microRNA (miRNA) expression profile of extracellular vesicles is a potential tool for clinical practice. Despite the key role of vascular smooth muscle cells (VSMC) in cardiovascular pathology, there is limited information about the presence of miRNAs in microparticles secreted by this cell type, including human coronary artery smooth muscle cells (HCASMC). Here, we tested whether HCASMC-derived microparticles contain miRNAs and the value of these miRNAs as biomarkers.
MethodsHCASMC and explants from atherosclerotic or non-atherosclerotic areas were obtained from coronary arteries of patients undergoing heart transplant. Plasma samples were collected from: normocholesterolemic controls (N=12) and familial hypercholesterolemia (FH) patients (N=12). Both groups were strictly matched for age, sex and cardiovascular risk factors. Microparticle (0.1–1μm) isolation and characterization was performed using standard techniques. VSMC-enriched miRNAs expression (miR-21-5p, -143-3p, -145-5p, -221-3p and -222-3p) was analyzed using RT-qPCR.
ResultsTotal RNA isolated from HCASMC-derived microparticles contained small RNAs, including VSMC-enriched miRNAs. Exposition of HCASMC to pathophysiological conditions, such as hypercholesterolemia, induced a decrease in the expression level of miR-143-3p and miR-222-3p in microparticles, not in cells. Expression levels of miR-222-3p were lower in circulating microparticles from FH patients compared to normocholesterolemic controls. Microparticles derived from atherosclerotic plaque areas showed a decreased level of miR-143-3p and miR-222-3p compared to non-atherosclerotic areas.
ConclusionsWe demonstrated for the first time that microparticles secreted by HCASMC contain microRNAs. Hypercholesterolemia alters the microRNA profile of HCASMC-derived microparticles. The miRNA signature of HCASMC-derived microparticles is a source of cardiovascular biomarkers.
Los datos sobre la presencia de microARN en micropartículas liberadas por células de músculo liso vascular (VSMC) y en particular de las células de músculo liso de arteria coronaria humana (HCASMC) son limitados. Por ello, hemos analizado el contenido de miARN en micropartículas liberadas por HCASMC y su posible potencial como biomarcadores.
MétodosLos explantes de arterias coronarias se obtuvieron de pacientes sometidos a trasplante de corazón. Las muestras de plasma se obtuvieron de 2 grupos de estudio: controles normocolesterolémicos (n=12) y pacientes con hipercolesterolemia familiar (HF) (n=12). El aislamiento de micropartículas (0,1-1μm) se llevó a cabo mediante técnicas estándar. La expresión de los miARN típicos de VSMC (miR-21-5p, -143-3p, -145-5p, -221-3p y -222-3p) se analizó mediante RT-qPCR.
ResultadosEl ARN total aislado a partir de micropartículas procedentes de HCASMC presentó miARN típicos de VSMC. La exposición de HCASMC a condiciones de hipercolesterolemia indujo la reducción en la expresión de miR-143-3p y de miR-222-3p en las micropartículas. Los niveles de expresión de miR-222-3p en micropartículas circulantes fueron inferiores en pacientes de HF en comparación con controles normocolesterolémicos. Las micropartículas liberadas por áreas de placa aterosclerótica mostraron una disminución de los niveles de miR-143-3p y de miR-222-3p en comparación con las de áreas no ateroscleróticas.
ConclusionesDemostramos la presencia de microARN en micropartículas liberadas por HCASMC. La exposición celular a hipercolesterolemia altera el perfil de microARN de estas micropartículas. El perfil de microARN de micropartículas liberadas por HCASMC es una fuente de biomarcadores.
microRNAs (miRNAs) are small, single-stranded, non-coding RNA molecules (∼22 nucleotides) involved in the epigenetic regulation by binding to the target mRNA. miRNAs have emerged as key regulators of a variety of regulatory pathways involved in cellular development, differentiation, metabolism, homeostasis and response to stress.1 Genetic studies have demonstrated that miRNAs are essential for cardiovascular physiology.2 Dysregulation of intracellular miRNA expression is a hallmark of a wide array of cardiovascular disorders.3 In addition to their intracellular location, miRNAs have been found in almost all body fluids either transported in microvesicles (microparticles, exosomes and apoptotic bodies) or non-vesicle protein/lipoprotein complexes.4,5 Extracellular miRNAs participate in cell-to-cell communication.6 Cells actively export miRNA which act as autocrine, paracrine and endocrine factors by regulating gene expression of recipient cells.7,8
The release of miRNAs to extracellular milieu and bloodstream in response to cell stress or damage supports its study as biomarkers of disease. Circulating miRNAs (c-miRNAs) have attractive biochemical properties to become excellent clinical indicators,9 including high tissue specificity, rapid release kinetics, great stability and long half-life. miRNA evaluation is relatively inexpensive using standard techniques already established in clinical laboratories and its analysis is performed with higher sensitivity and specificity than protein-based biomarkers. To date, several existing lines of evidence have demonstrated that circulating miRNAs could be used as biomarkers of cardiovascular conditions, including atherosclerosis, acute coronary syndrome and heart failure.10 Some miRNAs exhibit higher diagnostic and prognostic value that the established gold standard, such as troponins and natriuretic peptides.11 The clinical application of c-miRNA as biological markers of cardiovascular disease has been proposed in the short-medium term.12
Vascular smooth muscle cells (VSMC) are the main constitutive stromal cells of the vasculature. VSMC play a key role in maintaining vessel wall integrity and regulating arterial tone. Indeed, maladaptive phenotypic modulation of VSMC contributes to a number of vascular-related pathological conditions including atherosclerosis, aneurysm and restenosis.13 Previous data from our group showed that human coronary artery smooth muscle cells (HCASMC) are a potential source of biomarkers for monitoring vascular physiology and disease.14 In this context, recent studies have demonstrated that miRNA-containing microvesicles from a variety of cell types could be used as molecular signatures of disease.15 Surprisingly, the presence of miRNAs in HCASMC-derived microvesicles has not been previously addressed. Here, we hypothesized that microparticles released from HCASMC contain miRNAs and that the miRNA profile of HCASMC-derived microparticles has a potential clinical value as biomarker. Our study identifies the presence of miRNAs in HCASMC-derived microparticles and characterizes the expression pattern of VSMC-enriched miRNAs in response to hypercholesterolemia conditions.
Patients and methodsHuman coronary artery smooth muscle cell isolation and culturePrimary cultures of HCASMC were obtained from the media layer of macroscopically healthy coronary artery segments collected from patients undergoing cardiac transplantation at Hospital de la Santa Creu i Sant Pau, Barcelona, Spain. HCASMC were isolated by a modification of the explant technique, as previously described.16 Cells used in the present experiments were between the fourth and sixth passage. HCASMC at these passages appeared as a relatively homogeneous cell population, showing a hill-and-valley pattern at confluence. Western blot analysis for specific differentiation markers revealed a positive band for α-actin (45kDa) and calponin (33kDa). Cell monolayers were grown in medium M199 (Life Technologies) supplemented with 20% fetal bovine serum (FBS) and 2% human serum, 2mmol/L l-glutamine, 100U/mL penicillin G, and 100μg/mL streptomycin. Cells quiescence was induced by maintaining the cell culture for 24h in medium with 0.2% FBS. HCASMC were incubated in absence or presence of nLDL and agLDL in medium deprived of serum.
