Under normal conditions, the endothelium prevents vascular spasms, plaque adhesion and cellular proliferation through the release of substances such as endothelium-dependent relaxation factor (EDRF-NO) and prostacyclin (PGI2)1. Hypercholesterolemia causes endothelial dysfunction and atherosclerotic disease in humans and animals2,3. Such dysfunction results in vasoconstriction and plaque adhesion and aggregation that can cause atherosclerotic ischemic cardiopathy, including angina and acute myocardial infarction2,4.
Clinically, a reduction in the plasma cholesterol level decreases the morbidity and mortality associated with cardiovascular diseases, probably by reversing or attenuating endothelial dysfunction rather than by decreasing the size of the coronary atherosclerotic plaque, which is only moderately affected5,6. Similarly, the endothelial dysfunction caused by hypercholesterolemia can be reversed by treatment with simvastatin and pravastatin, two inhibitors of HMG-CoA redutase, the key enzyme in the synthesis of cholesterol7,8. This reversal of endothelial dysfunction is dependent on a decrease in the plasma cholesterol concentration. Indeed, numerous therapies designed to reduce the plasma cholesterol level can reverse the endothelial dysfunction and atherosclerosis associated with hyper-cholesterolemia9,10. This restoration of endothelial function prevents or attenuates myocardial ischemia by correcting the associated abnormal coronary vasoreactivity and plaque adhesion.
Lipid peroxidation plays a role in atherogenesis, and the reversal of endothelial dysfunction partly involves the inhibition of oxidative modifications to low-density lipoproteins (LDL-c) in the arterial wall11. Vitamin E increases the resistance of LDL-c to oxidation, reverts endothelial dysfunction and reduces the risk of coronary disease without a corresponding reduction in the plasma cholesterol concentration or size of the atherosclerotic plaque in humans12. In hyperlipidemic rabbits, the progression of atherosclerosis can also be attenuated by other antioxidants13. These studies indicate that oxidative modifications to subendothelial LDL-c by free radicals can result in endothelial dysfunction and atherogenesis14. The endothelial dysfunction associated with hypercholesterolemia also involves an inflammatory reaction since individuals with acute coronary syndrome have increased numbers of circulating inflammatory cells and enhanced levels of proinflammatory cytokines and acute-phase proteins15.
Although an increase in the plasma cholesterol concentration causes endothelial dysfunction and atherosclerosis through the action of free radicals and inflammatory mediators, the precise relationship between cholesterol levels and the onset of endothelial dysfunction remains unclear. In this work, we examined the relationship between the degree of endothelial dysfunction and the levels of cholesterol and lipid peroxidation in the aortic wall of hypercholesterolemic rabbits.
MethodsPreparation of animals
Fifty male New Zealand white rabbits 16-20 weeks old and weighing 3.0-3.5 kg obtained from were used. All of the procedures involving animals were done according to the guidelines described in the Care and Use of Laboratory Animals (NIH Publication, no 85-23, 1985 revision). The rabbits were numbered and housed individually at 25oC with free access to water, and were fed a fixed amount (60 g/day) of Purina® chow (Nestlé do Brasil Ltda.) of the following composition (g/100 g of product): proteins 20.00, carbohydrates 45.00, fibers 16.00, fat 5.00, and ashes 14.00. In all cases, except for the rabbits in group 1 (G1), the diet was enriched with 0.5% cholesterol (Vetec, Rostock,Germany) and 10% coconut oil (Refino de Óleos Brasil Ltda). Weekly determinations of the plasma total cholesterol concentrations were used to allocate the rabbits to one of ten groups (G1 to G10, n=5 each), with class intervals of 100 mg/dl, as follows: Group 1: <100 mg/dl, Group 2: 100-199 mg/dl, Group 3: 200-299 mg/dl, Group 4: 300-399 mg/dl, Group 5: 400-499 mg/dl, Group 6: 500-599 mg/dl, Group 7: 600-699 mg/dl, Group 8: 700-799 mg/dl, Group 9: 800-899 mg/dl and Group 10: > 900mg/dl. Blood samples were obtained from a marginal ear vein and the plasma cholesterol concentrations were determined colorimetrically using a commercial kit, as described below.
