (Lipitor®, Pfizer S.A.) and the paclitaxel-eluting stent (PES; Taxus Express Stent®, Boston Scientific Corp.) on atherosclerotic plaques in the aortas of adult New Zealand (NZ) rabbits with advanced atherosclerosis. The protocol was approved by the Animal Research Ethics Committee of the Fundación CardioInfantil.
Experimental modelThe study included 60 NZ rabbits which were placed on a pulse diet with a 1% cholesterol supplement (Purina®), for a period of three months on two occasions, alternating with a normal diet for one year. The atherosclerotic lesions previously observed with this plan include advanced plaques with a lipid rich core, cholesterol crystals, calcification and formation of a fibrous capsule.
Experimental protocolAfter one year of a hypercholesterolemic diet, an experimental study was designed with the 60 animals which were distributed in four observation groups: Group 1 (n=15): Control. Group 2 (n=15): Animals with PES implant. Group 3 (n=15): Atorvastatin 2.5mg/day po+PES implant, and Group 4 (n=15): Atorvastatin 2.5mg/day po. Follow-up was carried out at 30, 60 and 90 days for which 5 animals from each group were sacrificed at each cut-off day.
Paclitaxel-eluting stent implantTwo sequential stents were implanted in the thoracic aorta of each rabbit (Groups 2 and 3). All the animals received aspirin at 10mg/kg/day po beginning the night prior to the procedure and up to one month following. IV ketamine and xylazine were used for sedation and anesthesia induction. General anesthesia was maintained with PRN IV ketamine doses and oxygen support by mask. Arterial access was obtained via arteriotomy of the common femoral artery, through which a 4 Fr. introducer was implanted. Complete anticoagulation was obtained with heparin 100IU/kg, IV. A retrograde distal aortogram was carried out manually through the introducer, using non-ionic contrast media (Ioversol 68%, Optiray®320; Mallinckrodt). A 0.014in. guide was advanced under fluoroscopic guidance up to the ascending thoracic aorta. Two sequential PES were implanted in the descending thoracic aortic segment proximal to the left renal artery at a pressure of 12–14atm, obtaining a stent:artery ratio of 1:1. A distal aortogram was carried out again, to angiographically confirm the adequate apposition of the stents and identify possible complications. At the end of the procedure the introducer was removed and the femoral artery ligated. All the animals were observed during recovery and continued their follow up and clinical monitoring.
SacrificeFive animals from each group were sacrificed at 30, 60 and 90 days. Following sedation with ketamine and xylazine IM, an IV was started in a marginal ear vein and sodium pentothal 150mg/kg IV was administered to obtain a barbiturate coma, respiratory arrest and death without any kind of suffering. Following this, arterial dissection and aortic isolation were carried out.
Pathology preparationImmediately following euthanasia, the proximal aorta was accessed and subjected to perfusion and fixation with buffer phosphate solution at 60–80mmHg for 5min. The aortas of animals without PES (Groups 1 and 4) were also embedded in paraffin and subjected to histologic cuts. The segments with stent implants (Groups 2 and 3) were carefully dissected and embedded in methylmethacrylate and then cut at 4–5μm intervals with a stainless steel blade (Boston Scientific Corp. Maple Grove, MN). All the histologic segments were stained with the elastic trichrome method.
Morphometric analysesAn individual analysis of the segments was carried out using a light microscope (Olympus BX50). The segments were measured using digital morphometry and computerized planimetry (Media Cybernetics® Image-Pro Plus TM).
MorphometryEach aortic segment examined was systematically placed in the center of the objective field using a 2× magnification. The vessel area is equal to the area within the external elastic lamina (mm2), the lumen area to the area within the endothelial surface (mm2), and the atherosclerotic plaque area to the area included between the internal elastic lamina and the arterial lumen (mm2). The lumen diameter is equal to the largest segment between the endothelial borders (mm). Ratios were calculated for atherosclerotic plaque area/vessel area and atherosclerotic plaque area/media area.
Strut analysisWe quantified the mean vascular trauma score (total score/total struts) under high power lenses (40×).20
Statistical analysesThe results are presented as averages±standard error. The majority of the morphometric data follows a non-Gaussian distribution and was compared using Mann–Whitney U non-parametric tests. Data with a Gaussian distribution were compared using a two-tailed t-test. Dichotomous variables were compared using the Chi-square test. In order to determine the difference in the total number of struts and the mean trauma score between treatments (PES vs. PES+Atorvastatin) Student's t-tests for non-homogenous variance were carried out. Significance was established as a p-value=0.05. The statistical package for social science (SPSS) 11.5 was used for the analyses.
Ethical aspectsThe protocol was approved by the Experimental Research Committee of the Instituto de Cardiología Fundación CardioInfantil and good laboratory practices for the care of animals based on the Guide for the Care and Use of Laboratory Animals, U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, were followed.21 All procedures carried out in this study were designed to avoid discomfort, suffering and pain for the animals. All aspects of this study that could cause suffering or momentary or mild pain were carried out under the effects of sedatives, analgesics or anesthetics.
ResultsSixty animals which received a diet with a cholesterol supplement were used. Group 1 (15 animals) was the control group, which did not receive atorvastatin nor PES implants. Thirty animals received two PES (Groups 2 and 3). Thirty animals received atorvastatin (Groups 3 and 4). The PES implant was successful in all the animals. One animal from Group 3 died at 2 weeks due to a respiratory infection. There was no evidence of local infection at the wound site, kidney failure, paraplegia or neural lesions due to the surgical procedure in the treated extremity. There was no clinical evidence of stent thrombosis nor hepatic toxicity due to atorvastatin.