Explants of coronary media layersExplants of media layers from atherosclerotic plaque segments and from non-atherosclerotic areas of the same coronary artery and patient were collected and seeded in different wells as previously described.14,16
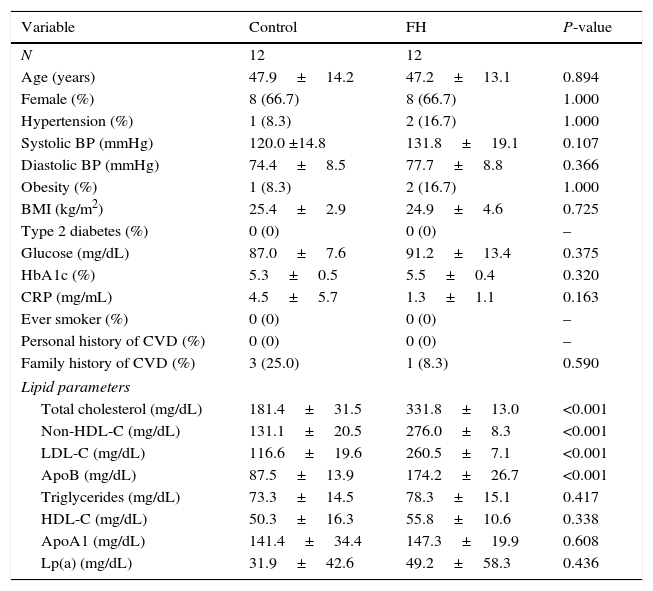
Study populationSelected subjects (N=12) were unrelated adults 29–67 years of age with the diagnosis of heterozygous familial hypercholesterolemia (FH): LDL-C above the 95th percentile of the Spanish population,17 triglycerides below 200mg/dL, primary cause, and familial presentation (at least one first-degree relative with the same phenotype) from the Lipid Clinic at Hospital Universitario Miguel Servet, Zaragoza, Spain. In all subjects, the presence of a functional mutation in the LDL receptor gene (LDLR) was confirmed as previously described.18 Secondary causes of hypercholesterolemia including: poorly controlled type 2 diabetes (HbA1c>8%), renal disease with glomerular filtration rate <30mL/min and/or macroalbuminuria, liver diseases (ALT>3 times upper normal limit), hypothyroidism (TSH>6mIU/L), pregnancy, autoimmune diseases and protease inhibitors were exclusion criteria. The same number of normolipidemic controls (LDL cholesterol under the 75th percentile and triglycerides <200mg/dL) were selected. This control group consisted of healthy, unrelated men and women volunteers aged 25–64 years, who underwent a medical examination at the Hospital Universitario Miguel Servet of Zaragoza. Exclusion criteria for normolipemic subjects were personal or parental history of premature cardiovascular disease or dyslipidemia, current acute illness, or use of drugs that might influence glucose or lipid metabolism. Blood sampling was performed in cases and controls after at least 10h fasting, and without lipid-lowering drug in the previous 6 weeks. Blood was collected in citrate-anticoagulated tubes. Patients and controls were strictly matched based on the following criteria: age, sex and cardiovascular risk factor profile (hypertension, diabetes, smoking, obesity, CRP, family history of cardiovascular disease, personal history of cardiovascular disease, triglyceride and Lp(a)).
The study was performed in full compliance with the Declaration of Helsinki and was approved by the corresponding medical ethics committees. Written informed consent was obtained from all participants.
LDL isolation and modificationHuman nLDL (d1.019–d1.063g/mL) was obtained from pooled sera of normocholesterolemic volunteers by sequential ultracentrifugation in KBr density gradient.16 Briefly, VLDL were first discarded after spinning plasma at 36,000rpm for 18h at 4°C using a fixed angle rotor (50.2 Ti, Beckman) mounted on an Optima L-100 XP ultracentrifuge (Beckman). Subsequently, VLDL-free plasma was layered with 1.063g/mL KBr solution and centrifuged at 36,000rpm for 18h at 4°C. LDLs were dialyzed against 0.02M Trizma, 0.15M NaCl, 1mM EDTA, pH 7.5, for 18h and then against normal saline for 2h. Finally, isolated LDLs were filter-sterilized (0.22-μm Millex-GV filter unit, Millipore). nLDL preparations were less than 24h old and without detectable levels of endotoxin (Bio Whittaker). Thiobarbituric acid reactive substances in nLDL were measured as an indirect evaluation of lipid peroxidation and were <1.2mmol malonaldehyde per mg protein. AgLDL was prepared by vortexing nLDL in PBS at room temperature. The formation of agLDL by vortexing was monitored by measuring the turbidimetry (at 680nm), as previously described.16 The percentage of nLDL in aggregated form was calculated by measuring the fraction of protein recovered in the pellet obtained after centrifugation at 10,000×g for 10min. The different fractions were analyzed by agarose electrophoresis (Beckmann). No significant alterations of thiobarbituric acid reactive substances levels against nLDL were detected after LDL aggregation.
Determination of free and cholesteryl ester contentHCASMC were exhaustively washed and harvested in NaOH 0.1M following the LDL, or agLDL incubation period. Lipids were extracted as previously described.16 Cholesteryl esters (CE) and free cholesterol (FC) content was analyzed by thin layer chromatography. The spots corresponding to CE and FC in the thin layer chromatography were quantified by densitometry against the standard curve of cholesterol palmitate and cholesterol, respectively, using a computing densitometer (Molecular dynamics).