The necessary time to obtain the wanted cholesterolemia was largely variable due to the animals metabolic individualities, each group submitted to diet average seven days plus in total the ten weeks. Therefore, since the free demand diet was started, a blood sample of each animal was weekly obtained via punction from the ear marginal vein. The plasma was separated from each sample by centrifuge and the total plasmatic cholesterol concentration was measured utilizing a commercial enzymatic kit and a spectrophotometer. Since the value established for a certain group was reached, the animal was included considered the age 16-20 weeks and weight 3,0-3,5 kg.
At the end of the treatment, the rabbits were killed by cervical dislocation and a blood sample was collected by cardiac puncture for the quantification of plasma LDL-c. After a median thoracotomy, the aorta was removed for biochemical analyses measurement of cholesterol and lipid peroxidation and functional studies, as described below.
Quantification of plasma total cholesterol
Plasma total cholesterol was measured spectrophotometrically 500 nm (Thermo Spectronic, Rochester, USA) using a commercial enzyme-based kit (In Vitro Diagnostica, Germany) and the results were expressed in mg/dL.
Quantification of plasma LDL-CU
Blood was collected by cardiac punction into plastic tubes containing EDTA at pH 7.4 and centrifuged to 3,000 rpm for 15 min at 10oC. The resulting plasma was processed according to Havel and Bragdon16, which includes two sequential steps of ultracentrifugation (total of 38 h). Initially, 8 mL of plasma from each sample was centrifugated to 40,000 rpm for 18 h at 4oC (Beckman ultracentrifuge, model L-8) to separate the chylomicrons and very low density lipoproteins (VLDL). These lipoproteins were removed with a pipette and the density of the lower layer was adjusted to 1.063 g/mol with solid KBr prior to a second centrifugation at 40,000 rpm for 20 h at 4oC to obtain LDL-c. The plasma concentration of LDL-c was measured enzymatically and the results were expressed in mg/dl. Fried-wald´s formula was used to confirm the results.
Cholesterol content of the aortic wall
The cholesterol content of the aortic wall was measured as described by Naito and David17. Tissue samples were desiccated and then homogenized at 4oC in 5 ml of 13 mmol/L Tris-HCl, pH 7.4, containing 10 mmol/L NaN3. Total lipids were extracted from this mixture and homogenized in 10 volumes of chloroform:methanol (2:1, v/v) containing 0.001% butylated hydroxytoluene as antioxidant. The cholesterol content of the aortic wall was determined enzymatically as described above and the results expressed in mg/g dried tissue.
Lipid peroxidation in the aortic wall
Lipid peroxidation in the aortic wall was determined indirectly by measuring the content of malondialdehyde (MDA), one of the final products of lipid peroxidation. Aortic tissue was homogenized in 10% trichloroacetic acid 100mg of tis-sue/ml of TCA (Sigma Chemical Co., St. Louis, MO, USA) and then centrifuged. One volume of supernatant was mixed with an equal volume of 0.67% (v/v) thiobarbituric acid (Sigma), and the mixture was heated at 100 oC for 20 min. The MDA concentration was calculated as described by Bueg and Aust18 based on the absorbance at 532 nm and a molar extinction coefficient of 1.49x10–5. The results were expressed in nmol/mg of dried tissue.