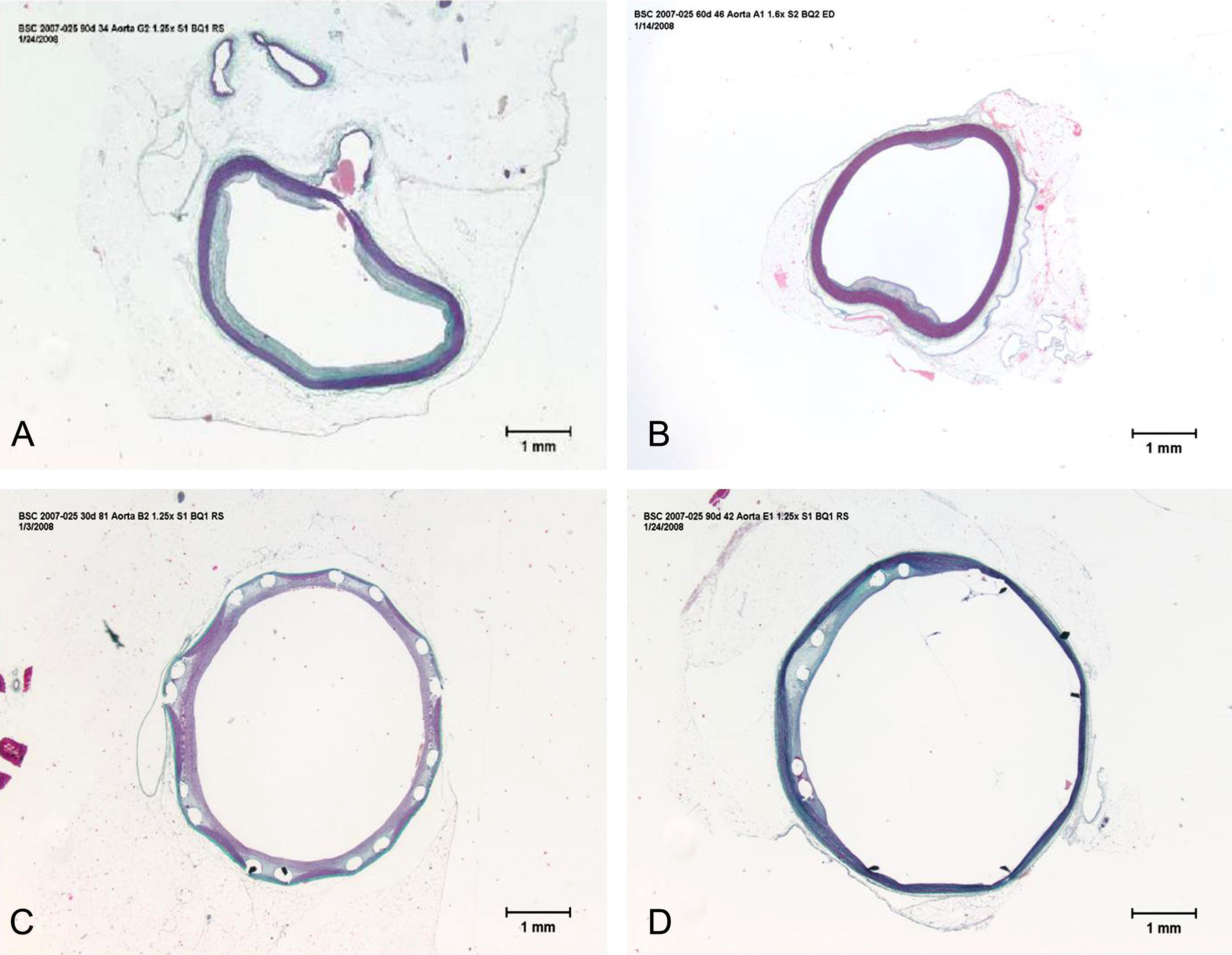
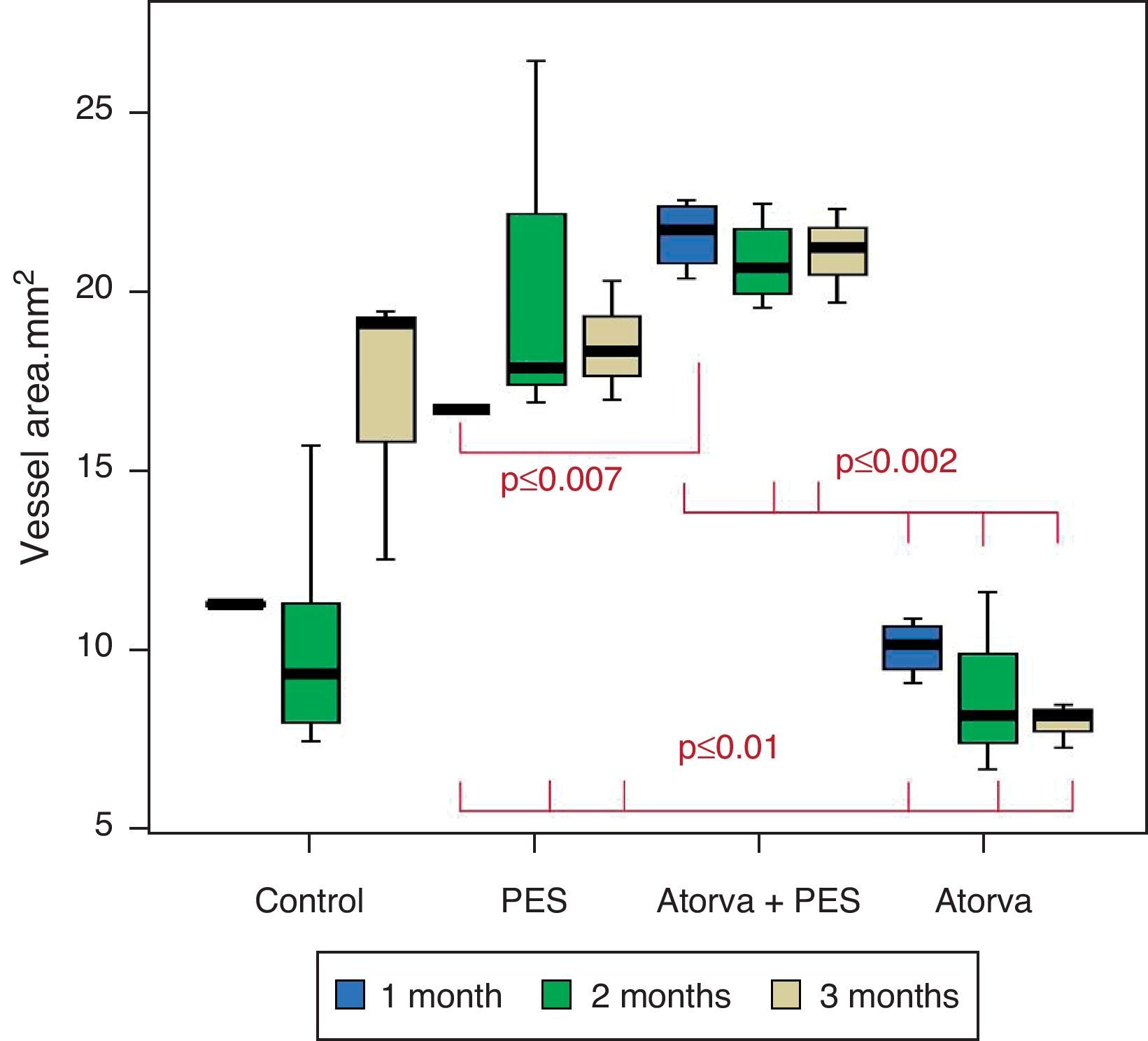
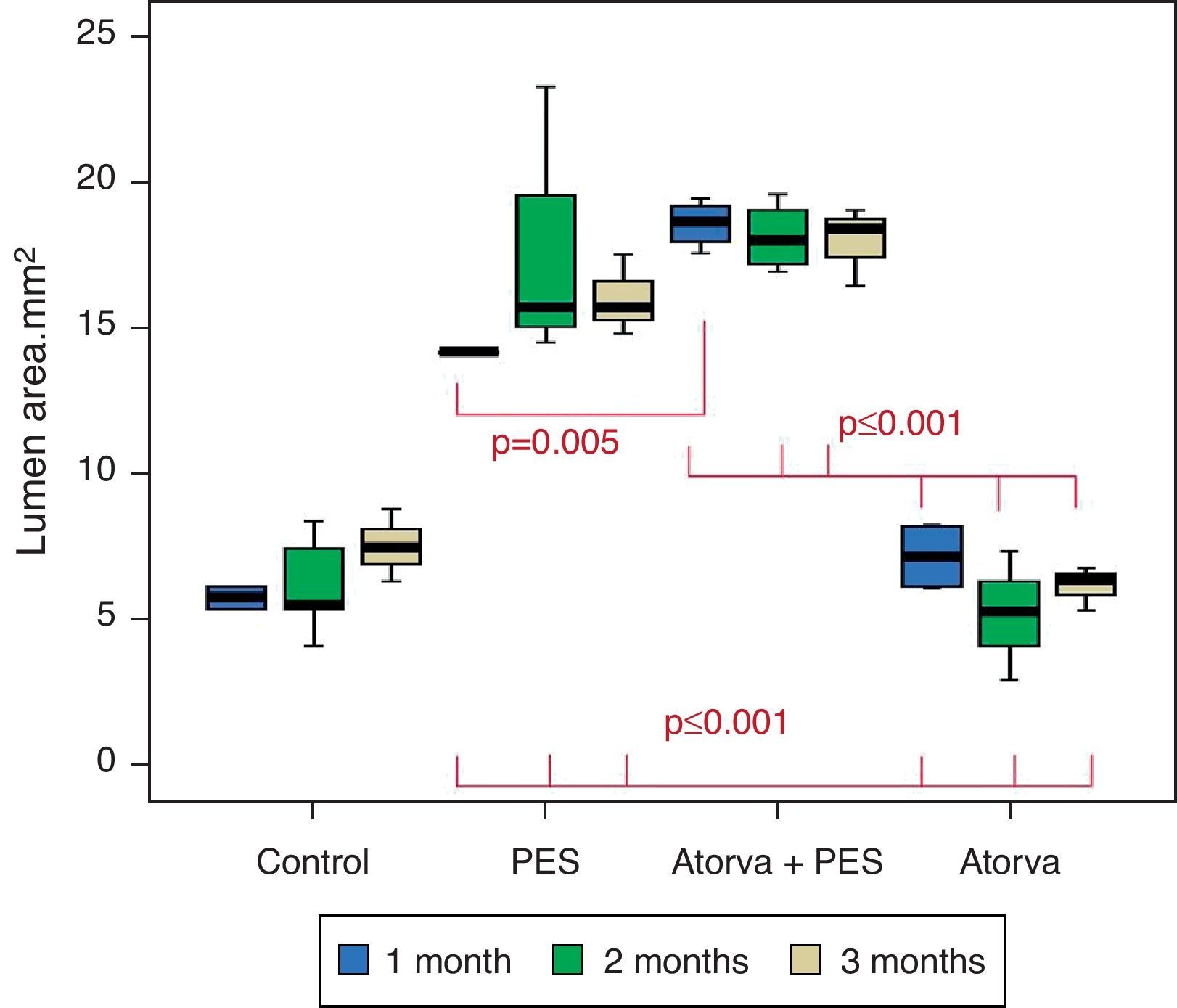
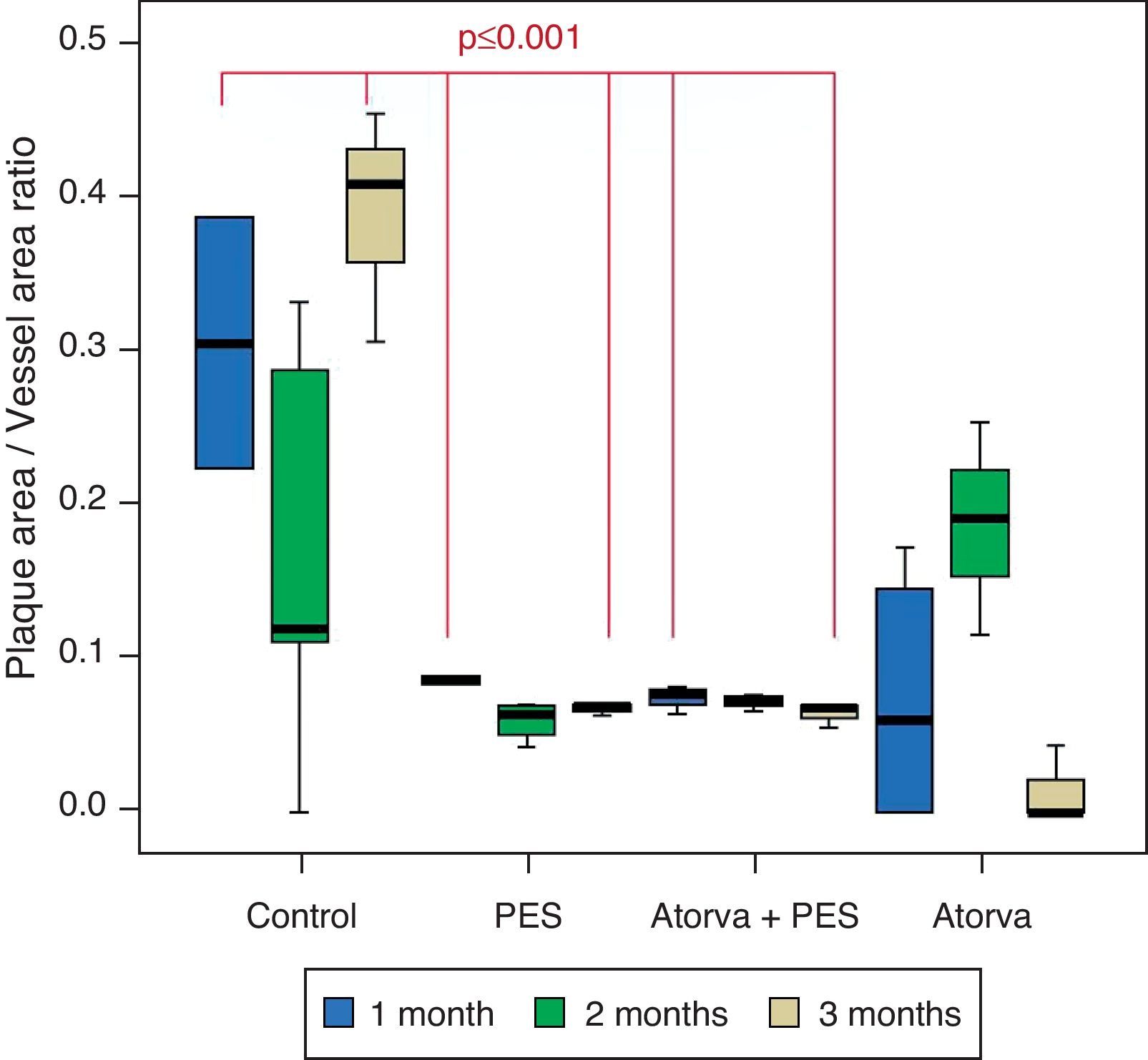
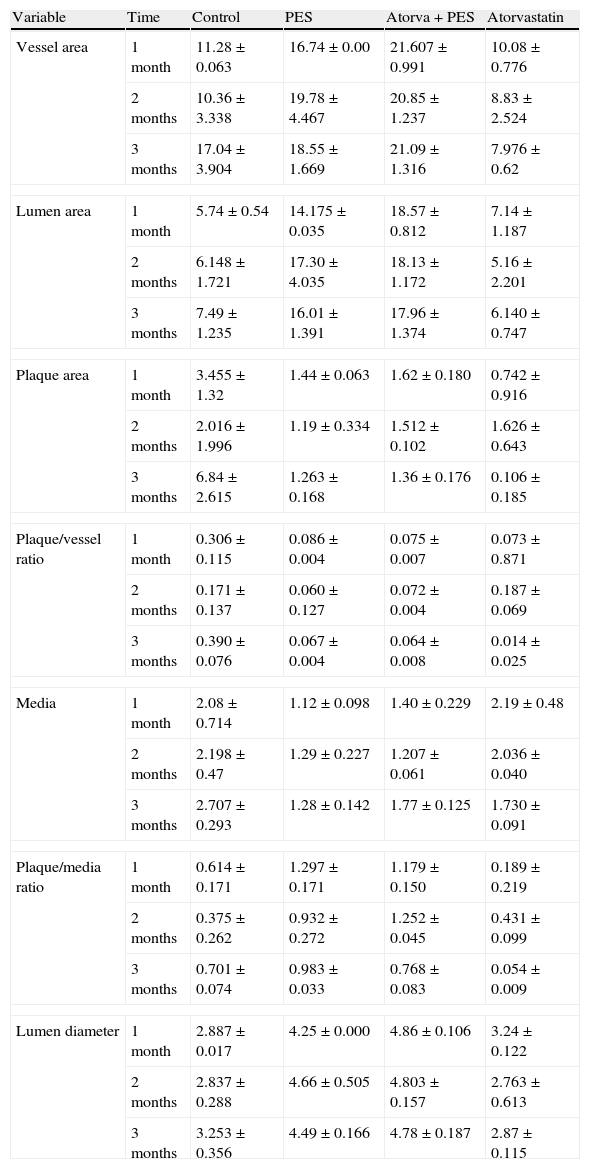
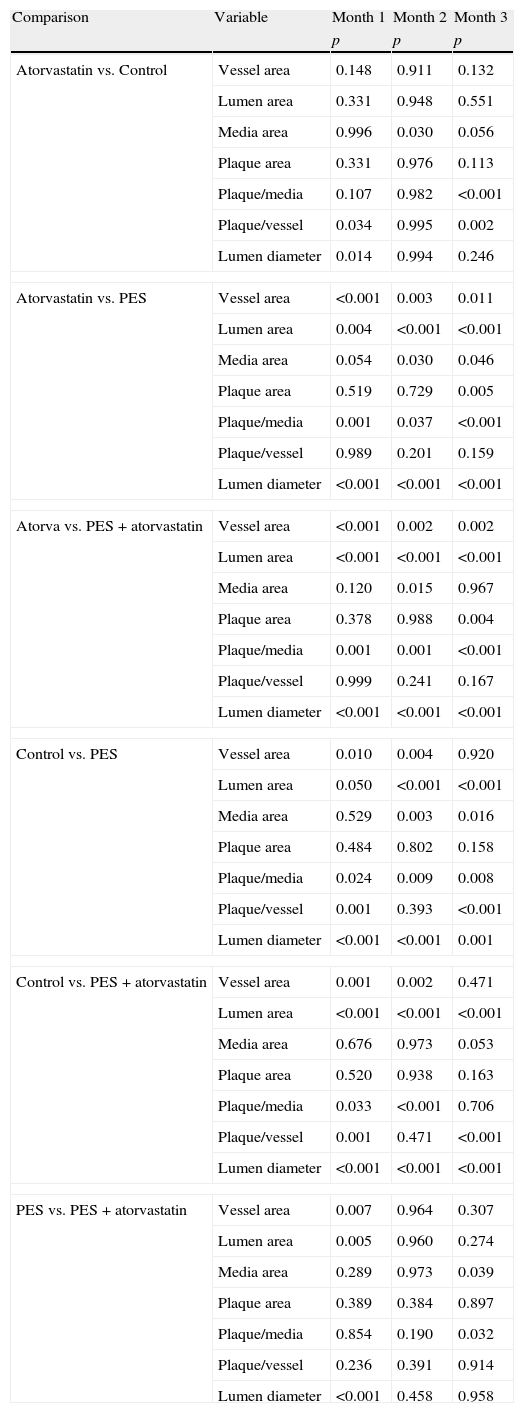
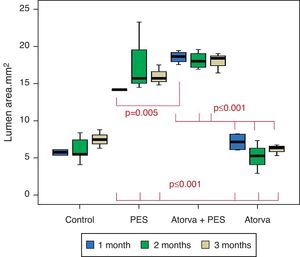
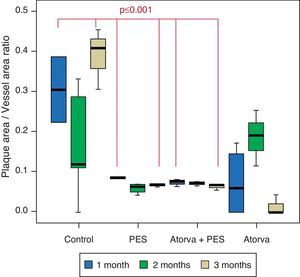
Morphometric analysesThe results of the histo-morphometric analyses are shown in Table 1. Group 1-Control showed development of advanced atherosclerotic plaque with cholesterol deposits, lipid nucleus formation and fibrotic capsule (Fig. 1). No significant differences were found (p<0.05) in all measurements at 1, 2 and 3 months of follow-up. In Group 2-PES, differences were found in the atherosclerotic plaque area/vessel area ratio between month 1 at 0.086±0.004 and month 2 at 0.060±0.127 (p=0.043). In Group 3-atorvastatin+PES, significant differences were found in the atherosclerotic plaque area/media area ratio between month 1 at 1.17±0.15 and month 3 at 0.76±0.08 (p=0.016) and between month 2 at 1.25±0.04 and month 3 at 0.76±0.08 (p=0.007) respectively. In Group 4-Atorvastatin a marked tendency was found to reduce the atherosclerotic plaque area/vessel area ratio and the atherosclerotic plaque area/media area ratio between month 1 and month 3 (p=0.048).
