A nutraceutical combination containing berberine, policosanol, and red yeast rice, largely marketed in Europe (Armolipid Plus®) (AP), has been reported to induce significant improvements in plasma lipids, insulin resistance and other components of the metabolic syndrome. However, literature study designs and results were heterogeneous and it was thus necessary to systematically review and meta-analyse all relevant randomised clinical trials (RCTs) to explore and quantify the effects of the dietary supplement AP on lipid profile. The aim of our meta-analysis was the evaluation of the effect of AP on lipid profile.
Methods and resultsWe conducted a structures search on PubMed and Google Scholar to identify eligible articles published prior to 2015. Eleven RCTs were subjected to meta-analysis by means of random effects models using the Standardised Mean Differences approach (Hedges’ method) and the Mean Differences approach as a sensitivity analysis. Data from 11 randomised clinical trials, corresponding to 1970 nutraceutical combination and 1954 control patients (3924 total patients), were included after the peer evaluation and data extraction of two independent evaluators. Heterogeneity was significant in all models. A significant effect was found for all lipid parameters. The effect size (relative change from baseline (%)) was −1.3 (9.9%) for total cholesterol, −1.17 (−13.7%) for LDL-c, +0.17 (+3.7%) for HDL-c and −0.24 (−7.0%) for Triglycerides.
ConclusionThis meta-analysis confirms that the nutraceutical combination containing berberine, policosanol, and red yeast rice has shown to be an effective product for the improvement of the lipid profile.
Se ha observado que una combinación de productos nutricéuticos con berberina, policosanol y arroz de levadura roja, altamente comercializada en Europa (Armolipid Plus®) (AP), induce mejoras significativas del lipidograma, resistencia a la insulina y otros componentes del síndrome metabólico. Sin embargo, los diseños y resultados del estudio de la literatura fueron heterogéneos y por tanto es necesario realizar una revisión y metaanálisis sistemático de todos los ensayos clínicos aleatorizados (ECA) relevantes para explorar y cuantificar los efectos del suplemento dietético AP en el lipidograma. El objetivo de nuestro metaanálisis fue la evaluación del efecto de AP en el lipidograma.
Métodos y resultadosSe llevó a cabo una búsqueda estructurada en PubMed y Google Scholar para identificar los artículos aptos para publicación antes del 2015. Se sometieron once ECA a metaanálisis por medio de modelos de efectos aleatorios utilizando el enfoque de diferencias medias estandarizadas (método Hedges) y el enfoque de diferencias medias estandarizadas como análisis de la sensibilidad. Los datos de los once ensayos clínicos aleatorizados, correspondientes a 1970 pacientes con combinación de productos nutricéuticos y 1954 pacientes de control (3924 pacientes en total), se incluyeron después de la evaluación científica externa y la extracción de datos de dos evaluadores independientes. La heterogeneidad fue significativa en todos los modelos. Se encontró un efecto significativo para todos los parámetros lipídicos. El tamaño del efecto (cambio relativo conforme a los valores de referencia, %) fue de -1,3 (9,9%) para el colesterol total, -1,17 (-13,7%) para el colesterol LDL, +0,17 (+3,7%) para el colesterol HDL y de -0,24 (-7%) para los triglicéridos.
ConclusiónEste metaanálisis confirma que la combinación de productos nitrucéuticos con berberina, policosanol y arroz de levadura roja es un producto eficaz para la mejora del lipidograma.
Cardiovascular diseases (CVD) are the principal cause of death worldwide.1 CVD are the result of complex processes in which several classical risk factors such as abnormal lipid profile, elevated blood pressure (BP), smoking, diabetes, abdominal obesity,2 as well as other emerging risk factors such as oxidative stress, inflammation, endothelial dysfunction, antithrombotic activity and insulin resistance play major roles in its onset and development.3–5 Among all these existing risk factors for CVD, high cholesterol levels and atherogenic dyslipidaemia (high triglycerides and/or low HDL-cholesterol) are considered preeminents6 and his management must be placed to modify cardiovascular risk.