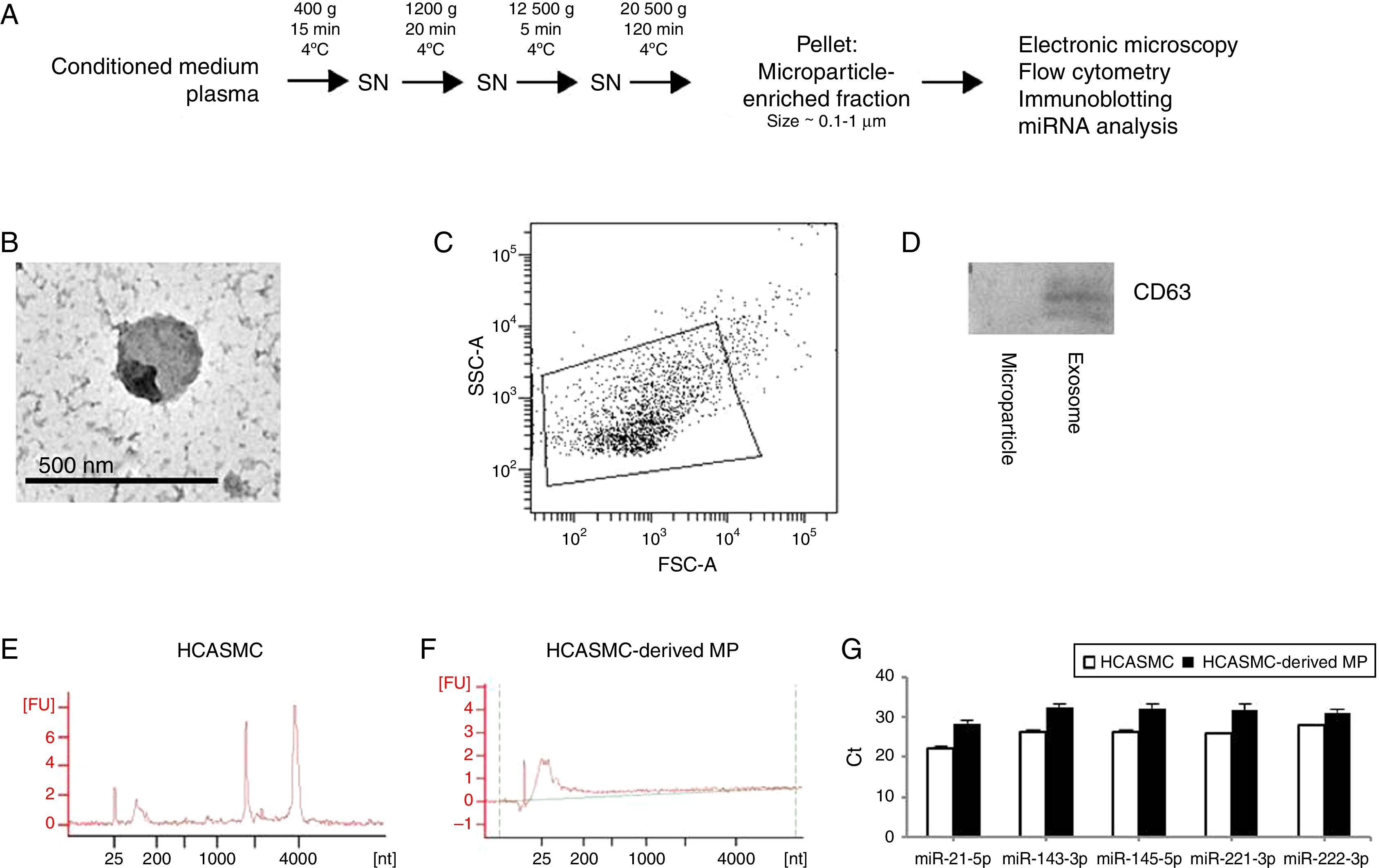
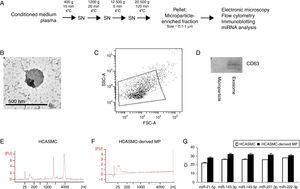
Isolation of microparticlesMicroparticle isolation protocol is showed in Fig. 1A. Briefly, supernatant was centrifuged at 400×g for 15min at 4°C, 1200×g for 20min at 4°C and 12,500×g for 5min at 4°C. For microparticle purification (0.1–1μm), the supernatant was centrifuged at 20,500×g for 120min at 4°C. Identical centrifugation steps were performed to obtain microparticles from conditioned medium or plasma. The pelleted microparticles were carefully resuspended in PBS and stored at −80°C. Purity and characteristics of the isolated microparticles were evaluated using transmission electron microscopy (TEM), flow cytometric analysis and immunoblotting.
HCASMC-derived microparticles contain microRNAs. Extracellular vesicles were isolated using repeated centrifugation and ultracentrifugation. (A) Microparticle isolation protocol. (B) Electron micrograph of microparticle-enriched fraction. Scale bar=500nm. (C) Determination of forward scatter (FSC) and side-scatter (SSC) characteristics of Annexin V-positive microparticles. (D) Western blot against CD63 in purified HCASMC microparticle- and exosome-enriched fractions. (E) Total RNA of cells was detected using Bioanalyzer nano analysis. (F) Total RNA of microparticle-enriched fraction was detected using Bioanalyzer pico analysis. (G) Expression of VSMC-enriched miRNAs in the microparticle-enriched fraction.
Detection of RNA was performed using Agilent 2100 Bioanalyzer® (Agilent Technologies). RNA was analyzed with the Agilent RNA 6000 Nano kit (Agilent Technologies) for cells, and Agilent RNA 6000 Pico kit (Agilent Technologies) for microparticles.
Gene expressionTotal RNA was isolated by TriPure™ isolation Reagent (Roche), according to the manufacturer's instructions. Total RNA concentration was determined with a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). Gene expression analyses of low density lipoprotein receptor-related protein 1 (LRP1), sterol regulatory element-binding protein 2 (SREBP-2), 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoAR) and LDLR were performed at mRNA level by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-qPCR). Predesigned assays were: LRP1 (Hs00233899_m1, Applied Biosystems), SREBP2 (Hs00190237_m1, Applied Biosystems), HMG-CoAR (Hs00168352_m1, Applied Biosystems) and LDLR (Hs00181192_m1, Applied Biosystems). Human GAPDH (4326317E, Applied Biosystems) was used as endogenous control. Taqman real-time PCR was performed with 1μL/well of reverse transcription products (1μg total RNA) in 10μL of TaqMan PCR Master Mix (Applied Biosystems), with the primers at 300nmol/L and the probe at 200nmol/L. PCR was performed at 95°C for 10min (for AmpliTaq Gold activation); and then run for 40 cycles at 95°C for 15s and 60°C for 1min. The threshold cycle (Ct) values were determined and normalized to endogenous control.
microRNA expressionQuantitative miRNA analysis was restricted to the VSMC-enriched miRNAs: miR-21-5p, miR-143-3p, miR-145-5p, miR-221-3p and miR-222-3p. These miRNAs have been associated with VSMC (patho-)physiology, including proliferation, differentiation, phenotype regulation, migration and apoptosis.19,20 All smooth muscle-enriched miRNAs have been previously examined in studies focused on cardiovascular biomarkers,21–24 and some of them, miR-21-5p and miR-145-5p, have been proposed as clinical indicator of different cardiovascular conditions.23,24
Total RNA extraction was performed using the mirVana PARIS kit (Thermo Fisher) for microparticles and nLDL, and the mirVana miRNA Isolation kit (Thermo Fisher) for cells, according to the manufacturer's instructions. RNA spike-in kit (Exiqon) was used in all extractions to determine the quality of the sample. When total RNA was extracted from microparticles, synthetic Caenorhabditis elegans miR-39-3p (cel-miR-39-3p), lacking sequence homology to human miRNAs, was added as an external standard. Cel-miR-39-3p was spiked into samples during RNA isolation after incubation with the denaturing solution. The mixture was supplemented with 1μg of MS2 carrier RNA (Roche) to improve miRNA yield, as recommended by the manufacturer. cDNA was synthesized using the universal cDNA synthesis kit II (Exiqon). miRNAs were quantified by RT-qPCR using the ExiLENT SYBR Green master mix (Exiqon) and a 7900HT Fast Real-Time PCR System (Applied Biosystems), with the following cycling conditions, 10min at 95°C, 40 cycles of 10s at 95°C and 1min at 60°C, followed by a melting curve analysis. The threshold cycle (Ct) was defined as the fractional cycle number at which the fluorescence exceeded a given threshold. All miRNAs were considered to be expressed when Ct values were less than 35. Relative quantification was performed using the 2−ΔCt method, where ΔC=mean Cttarget−mean Ctnormalizationcontrol.