Table of average results and Kruskall Wallis analysis for evaluating differences between treatments.
| Variable | Time | Control | PES | Atorva+PES | Atorvastatin |
| Vessel area | 1 month | 11.28±0.063 | 16.74±0.00 | 21.607±0.991 | 10.08±0.776 |
| 2 months | 10.36±3.338 | 19.78±4.467 | 20.85±1.237 | 8.83±2.524 | |
| 3 months | 17.04±3.904 | 18.55±1.669 | 21.09±1.316 | 7.976±0.62 | |
| Lumen area | 1 month | 5.74±0.54 | 14.175±0.035 | 18.57±0.812 | 7.14±1.187 |
| 2 months | 6.148±1.721 | 17.30±4.035 | 18.13±1.172 | 5.16±2.201 | |
| 3 months | 7.49±1.235 | 16.01±1.391 | 17.96±1.374 | 6.140±0.747 | |
| Plaque area | 1 month | 3.455±1.32 | 1.44±0.063 | 1.62±0.180 | 0.742±0.916 |
| 2 months | 2.016±1.996 | 1.19±0.334 | 1.512±0.102 | 1.626±0.643 | |
| 3 months | 6.84±2.615 | 1.263±0.168 | 1.36±0.176 | 0.106±0.185 | |
| Plaque/vessel ratio | 1 month | 0.306±0.115 | 0.086±0.004 | 0.075±0.007 | 0.073±0.871 |
| 2 months | 0.171±0.137 | 0.060±0.127 | 0.072±0.004 | 0.187±0.069 | |
| 3 months | 0.390±0.076 | 0.067±0.004 | 0.064±0.008 | 0.014±0.025 | |
| Media | 1 month | 2.08±0.714 | 1.12±0.098 | 1.40±0.229 | 2.19±0.48 |
| 2 months | 2.198±0.47 | 1.29±0.227 | 1.207±0.061 | 2.036±0.040 | |
| 3 months | 2.707±0.293 | 1.28±0.142 | 1.77±0.125 | 1.730±0.091 | |
| Plaque/media ratio | 1 month | 0.614±0.171 | 1.297±0.171 | 1.179±0.150 | 0.189±0.219 |
| 2 months | 0.375±0.262 | 0.932±0.272 | 1.252±0.045 | 0.431±0.099 | |
| 3 months | 0.701±0.074 | 0.983±0.033 | 0.768±0.083 | 0.054±0.009 | |
| Lumen diameter | 1 month | 2.887±0.017 | 4.25±0.000 | 4.86±0.106 | 3.24±0.122 |
| 2 months | 2.837±0.288 | 4.66±0.505 | 4.803±0.157 | 2.763±0.613 | |
| 3 months | 3.253±0.356 | 4.49±0.166 | 4.78±0.187 | 2.87±0.115 | |
The results of the histomorphometric analyses and their comparison between groups are shown in Tables 1 and 2.
Post hoc tests. p-Value comparison between months of follow-up for each treatment.
| Comparison | Variable | Month 1 | Month 2 | Month 3 |
| p | p | p | ||
| Atorvastatin vs. Control | Vessel area | 0.148 | 0.911 | 0.132 |
| Lumen area | 0.331 | 0.948 | 0.551 | |
| Media area | 0.996 | 0.030 | 0.056 | |
| Plaque area | 0.331 | 0.976 | 0.113 | |
| Plaque/media | 0.107 | 0.982 | <0.001 | |
| Plaque/vessel | 0.034 | 0.995 | 0.002 | |
| Lumen diameter | 0.014 | 0.994 | 0.246 | |
| Atorvastatin vs. PES | Vessel area | <0.001 | 0.003 | 0.011 |
| Lumen area | 0.004 | <0.001 | <0.001 | |
| Media area | 0.054 | 0.030 | 0.046 | |
| Plaque area | 0.519 | 0.729 | 0.005 | |
| Plaque/media | 0.001 | 0.037 | <0.001 | |
| Plaque/vessel | 0.989 | 0.201 | 0.159 | |
| Lumen diameter | <0.001 | <0.001 | <0.001 | |
| Atorva vs. PES+atorvastatin | Vessel area | <0.001 | 0.002 | 0.002 |
| Lumen area | <0.001 | <0.001 | <0.001 | |
| Media area | 0.120 | 0.015 | 0.967 | |
| Plaque area | 0.378 | 0.988 | 0.004 | |
| Plaque/media | 0.001 | 0.001 | <0.001 | |
| Plaque/vessel | 0.999 | 0.241 | 0.167 | |
| Lumen diameter | <0.001 | <0.001 | <0.001 | |
| Control vs. PES | Vessel area | 0.010 | 0.004 | 0.920 |
| Lumen area | 0.050 | <0.001 | <0.001 | |
| Media area | 0.529 | 0.003 | 0.016 | |
| Plaque area | 0.484 | 0.802 | 0.158 | |
| Plaque/media | 0.024 | 0.009 | 0.008 | |
| Plaque/vessel | 0.001 | 0.393 | <0.001 | |
| Lumen diameter | <0.001 | <0.001 | 0.001 | |
| Control vs. PES+atorvastatin | Vessel area | 0.001 | 0.002 | 0.471 |
| Lumen area | <0.001 | <0.001 | <0.001 | |
| Media area | 0.676 | 0.973 | 0.053 | |
| Plaque area | 0.520 | 0.938 | 0.163 | |
| Plaque/media | 0.033 | <0.001 | 0.706 | |
| Plaque/vessel | 0.001 | 0.471 | <0.001 | |
| Lumen diameter | <0.001 | <0.001 | <0.001 | |
| PES vs. PES+atorvastatin | Vessel area | 0.007 | 0.964 | 0.307 |
| Lumen area | 0.005 | 0.960 | 0.274 | |
| Media area | 0.289 | 0.973 | 0.039 | |
| Plaque area | 0.389 | 0.384 | 0.897 | |
| Plaque/media | 0.854 | 0.190 | 0.032 | |
| Plaque/vessel | 0.236 | 0.391 | 0.914 | |
| Lumen diameter | <0.001 | 0.458 | 0.958 | |
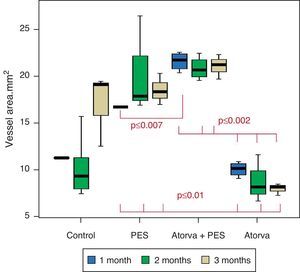
Control vs. Atorvastatin: The results show how the morphometric vascular effects of atorvastatin begin to be seen as the follow-up time progresses, showing a tendency to increase the lumen area and the lumen diameter (p=0.014); to reduce the plaque area, plaque/vessel ratio (p=0.002), media area (p=0.030), and the plaque/media ratio (p<0.001) (Figs. 2–4).