Functional studies
Endothelial function was assessed by studying the endothe-lium-dependent relaxation of aortic rings from control and hypercholesterolemic rabbits. The aorta was cleaned of conjunctive tissue and a segment approximately 5 mm long (with intact endothelium) was suspended by two hooks in a 10 ml glass chamber containing warmed (37 oC), aerated (95% O2-5% CO2) Krebs-Henseleit solution (composition in mmol/L: NaCl 113, KCl 4.74, CaCl2 21.9, NaHCO3 25, MgSO4 0.44, KH2PO4 1.18, EDTA 0.03, glucose 11, pH 7.4) (Merck Chemicals, Darmstadt, Germany). The segments were allowed to equilibrate for 60 min at a resting tension of 1 g, with the Krebs-Henseleit solution being changed every 20 min. The tissue responses to exogenously added test substances were recorded via a linear voltage displacement transducer coupled to a Narcotrace 40 recorder. The tissues were pre-contracted with norepinephrine (10-7 mol/L) (Sigma) and, after maximal contraction, a cumulative concentration-response curve was obtained for relaxations induced by acetylcholine (10–8 to 10–5.5 mol/L; Sigma). The preparations were subsequently washed and re-equilibrated, after a period of 30 min, the segment was contracted again with noradrenalin (10–7 mol/L), and another dosage-effect curve was obtained with sodium nitroprusside (10–8 to 10–5,5 mol/L) (Sigma Chemical Co., St. Louis, USA). Sodium nitroprusside was used to verify the relaxation of the smooth muscle.
Statistical analysis
The results were expressed as the mean ± standard deviation. Statistical comparisons were done using analysis of variance (ANOVA)19 followed by the Tukey test, with a value of P<.05 indicating significance. When required, the data were rank transformed prior to analysis. The sample sizes required were determined based on preliminary experiments and analysis by ANOVA.
ResultsTable 1 shows the mean values for the variables measured in the control and hypercholesterolemic rabbits. These data are also shown graphically in figures 1-4.
Table 1. Mean values ± standard deviation for the variables measured in each of the experimental groups
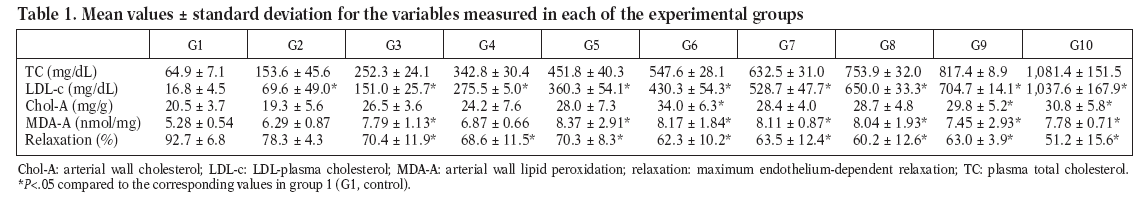
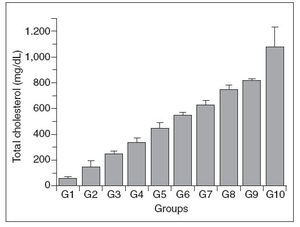
Figure 1. Total plasma cholesterol concentration in control (G1) and htypercholesterolemic (G2-G10) rabbits. The values are the mean ± standard deviation of n = 5 each.
Total plasma cholesterol
Concerning the variable total plasmatic cholesterol, the statitistic test was not applied among the groups because its values was preestablished (100 mg/dl/group) and were utilized as parameter for the comparison with the other variables.
Plasma LDL-C
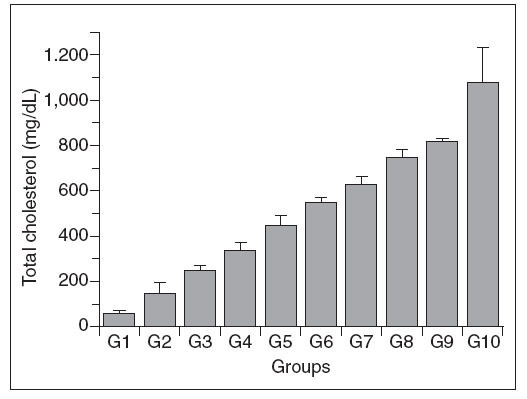
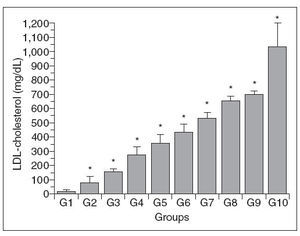
Figure 2 shows that there was a progressive in-crease in the plasma LDL-c concentration in the different groups, with a pattern similar to that seen for plasma total cholesterol. However, there were no significant differences in the LDL-c concentrations of the following pairs of groups (4-5, 5-6, 6-7, 7-8, 8-9 and 9-10).