Small nuclear U6, small nucleolar RNA SNORD48, miR-103a-3p and miR-191-5p were examined as internal normalization controls, as recommended by the manufacturer. Only SNORD48 fulfilled the following criteria: detectable in all samples and low dispersion of expression levels;25 and therefore, was used for normalization. As there is no known generally accepted standards for extracellular miRNAs, an internal control (SNORD48) and external control (c-miR-39-3p) were used for normalization of miRNA expression in microparticles, as previously proposed.26
Statistical analysisThe statistical software package SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Descriptive statistics were used to characterize study populations and to analyze the studied parameters. Data were presented as the mean±SD for continuous variables and as frequencies (percentages) for categorical variables. The normality of the data was analyzed using the Kolmogorov–Smirnov test. Continuous variables were compared between groups using a Student's t-test for independent samples, one-way ANOVA followed by Tukey's post hoc test for comparison between each subgroup and ANCOVA with adjustments for SBP and CRP to take into account differences between study groups. Differences were considered statistically significant when P<0.050.
ResultsMicroparticles secreted from HCASMC contain miRNAsMicroparticle-enriched fractions were prepared from conditioned medium of HCASMC (Fig. 1A). To confirm that the extracellular vesicles studied are microparticles, the microparticle-enriched fraction was subjected to electron microscopy (Fig. 1B) and flow cytometric analysis (Fig. 1C). Electron micrographs and flow cytometric analysis revealed vesicles with a size approximately 0.1–1.0μm, consistently with previously described microparticles. Accordingly, microparticle-enriched fraction was negative for the exosomal protein marker CD63 (Fig. 1D).
To characterize the microparticle RNA profile, we analyzed the total RNA extracted from microparticles and their donor cells using Agilent 2100 Bioanalyzer. As shown in Fig. 1E and F, total RNA profile was essentially different between HCASMC and HCASMC-derived microparticles. Cellular RNA profile showed a peak of small RNA and the peaks of 18S and 28S ribosomal RNAs. Analysis of electropherogram revealed that RNA isolated from microparticles contained small RNA, but was devoid of long RNA. The small RNA fraction is likely miRNA as evidence by its approximate length (∼25 nucleotides). In order to corroborate the presence of miRNAs, we analyzed the expression levels of five VSMC-enriched miRNAs: miR-21-5p, -143-3p, -145-5p, -221-3p and -222-3p by RT-qPCR. Microparticles derived from HCASMC contained detectable levels of all miRNAs examined (Fig. 1G).
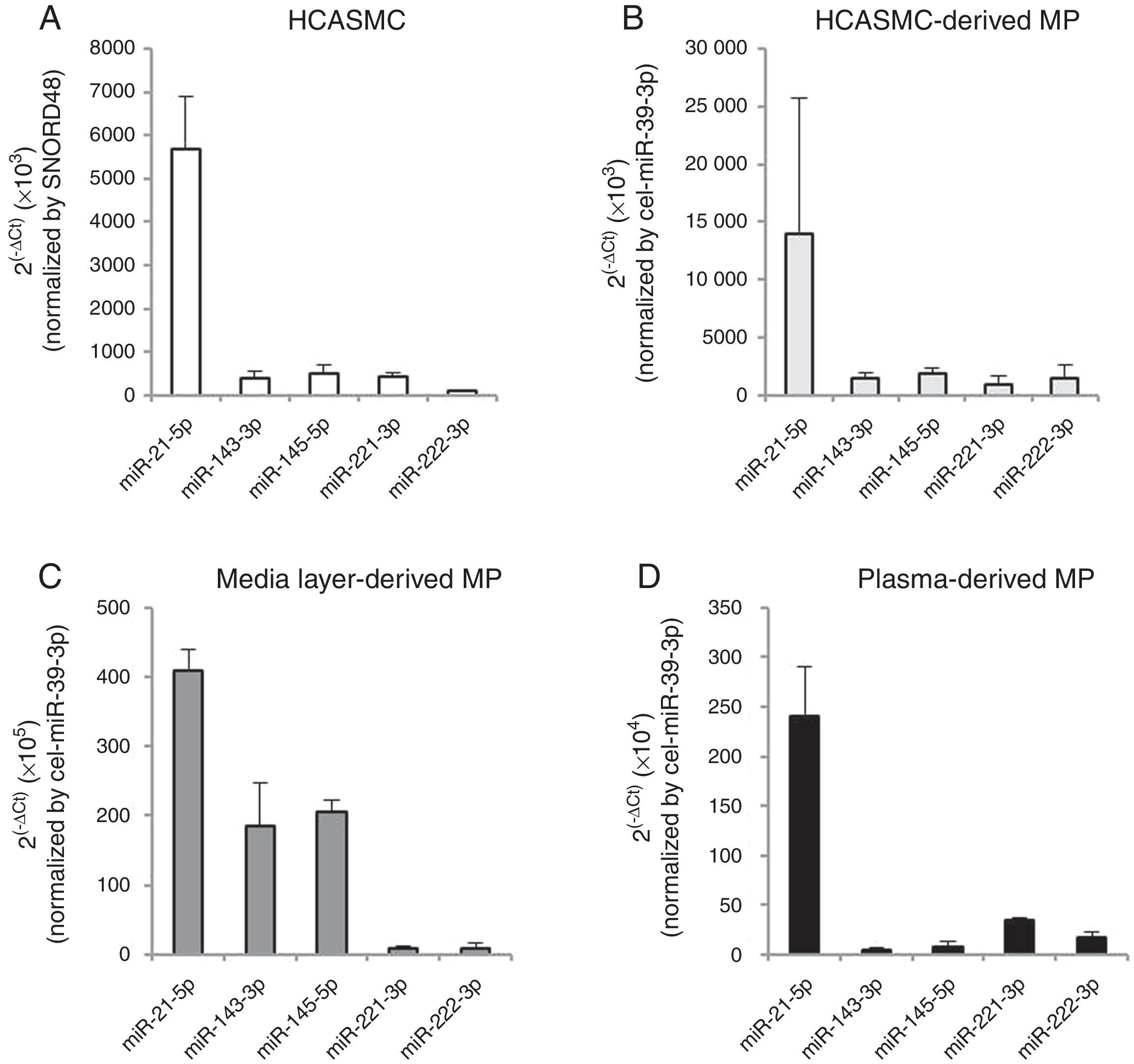
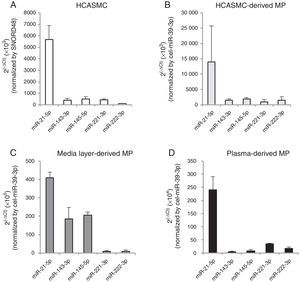
The VSMC-enriched miRNA profile was qualitatively compared between cells and microparticles. As shown in Fig. 2A and B, the expression pattern of miR-21-5p, -143-3p, -145-5p, -221-3p and -222-3p was similar in both HCASMC and its secreted microparticles. Based on ranking analysis, miR-21-5p was the highest expressed in both types of samples.