Atorvastatin vs. PES: The results show how the morphometric vascular effects of the PES induce a significant increase in vessel area at one month (p<0.001), two months (p=0.003) and three months (p=0.011). The lumen area also increased at one month (p=0.004), two months (p<0.001) and three months (p<0.001) (Fig. 4). In addition, a reduction in media area is also seen at one month (p=0.054), two months (p=0.030) and three months (p=0.046). The vessel diameter increases with the use of PES at one month (p<0.001), two months (p<0.001) and three months (p<0.001). However, it was shown that atorvastatin significantly reduces the evident atherosclerotic plaque area at one month and three months compared to PES (0.10±0.18 vs. 1.26±0.16mm2 (p=0.005).
PES vs. PES+Atorvastatin: it is observed that the simultaneous use of atorvastatin and PES induces early on a larger lumen diameter (p<0.001), an increase in the vessel area (p=0.007), an increase in the lumen area (p=0.005) and an important reduction in the plaque/media ratio (p=0.032).
Strut analysisTwenty-nine animals from the two PES groups were analyzed (one animal from Group 3 died), with a total of 58 PES. Four sections were made of each stent and 1926 struts were analyzed. In Group 2 (PES) an average of 19.2±6.8 struts/section were analyzed, with a mean trauma score of 1.49±0.47. In Group 3 (PES+atorvastatin) an average of 20.0±6.89 struts/section were analyzed (p=0.34), with a mean trauma score of 1.45±0.39 (p=0.36).
DiscussionThis experimental study of atherosclerotic animals demonstrates the local effects on the arterial wall of systemic treatment with atorvastatin (high dose) and paclitaxel eluting stents; likewise, very importantly, the synergistic effects of both therapies, increase in lumen area, vessel area, and plaque regression, which has important implications to keep in mind in future studies.
The results of this study demonstrate the safety and efficacy of the therapies employed (atorvastatin and PES). The control group showed the development of advanced atherosclerotic plaque by the presence of a lipid nucleus and a fibrotic capsule. The PES segments analyzed showed how the stents have a significant mechanical effect increasing the lumen area and diameter and the vessel area, and a moderate effect on reducing atherosclerotic plaque. With the use of atorvastatin, the mechanical effect is less evident, but with an important effect on the atherosclerotic plaque, inducing a significant regression. The simultaneous use of PES and atorvastatin potentiates the mechanical and biologic effect of both therapies demonstrated by a synergistic effect in maintaining the lumen and vessel area with an important reduction in atherosclerotic plaque.
Plaque regressionTreatment with statins has been shown to reduce major cardiac events in patients with severe coronary disease in different clinical studies.22,23 Angiographic studies have shown minimal changes in the arterial lumen in patients treated with statins.24 However, various studies with follow-up of plaque volume using intravascular ultrasound (IVUS) have demonstrated the benefit of the treatment, inducing regression or non-progression of coronary plaque size.9,25 Volumetric analyses of plaque using higher resolution techniques, such as VH-IVUS,26 demonstrated that statins are associated with significant changes in the lipid nucleus and the fibro-lipid plaque volume in patients with coronary disease. In patients treated with atorvastatin, intracoronary angioscopy analyses also demonstrated early loss of the yellow plaque color, and the volumetric analyses evaluated with IVUS showed significant regression changes, suggesting a potential effect on vulnerability reduction.27
The vascular mechanisms of action of the statins, although not clearly understood, seem to indicate that the hypolipemic and pleiotropic effects change the plaque composition, and significantly reduce the lipid and inflammatory component, stabilizing the plaque and recovering endothelial function.28 Previous findings29 have shown that an ongoing statin treatment can contribute to plaque stabilization in non-fatal coronary syndromes by reducing intra-plaque neo-angiogenesis and hemorrhage, reducing the lipid and macrophage content, and, among other things, increasing the local collagen content. Paclitaxel also possesses significant vascular effects on the vascular wall, given its insolubility in water and its lipophilic nature, which allows it to increase cellular capture of the drug through the membranes. Paclitaxel in vitro30 and in-vivo31 studies demonstrated that it inhibits the migration and proliferation of smooth muscle cells, an important component of atherosclerotic plaque.