Figure 2. Plasma LDL-c concentrations in control (G1) and hypercholesterolemic (G2-G10) rabbits. The values are the mean± standard deviation of n = 5 each. *P<.05 compared to G1.
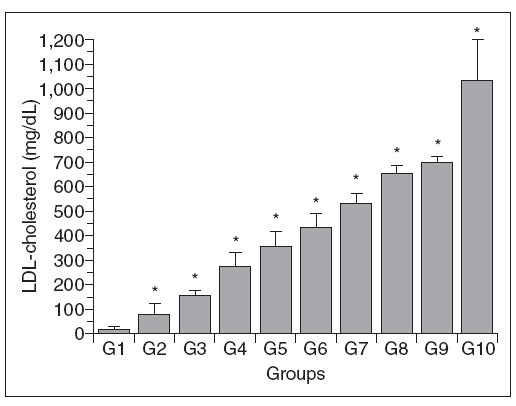
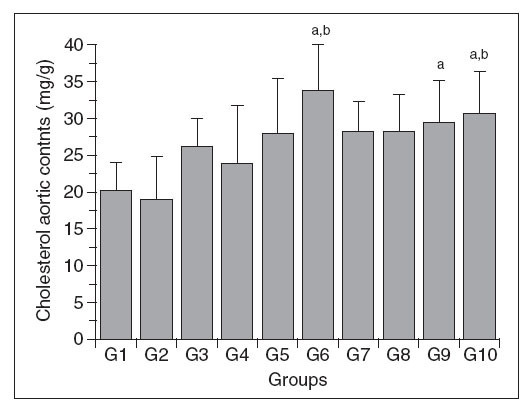
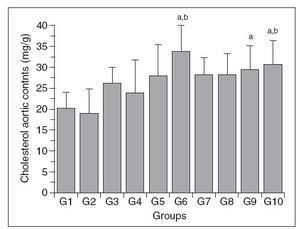
Figure 3. Aortic wall cholesterol content in control (G1) and hypercholesterolemic (G2-G10) rabbits. The values are the mean ± standard deviation of n = 5 each. aP<.05 compared to G1. bP<.05 compared to G2.
Aortic wall cholesterol (Chol-A)
The Chol-A content of the different groups is shown in Figure 2. The changes in this parameter were variable, with significant increases only in groups 6, 9 and 10 compared to group 1; groups 6 and 10 also differed from group 2, but there were no significant differences among the other groups. In addition, there was no direct relationship between the changes in Chol-A and the plasma total cholesterol levels.
Aortic wall lipid peroxidation (MDA-A)
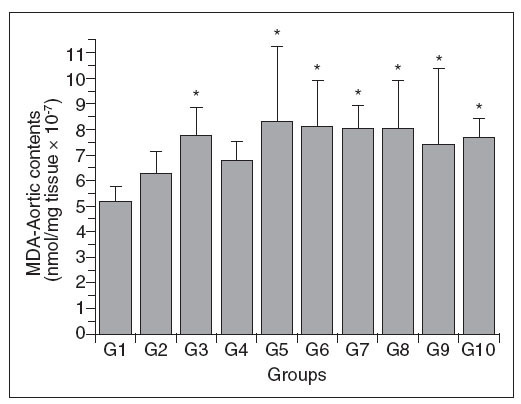
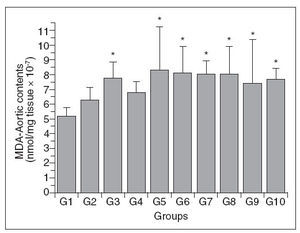
Figure 4 shows the aortic wall MDA content of the different groups. As with Chol-A, there was a variable response among the groups, with groups 3 and 5-10 having significantly higher levels than group 1; there were no differences among the other comparisons. The changes in MDA-A were not proportional to the increase in plasma total cholesterol in these same groups.
Figure 4. Aortic wall MDA content in control (G1) and hypercholesterolemic (G2-G10) rabbits. The values are the mean± standard deviation of n = 5 each. *P<.05 compared to G1.