miRNA expression profile in HCASMC (A), HCASMC-derived microparticles (B), coronary artery media layer-derived microparticles (C) and plasma-derived microparticles. Bar graph showing mean±SD. Relative quantification was performed using SNORD48 for normalization in HCASMC and cel-miR-39-3p for normalization in HCASMC-, coronary artery media layer- and plasma-derived microparticles. MP: microparticle.
To explore the physiological relevance of these in vitro results, we analyzed the VSMC-enriched miRNA profile in microparticles isolated from conditioned medium of non-atherosclerotic areas of coronary artery media layers (Fig. 2C), and plasma of healthy subjects (Fig. 2D). All VSMC-enriched miRNAs were detected in both types of samples. Enrichment in miR-143-3p and miR-145-5p was observed in microparticles isolated from conditioned medium of coronary artery media layers compared to HCASMC-derived microparticles. Microparticles isolated from plasma of healthy subjects showed a similar VSMC-enriched miRNA pattern to that observed in microparticles secreted from HCASMC. For both type of samples, miR-21-5p was also the highest expressed miRNA.
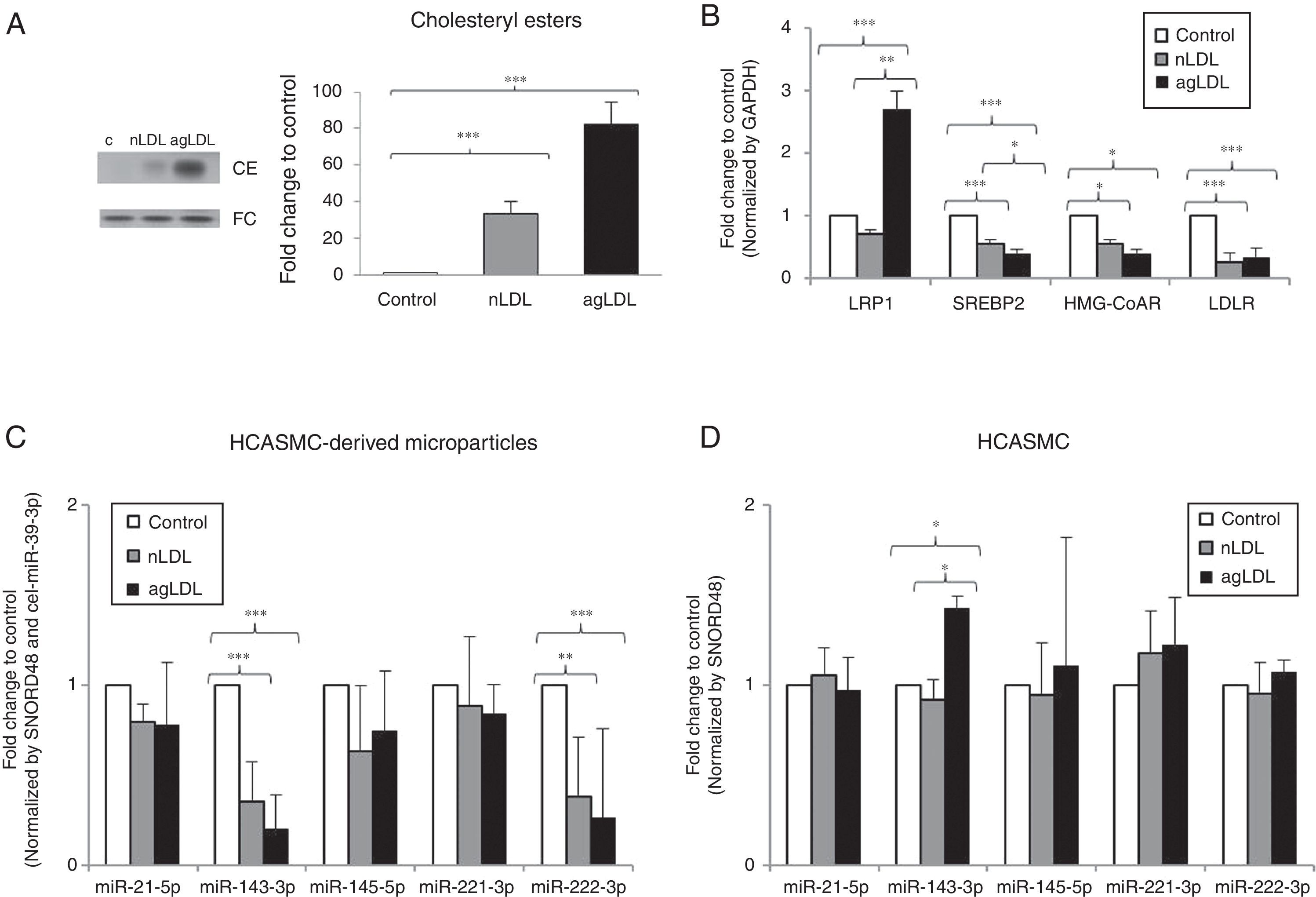
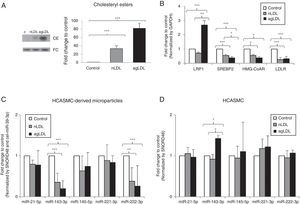
Hypercholesterolemia alters the miRNA profile of HCASMC-derived microparticlesOnce we demonstrated the presence of miRNAs in microparticles from HCASMC, we tested whether the miRNA profile of HCASMC-derived microparticles could be a potential source of biomarkers. To do that, we used a well-established in vitro model available in our group based on exposition of HCASMC to conditions mimicking hypercholesterolemia.27 Exposition of HCASMC to nLDL and agLDL increased intracellular CE levels (Fig. 3A). To examine the impact of exposition to hypercholesterolemia, we analyzed the mRNA expression of genes closely related to cholesterol homeostasis. As previously reported, agLDL strongly increased LRP1 mRNA levels in HCASMC (Fig. 3B). Both nLDL and agLDL reduced gene expression of SREBP2, HMG-CoAR and LDLR (Fig. 3B). Intracellular neutral lipid accumulation did not induce cell death (data not shown), apoptosis or necrosis, in agreement with previous results from our group.27
Effect of conditions mimicking hypercholesterolemia on microparticle and cellular miRNA expression profile. HCASMC were incubated for 24h in the absence or presence of nLDL or agLDL (100μg/mL) and cells/microparticles were collected for lipid and molecular studies. (A) Thin layer chromatography and bar graphs showing cholesteryl ester (CE) and free cholesterol (FC) bands and their quantification, respectively. (B) Real time PCR quantification of LRP1, SREBP2, HMG-CoAR and LDLR mRNA. (C) Real time quantification of HCASMC-derived microparticles miRNAs after exposure to nLDL and agLDL. Relative quantification was performed using GAPDH for normalization in HCASMC. (D) Real time quantification of HCASMC miRNAs after exposure to nLDL and agLDL. Results are expressed as a relative to control cells (incubated in absence of LDL or agLDL). Relative quantification was performed using SNORD48 and cel-miR-39-3p for normalization in HCASMC-derived microparticles, and SNORD48 for normalization in HCASMC. Data are shown as mean±SD. Differences between groups were analyzed using a Student's t-test for independent samples. *P<0.050; **P<0.010; ***P<0.001. MP: microparticle.