In previous observations carried out by our group, we confirmed atorvastatin's effect in inducing early atherosclerotic plaque regression in a hypercholesterolemic rabbit animal model32 and the effect of everolimus eluting stents and beta estradiol eluting stents (Guidant – Santa Clara, CA, USA), in an advanced atherosclerosis animal model18 demonstrating how at 28 days of follow-up the DES induced the reduction of plaque area and lipid area and an increase in the fibrotic capsule. In the present study we also demonstrate how the PES reduce the volume of atherosclerotic plaque in the treated segment, principally in the first weeks post-implant, up to 30%. Treatment with atorvastatin alone reduced plaque volume rapidly in the first weeks, up to 86%, and the use of atorvastatin and PES synergistically reduced atherosclerotic plaque volume up to 17% at 3 months follow-up.
Statins in percutaneous coronary interventionsInflammation is a central phenomenon in the pathogenesis of atherothrombotic events. Systemic inflammation markers, such as C-reactive protein (CRP), have been correlated with the presence and prognosis of acute coronary syndromes (ACS) without ST segment elevation.33,34 Patients with ACS without ST elevation, who have elevated CRP levels and undergo a percutaneous coronary intervention (PCI), have an increased incidence of death or non-fatal infarct at 6 months post-procedure compared to patients who have normal CRP levels.35 Elevated CRP levels prior to a PCI procedure in ACS without ST elevation, predict mortality at 5 years follow-up.36 Likewise, the inflammatory response following PCI and stents in humans has shown a prevalence of lymphocyte/macrophage infiltration, playing an important role in the formation of early and late thrombi, reendothelialization delay, and neointimal proliferation.37 Neointimal infiltration by macrophages has been directly related to intra-stent restenosis.38
Both for ACS and for PCI, the magnitude of vascular inflammation is an adverse factor and is associated with late clinical results (death, recurrent MI, ischemia or restenosis). Many pharmacologic treatments with demonstrated efficacy in the treatment of ACS have anti-inflammatory properties, which differ according to their mechanism of action (aspirin, clopidogrel, low molecular weight heparins, GpIIb/IIIa inhibitors, statins ACE inhibitors, etc.). Clinicians continue to work with the generated hypothesis that the modulation of vascular inflammation obtained with these medications, at least in part, contributes to a benefit for the patient.39
The anti-inflammatory effect of the statins is seen very early on following coronary stent implantation, as CRP levels are reduced within 24–48h following the intervention.40 In the same way, those patients who received atorvastatin 80mg/day beginning at the time of intervention and stent implantation and continued with daily treatment also showed a reduction in CRP levels at 48h follow-up. Basic science and clinical observation support the presence of anti-inflammatory and pleiotropic effects of statin therapy that appear to be independent and different from the hypolipemic ones. These observations have clinical relevance, given that patients pre-treated with statins who undergo PCI have shown a reduction in post-procedure myocardial necrosis,41 intra-hospitalization myocardial necrosis,42 and long-term mortality (6–12 months).43 Recently, a significant reduction in peri-procedure myocardial infarction (9.5%) was published in a group of patients treated with elective PCI and atorvastatin 80mg/day, compared to a control group (15.8%; p=0.014).44 The ARMYDA-RECAPTURE trial45 suggests that administering an extra (recharge) dose with high atorvastatin doses improves clinical results (post-procedure infarct and major adverse cardiovascular events at 30 days) of patients with chronic statin therapy undergoing PCI. Prati et al.46 describe the effects of atorvastatin at a dose of 80mg/day in reducing neointimal proliferation and angiographic restenosis (late loss) in patients receiving stent implants. This evidence supports the use of moderate to high dose statin therapy in patients with coronary disease who require percutaneous myocardial revascularization procedures.
ConclusionsThe use of statins and DES are the best systemic and local therapies, respectively, available for the treatment of obstructive coronary disease in selected cases. The clinical evidence to date has demonstrated the benefits of the use of moderate and high-dose statins before, during and post-PCI procedures with stent implantations, in patients with stable coronary disease and ACS without ST elevation. The hypothesis that the anti-inflammatory and pleiotropic effects of the statins can modulate the local hypersensitivity reaction of the DES favors the idea of routinely using these two therapies in clinical practice. Our results confirm the potent mechanical effect of the DES, and the plaque regression of the statins in an animal model with advanced atherosclerosis.
Ethical disclosuresRight to privacy and informed consentThe authors declare that no patient data appears in this article.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
FundingThis study received partial funding from Pfizer Laboratories S.A. and Boston Scientific Corp.
Conflict of interestThe authors declare no conflict of interest.
The authors express their gratitude to Laboratorios Pfizer S.A. and Boston Scientific for their co-funding of this study. David Warnock, Principal Preclinical Research Associate (Boston Scientific Corporation. Natick, MA). Barbara A. Huibregtse, DVM; Brooks Johnson, Eric DoBrava (Plymouth, MN) and Paul S Seifert, PhD (Oregon House, CA).