Endothelial function
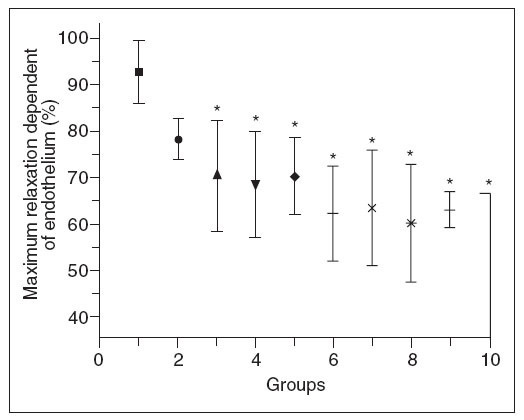
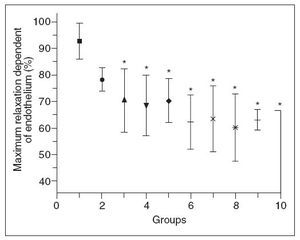
Figure 5 shows that there was a progressive decrease in the maximum endothelium-dependent relaxation of aortic rings from hypercholesterolemic rabbits compared to control rabbits. Inter-group comparisons showed that groups 3-10 differed significantly from group 1, and that groups 8-10 differed from group 2; there were no significant differences among the other comparisons. The decrease in relaxation dependent of endothelium was not proportional to the increase in plasma total cholesterol. No differences were observed in the concentration- effect curves for sodium nitroprusside between any of the groups of rabbits.
Figure 5. Endothelium-dependent relaxation of aortic rings from control (G1) and hypercholesterolemic (G2-G10) rabbits. The values are the mean ± standard deviation of n = 5 each. *P<.05 compared to G1.
DiscussionThe results of this study show that in male New Zealand white rabbits fed a diet enriched in 0.5% cholesterol and 10% coconut oil there was an the increase in plasma LDL-c that paralleled the increase in plasma total cholesterol. In addition, there was an accumulation of cholesterol and enhanced lipid peroxidation in the aortic wall, and a decrease in endothelial responsiveness. Although these results were unlikely to have been influenced by the age (16-20 weeks old) and weight (3-3.5 kg) of the rabbits, there was considerable individual variation in the levels of total cholesterol and LDL cholesterol. This finding agrees with Beynen et al1 who observed that a cholesterol-rich diet produced marked individual differences in the serum cholesterol levels, with some animals showing little increase (hyporesponders) while others showed a marked increase (hyperresponders).
Our results agree with the currently recognized stages of lipid transport and metabolism20. Lipids (triglycerides, phospholipids and cholesterol) are transported in the circulatory system by lipoprotransported as chylomicrons and distributed to adipose tissue, muscle, and liver, the principal storage organ for chylomicrons. Subsequently, lipids stored and/or absorbed by the liver are transported to adipose tissue and muscle by very low density lipoproteins (VLDL), which are transformed into intermediary density lipoproteins (IDL). The exchange of components of these particles with high density lipoproteins (HDL) reduces their size and transforms them into low density lipoproteins (LDL).
The removal of LDL-c from the circulation by hepatocytes involves receptor-mediated and non-receptor-mediated mechanisms. Under normal conditions, the removal of LDL-c by hepatocytes involves specific receptors in the plasma membra-20,21. Upon reaching the cell, the lipoprotein is degraded to yield amino acids and free cholesterol, the latter being used to produce cell membranes, steroid hormones and biliary acids. Negative feedback by excess free cholesterol tends to limit the intracellular concentration of this lipid by 1) inhibiting HMG-CoA reductase involved in the synthesis of cholesterol, 2) activating the enzyme ACAT, which is responsible for the conversion of free cholesterol into cholesterol esters for storage, and 3) inhibiting the production of new LDL-c receptors by suppressing the transcription of the corresponding gene. This highly regulated mechanism can increase the plasma level of LDL-c.