Consequently, lowering lipid levels is a fundamental goal in primary and secondary prevention of cardiovascular events. Lifestyle and diet changes are very important actions to improve lipid profile and to decrease cardiovascular risk and should precede any pharmacological intervention on dyslipidaemia. The treatment of dyslipidaemia with specific drugs is cost-effective in secondary prevention; but there is no rationale for the lifetime of hipolipidaemic drugs in primary prevention in subjects without cardiovascular symptoms and/or light/moderate cardiovascular risk, in whom therapeutic lifestyle changes appears to be better practicable and effective.7
In order to achieve a food supplement with beneficial effects on dyslipidaemias, different compositions have been used with natural products with hipolipidaemic activity. A dietary supplement is a product that is orally consumed and which is intended to supplement the usual diet to increase the intake of ingredients reputed to have clinical benefit. These supplements are, usually, an addition to the normal diet, and not as a conventional food or the sole item of a meal.8 A common strategy is the association of molecules with different mechanism of action to reduce the potential risk of dose-related adverse event without losing efficacy.
A dietary supplement (AP, Armolipid Plus®, Rottapharm S.L., Barcelona, Spain) has become commercially available and has generated considerable research interest. It combines well-defined, active, natural compounds such as red yeast rice extract, policosanol and berberine (which decrease cholesterol and triglycerides) folic acid (reduces homocysteine) and coenzyme Q10 and asthaxantine (anti-oxidants). AP has been reported to induce significant improvements in plasma lipids, insulin resistance and other components of the metabolic syndrome; the overall benefit appears to be an improvement in CVD risk in populations with hyperlipidaemia and medium-high CVD risk.9–12
Therefore, it was thus necessary to systematically review and meta-analyse all relevant RCTs to explore and quantify the effects of the dietary supplement Armolipid Plus® on lipid profile.
MethodsWe followed the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis).13
Search strategy and selection of trialsWe systematically searched PubMed and all pre 2015 literature examining the effects of dietary supplement Armolipid Plus® on lipid profile was included. No language or other restriction was imposed. The search terms used were: (a) (“berberine”[MeSH Terms] OR “berberine”[All Fields]) AND (“policosanol”[Supplementary Concept] OR “policosanol”[All Fields]), (b) (“dietary supplements”[MeSH Terms] OR (“dietary”[All Fields] AND “supplements”[All Fields]) OR “dietary supplements”[All Fields] OR “nutraceutical”[All Fields]) AND (“hypercholesterolaemia”[All Fields] OR “hypercholesterolemia”[MeSH Terms] OR “hypercholesterolemia”[All Fields]) AND (Clinical Trial[ptyp]), AND “humans” (MeSH Terms), (c) (“dietary supplements”[MeSH Terms] OR (“dietary”[All Fields] AND “supplements”[All Fields]) OR “dietary supplements”[All Fields] OR “nutraceutical”[All Fields]) AND “metabolic syndrome”[All Fields] AND (Clinical Trial[ptyp] AND “humans”[MeSH Terms]). We also conducted a search on Google Scholar (nutraceutical AND policosanol AND berberine) and In addition, we manually searched for relevant published studies and review articles.
Data extractionData were extracted from RCTs meeting the inclusion criteria. These data were the first author; year of publication; study design (blinding, type of control, description of drop-out and adverse events); ITT analysis available; number of subjects; the duration of intervention; and baseline, post-intervention and difference in lipid profiles measurements. The screening and selection of studies was conducted by the sponsor, and data extraction was performed independently by two reviewers. Disagreements in the evaluation of studies were largely were resolved through discussion.