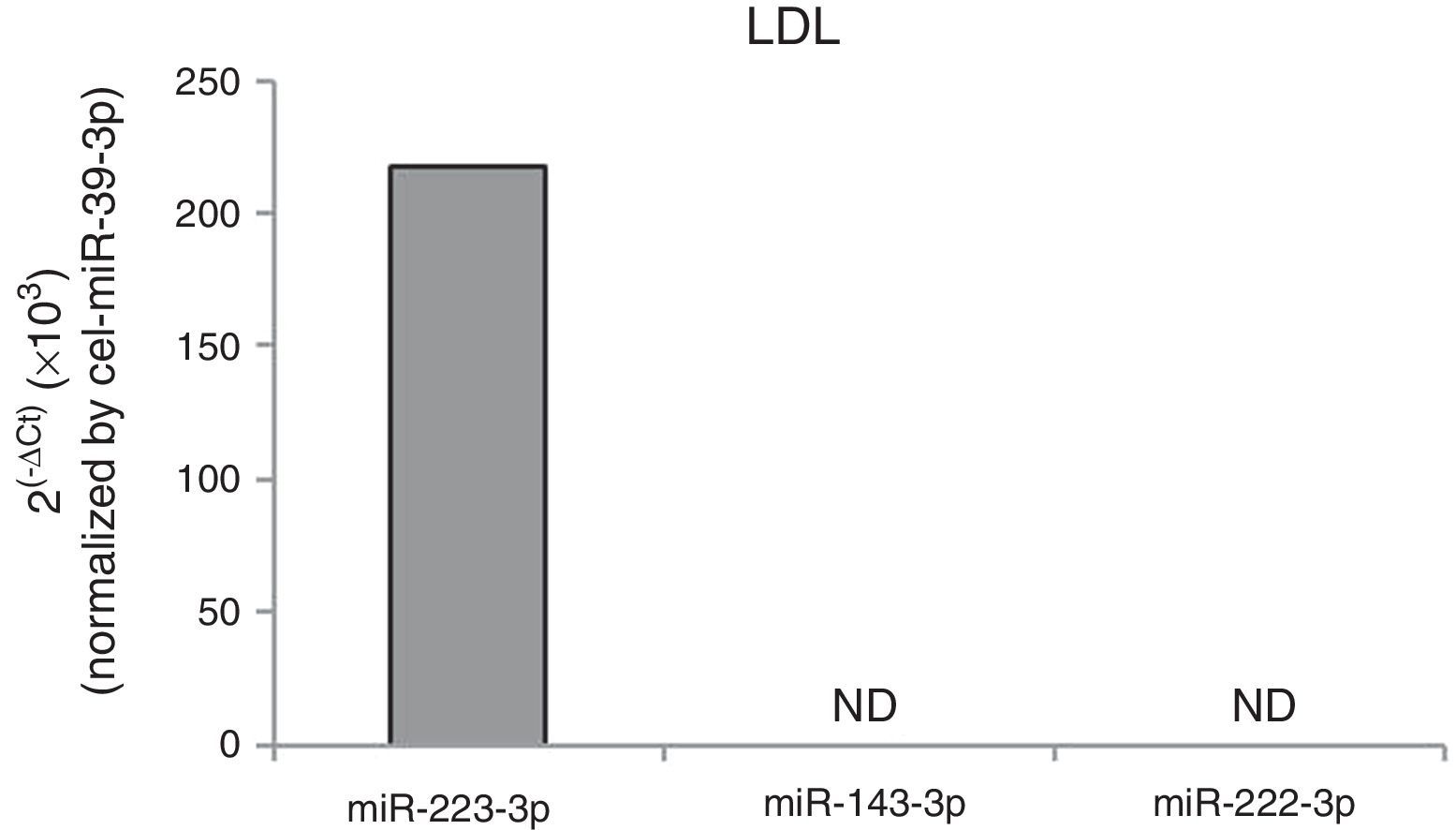
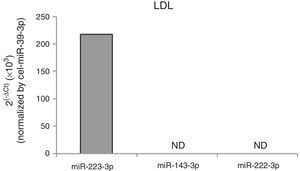
To determine whether cell exposition to hypercholesterolemia alters miRNA release from HCASMC, we examined the miRNA levels of HCASMC microparticles collected from conditioned medium before and after exposition to lipoproteins. Larger amounts of microparticles were observed after exposition to lipoproteins, with a higher increase in response to agLDL (fold change to control: nLDL=1.45±0.13, agLDL=1.71±0.29 microparticles/μl conditioned medium; P<0.050). Over 24h of cell exposition, both nLDL and agLDL decreased miR-143-3p (0.36-fold and 0.20-fold for nLDL and agLDL, respectively) and miR-222-3p (0.38-fold and 0.26-fold for nLDL and agLDL, respectively) expression levels in microparticles without differences between both conditions (Fig. 3C). Expression levels of miR-21-5p, -145-5p and -221-3p remained unchanged. We also evaluated the intracellular profiles of the VSMC-enriched miRNAs (Fig. 3D). AgLDL increased intracellular miR-143-3p expression levels compared to control or nLDL conditions (1.42-fold and 1.54-fold, respectively). No alteration in miR-21-5p, -145-5p, -221-3p or -222-3p intracellular expression levels was observed.miRNAs could be packaged in LDL.28 Therefore, we analyzed the presence of miR-143-3p and miR-222-3p in nLDL to control possible cross-contamination in our samples. Although we observed detectable expression levels of the miR-223, previously found associated with LDL,28 neither miR-143-3p, nor miR-222-3p were detected in nLDL (Fig. 4).