When there is a progressive increase in hypercholesterolemia, non-receptor-mediated mechanisms become involved, including in extrahepatic tissues. These processes include pinocytosis (transcytosis), phagocytosis mediated by scavenger receptors, filtration through paracellular gaps and lateral diffusion through transendothelial channels. These highly regulated mechanisms can increase the plasma LDL-c concentration20.
The cholesterol content of the aortic wall was significantly different from group 1 only at very high levels of hypercholesterolemia (groups 9 and 10) and therefore did not parallel the increase in plasma total cholesterol and plasma LDL-c. This limited uptake of cholesterol and LDL-c by the arterial wall via the non-receptor-mediated mechanisms mentioned above20 may protect the tissue from an excessive accumulation of intracellular cholesterol that could damage the cell.
There was no direct relationship between the in-crease in plasma total cholesterol and LDL-c concentrations and the cholesterol content or degree of lipid peroxidation in the aortic wall.
Hypercholesterolemia is a risk factor for endothelial dysfunction and is reflected in a loss of vascular reactivity. However, hypercholesterolemia and the presence of LDL-c in the arterial wall cannot cause endothelial dysfunction by themselves, unless the subendothelial cholesterol (LDL-c), undergoes oxidative modifications. The usual risk factors for atherosclerosis increase the production of reactive oxygen species (ROS) by endothelial cells, vascular smooth muscle cells and adventitial cells6,22. Hypercholesterolemia, diabetes mellitus, arterial hypertension, smoking, age and intolerance to nitrates increase the production of ROS, which induce the expression of adhesion molecules, stimulate the proliferation and migration of vascular smooth muscle cells, mediate apoptosis in endothelium, cause lipid oxidation, activate matrix metalloproteinases and alter the vasomotor activity. Nitric oxide (NO) is currently considered the main mediator responsible for endothelium-depen-dent relaxation and for normal endothelial func-23,24. A decrease in NO can result in endothelial dysfunction, including vasoconstriction, platelet activation and enhanced migration of LDL-c into the intima. Oxidative stress can reduce the bioavailability of NO and lead to endothelial dysfunction. Consequently, inhibitors of LDL oxidation may have a beneficial effect on eNOS activity in caveo-23,25. In agreement with this, experimental studies in hypercholesterolemia in rabbits have shown that endothelial dysfunction is mediated by LDL-c oxidation11,13.
In contrast, there was no corelation between the extent of vascular lipid peroxidation and the plasma total cholesterol or LDL-c concentrations. This lack of correlation suggests that there may be protective mechanisms operating in the vascular wall to prevent lipid peroxidation, despite increased levels of circulating cholesterol and LDL-c.
Using this same experimental model, Jorge et al7,11,27, demonstrated that LDL-c enters endothelial cells via endocytosis where it is oxidized or transported to the intima. This mechanism may be modulated by the intra- and extracelullar concentrations of lipoproteins. A regulated cellular uptake of LDL-c may prevent the intracellular accumulation of excessive amounts of lipids, despite elevated levels of plasma cholesterol and LDL-c.
Clinically, therapies used to treat hypercholesterolemia are aimed at preventing or reducing lipid peroxidation and preserving endothelial function, with a consequent decrease in the frequency of cardiovascular, cerebrovascular and peripheral events. Our results suggest that a beneficial effect on lipid peroxidation and endothelial dysfunction would be obtained only by reducing the hypercholesterolemia at very low values (as seen in group 3). The current strategies for reducing lipids in humans (particularly the use of older statins) tend to decrease cholesterol levels by ~30%, and could explain the limited success in preventing cardiovascular events reported by multicentric clinical studies22,28-30.
In conclusion, hypercholesterolemia results in endothelial dysfunction that is associated with the accumulation of cholesterol and enhanced lipid peroxidation in the blood vessel wall.
Correspondence: Prof. E.A. Almeida. Department of Internal Medicine. Faculty of Medical Sciences. State University of Campinas (UNICAMP). PO Box 6111, 13083-970 Campinas. São Paulo. Brazil. Correo electrónico: eros@fcm.unicamp.br
Recibido el 12 de julio de 2007 y aceptado el 2 de octubre de 2007.