Quantitative data synthesisAll pooling methods in this study were based on random effects models. Meta-analysis results and figures were elaborated by means of the “metacont” function of the “meta” package of the R software version 2.7.0 (R Development Core Team, Vienna, Austria).14
The main analysis was conducted using the Hedge's g Standardised Mean Differences (SMD) with corrections for small sample size.15 Since effect sizes, i.e. mean difference divided by the pooled SD, are non-dimensional measurements, the convention proposed by Cohen16,17 was used for the interpretation of the effect magnitude:small≈0.20, medium≈0.50 and large≈0.80. An additional sensitivity analyses was performed by using the difference of means weighted by inverse of variance method. Baseline values for the control group were pooled using a random effects model.
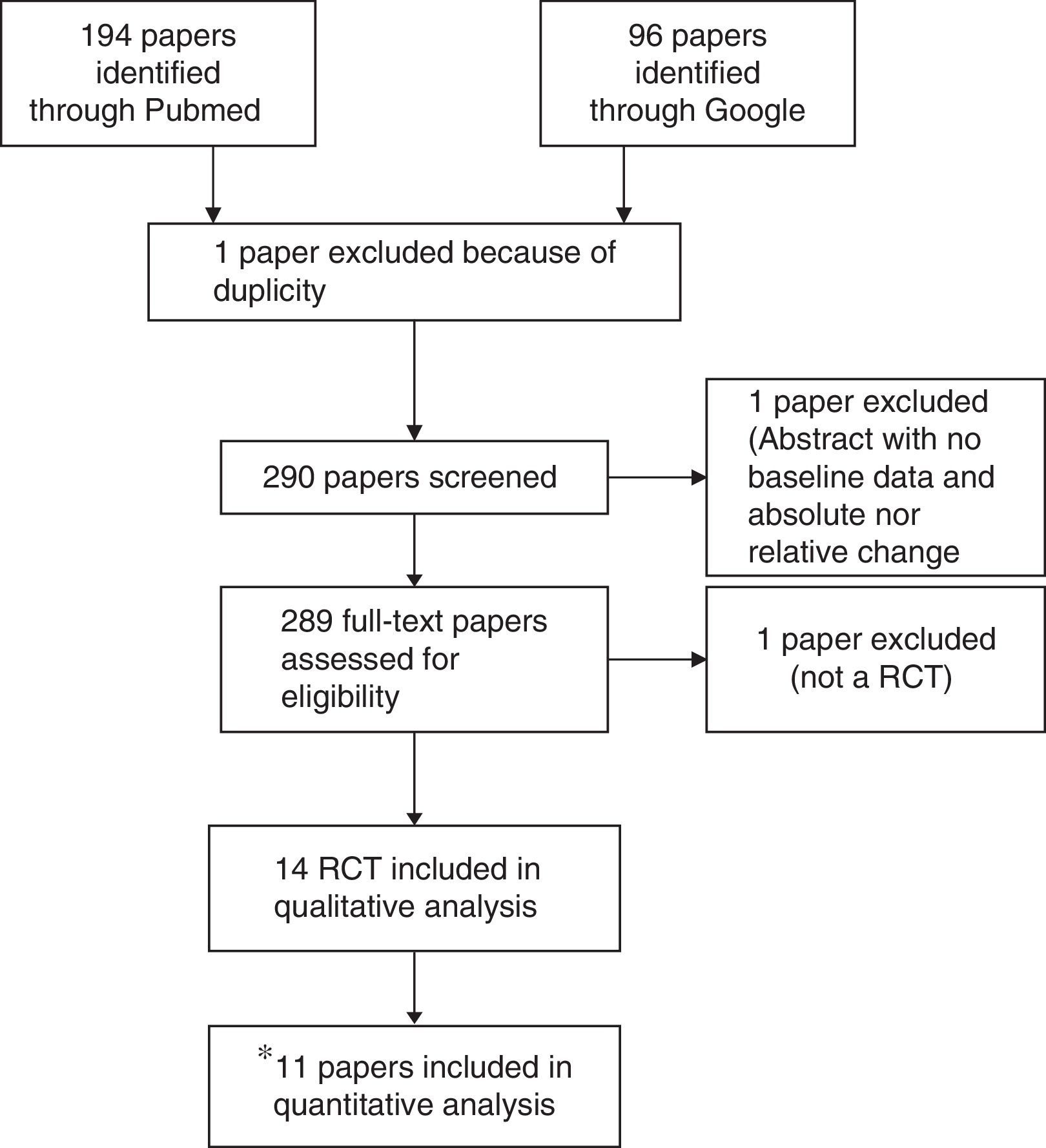
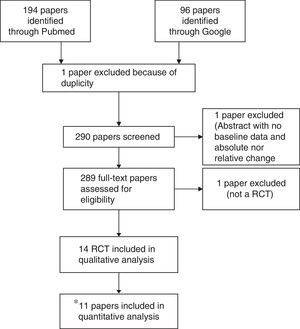
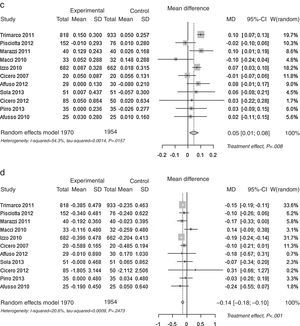
ResultsSearch resultsFrom the 290 initially identified papers, non-randomised clinical trial studies, or uncontrolled trials were excluded. Of the initially identified 14 studies,18–31 11 trials were valid according to the predefined criteria. One study was a meeting abstract (Pellicia 2012),29 which did not provide baseline and lipid change information to allow its inclusion. Other trial was a poster preliminary presentation (Puzo 