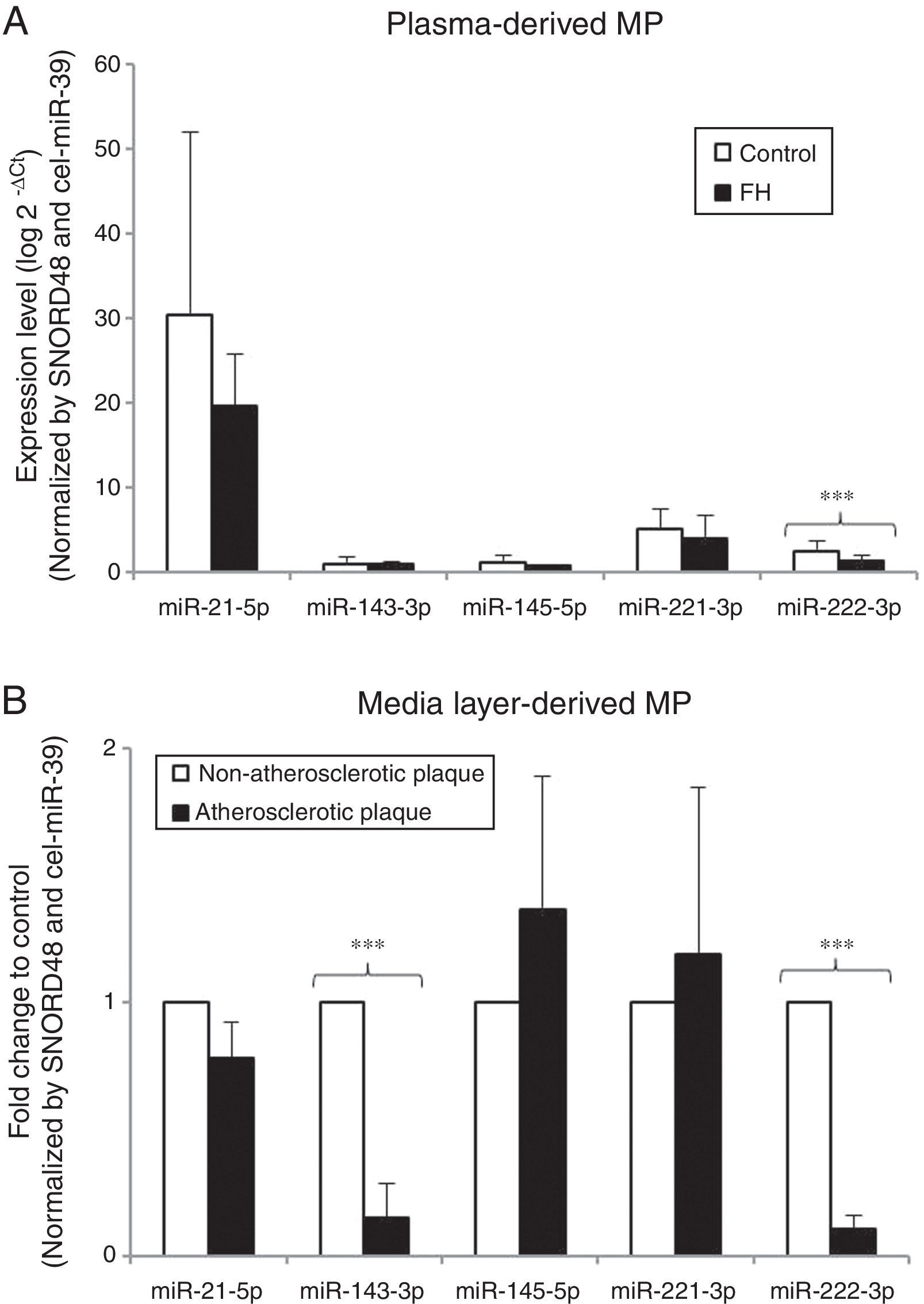
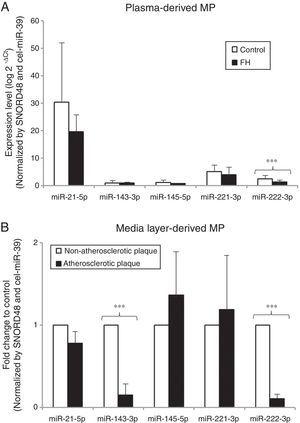
In vitro results suggest that hypercholesterolemia alters miRNA pattern of HCASMC-derived microparticles. To explore the biological relevance of current results, we next compared the miRNA profile of circulating microparticles isolated from strictly matched plasma samples of 12 normocholesterolemic subjects and 12 heterozygous FH patients. Table 1 summarizes the main clinical characteristics of the study groups. Control group was comparable in terms of age, sex and cardiovascular risk factors with the group of patients with FH. A higher level of plasma circulating microparticles was observed in FH patients than in control subjects (control group=2018.22 ± 592.95, FH group=4720.80 ± 1120.39microparticles/μl plasma; P<0.050). All miRNAs were detected in microparticles isolated from plasma of both control subjects and FH patients. miR-222-3p expression levels were lower in microparticles isolated from plasma of FH patients than in those isolated from plasma of normocholesterolemic subjects (0.54-fold) (Fig. 5A), even after adjusting by SBP and CRP (P<0.001 for both comparisons). There were no differences in plasma levels of miR-21-5p, -143-3p, -145-5p or -221-3p between groups.
Characteristics of study groups.
| Variable | Control | FH | P-value |
|---|---|---|---|
| N | 12 | 12 | |
| Age (years) | 47.9±14.2 | 47.2±13.1 | 0.894 |
| Female (%) | 8 (66.7) | 8 (66.7) | 1.000 |
| Hypertension (%) | 1 (8.3) | 2 (16.7) | 1.000 |
| Systolic BP (mmHg) | 120.0 ±14.8 | 131.8±19.1 | 0.107 |
| Diastolic BP (mmHg) | 74.4±8.5 | 77.7±8.8 | 0.366 |
| Obesity (%) | 1 (8.3) | 2 (16.7) | 1.000 |
| BMI (kg/m2) | 25.4±2.9 | 24.9±4.6 | 0.725 |
| Type 2 diabetes (%) | 0 (0) | 0 (0) | – |
| Glucose (mg/dL) | 87.0±7.6 | 91.2±13.4 | 0.375 |
| HbA1c (%) | 5.3±0.5 | 5.5±0.4 | 0.320 |
| CRP (mg/mL) | 4.5±5.7 | 1.3±1.1 | 0.163 |
| Ever smoker (%) | 0 (0) | 0 (0) | – |
| Personal history of CVD (%) | 0 (0) | 0 (0) | – |
| Family history of CVD (%) | 3 (25.0) | 1 (8.3) | 0.590 |
| Lipid parameters | |||
| Total cholesterol (mg/dL) | 181.4±31.5 | 331.8±13.0 | <0.001 |
| Non-HDL-C (mg/dL) | 131.1±20.5 | 276.0±8.3 | <0.001 |
| LDL-C (mg/dL) | 116.6±19.6 | 260.5±7.1 | <0.001 |
| ApoB (mg/dL) | 87.5±13.9 | 174.2±26.7 | <0.001 |
| Triglycerides (mg/dL) | 73.3±14.5 | 78.3±15.1 | 0.417 |
| HDL-C (mg/dL) | 50.3±16.3 | 55.8±10.6 | 0.338 |
| ApoA1 (mg/dL) | 141.4±34.4 | 147.3±19.9 | 0.608 |
| Lp(a) (mg/dL) | 31.9±42.6 | 49.2±58.3 | 0.436 |
Data are presented as mean±SD or frequency (%).
Evaluation of microRNA expression profile in microparticles as potential biomarker. (A) Real time quantification of plasma-derived microparticles from normocholesterolemic controls (N=12) and patients with FH (N=12). Results are expressed as arbitrary units. (B) miRNA profile in microparticles derived from atherosclerotic plaques and non-atherosclerotic areas of media layers explants from the same coronary artery and patient. Results are showed as fold-change relative to non-atherosclerotic area. Differences between groups were analyzed using a Student's t-test for independent samples. ***P<0.001. Relative quantification was performed using SNORD48 and cel-miR-39-3p for normalization.
To further examine the potential value of miRNA containing HCASMC-derived microparticles as biomarkers, we analyzed the VSMC-enriched miRNA profile of microparticles isolated from conditioned medium of atherosclerotic plaques and non-atherosclerotic areas from media layers of the same coronary artery and patient. The number of microparticles was higher in the conditioned medium from atherosclerotic areas than in the medium from non-atherosclerotic areas (fold change to control: atherosclerotic plaque=2.01±0.10 microparticles/μl conditioned medium; P<0.050). Consistently with in vitro experiments, miR-143-3p and miR-222-3p levels were lower in microparticles isolated from conditioned medium of atherosclerotic plaque segments than in that from non-atherosclerotic areas (0.14-fold and 0.10-fold respectively) (Fig. 5B). There were no differences in miR-21-5p, -145-5p or -221-3p expression levels between study groups.
DiscussionThe role of HCASMC in cardiovascular disease and the potential of microvesicle-associated miRNAs as biomarkers prompted us to investigate whether HCASMC release miRNAs in membrane vesicles. We demonstrated that HCASMC secrete miRNAs in microparticles and that the miRNA pattern of the released microparticles changes under hypercholesterolemia conditions.
The investigation of extracellular miRNAs in the cardiovascular system has gained attention during last decade. Previous studies reported that cardiovascular cells including monocytes,29 cardiomyocytes30 and fibroblasts7 release microvesicles containing miRNAs. Recent seminal studies have shown that VSMC are target cells for extracellular miRNAs.6,31 Despite the critical role of HCASMC-derived microvesicles in the vascular wall pathophysiology,27 there is no previous data about the non-coding RNA content of microvesicles secreted by HCASMC. Here, we revealed for the first time that the release of miRNA-containing microparticles represents a constitutive HCASMC property. Interestingly, our data add information to the debate about whether the miRNA profile of exported microvesicles reflects the miRNA profile of maternal cell.5 Exposition to hypercholesterolemia conditions was associated with a different profile of miRNAs in cells and microparticles which indicated the existence of a regulated mechanism for the release of both miRNAs. Similar to leucocytes, endothelial cells and platelets,32 HCASMC-derived microparticles do not reflect the abundance of miRNAs after stimulation of the maternal cell. miRNA packaging into microvesicles may be an actively regulated process highly dependent of environmental conditions to which is exposed the maternal cell.33
Because the content of microvesicles may be reflective of the physiological status of the cells and organs they originate from, its analysis is considered as valuable source of clinical indicators.34 Thus, once we demonstrated the presence of miRNAs in microparticles secreted by HCASMC, we examined the potential value of miRNA-containing microparticle as biomarker of disease. Our combined in vitro and patient-based approach suggested that the miRNA profile of HCASMC-derived microparticles, in particular reduced miR-222-3p expression levels could be an indicator of HCASMC response to hypercholesterolemia. Current results confirm and extend previous findings showing miR-222-3p as a potential biomarker of atherosclerotic-related conditions. Indeed, expression levels of miR-222 were decreased in the sclerotic intima samples from patients with arteriosclerosis obliterans,35 and peripheral blood and serum of patients with atherosclerosis.36,37 The expression level of miR-222 in microparticles, among others miRNAs, was significantly lower in serum obtained from coronary heart disease (CHD) patients compared to serum obtained from healthy subjects.38 Our data support studies showing a close association between reduced levels of circulating miRNAs and conditions intimately linked to hypercholesterolemia, such as CHD.23,38,39 Importantly, the decreased miR-222-3p content was not related with a decrease in the number of microparticles. Therefore, the decrease in miR-222-3p circulating levels could be associated, at least in part, with a reduced packaging of miRNA into microvesicles in lipid-loaded HCASMC.
Current results might provide new insights into pathological mechanisms of atherosclerosis. Microvesicles are implicated in horizontal transfer of information via its cargo of protein, RNA and DNA.34 Extracellular miRNAs contribute to intercellular signaling mechanisms by mediating the repression of mRNA targets of the recipient cell in the vascular microenvironment as well as distal cells.29 Microparticles derived from VSMC have been previously identified in human atherosclerotic plaques.40 Thus, it is conceivable that the selective packaging of both miR-143-3p and miR-222-3p is part of a cellular response to hypercholesterolemia-induced vascular stress. miR-143-3p and miR-222-3p are key regulators of diverse biological and pathological processes not only in VSMC, but also in surrounding cells such as endothelial cells or monocytes.19,20,41
In conclusion, our results support for first time that the miRNA profile of HCASMC-derived microparticles could be potentially useful as a source of accessible clinical biomarkers of cardiovascular disease. The discovery of miRNAs in HCASMC-derived microparticles raises intriguing possibilities for better understanding the pathogenesis of cardiovascular disease and possibly novel miRNA-based therapeutic strategies.
Ethical disclosureProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
FundingThis work was supported by the Spanish Atherosclerosis Foundation/Spanish Atherosclerosis Society (Basic Research Award 2013).
This work was also supported by FIS PI14/01729 from Instituto de Salud Carlos III, co-financed by the European Fund for Regional Development (E.F.R.D), Fundació Marató TV3 (201521 10), PI12/1087 from Spanish Ministry of Economy and Red de Investigación Cardiovascular (RIC)RD12/0042/0027. DdG-C was a recipient of Sara Borrell grant from the Instituto de Salud Carlos III (CD14/00109).
Author contributionsIdea and design: DdG-C, VLL-C; Collection of data: DdG-C, AC, FC; Data analysis and interpretation: DdG-C, AC; Writing of the draft of the article: DdG-C, VLL-C; Critical review of article: AC, FC; Approval of the final version to be published: DdG-C, AC, VLL-C, FC.
Conflict of interestsThe authors declare no conflicts of interest.
The authors thank the support of Dr. Javier Crespo, Dr. Maribel Balldellou, Dr. Sandra Camino, Jose Manuel Rebled and Dr. Pablo Castro.