2013)30 of a clinical trial paper which has been already submitted for peer review but not still published at the time of the meta-analysis (Solà 2013).26 Cicero 2013, which included hypercholesterolemic patients receiving all of them the nutraceutical, was excluded since it was a non-randomised comparison of patients with or without mild-to-moderate chronic kidney disease (CKD).31Fig. 1. A detailed description on study characteristics and the reasons for exclusions are included in Supplementary Table 1. Data from 11 published studies were included in this meta-analysis.
Flow diagram of study selection process. *One study was a meeting abstract (Pellicia 2012),28 which did not provide baseline and lipid change information to allow its inclusion. Other trial was a poster preliminary presentation (Puzo 2013)29 of a clinical trial paper which has been already submitted for peer review but not still published at the time of meta-analysis was done (Solà 2013).25 Cicero 2013, which included hypercholesterolemic patients receiving all of them the nutraceutical, was excluded since it was a non-randomised comparison of patients with or without mild-to-moderate chronic kidney disease (CKD).30
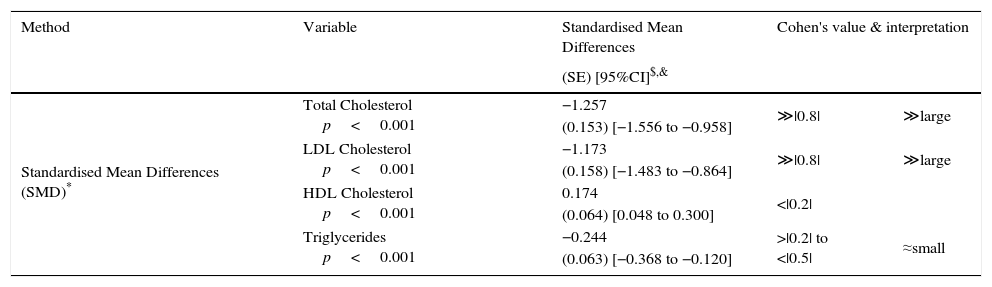
Pooled analysis of lipid variables.
| Method | Variable | Standardised Mean Differences | Cohen's value & interpretation | |
|---|---|---|---|---|
| (SE) [95%CI]$,& | ||||
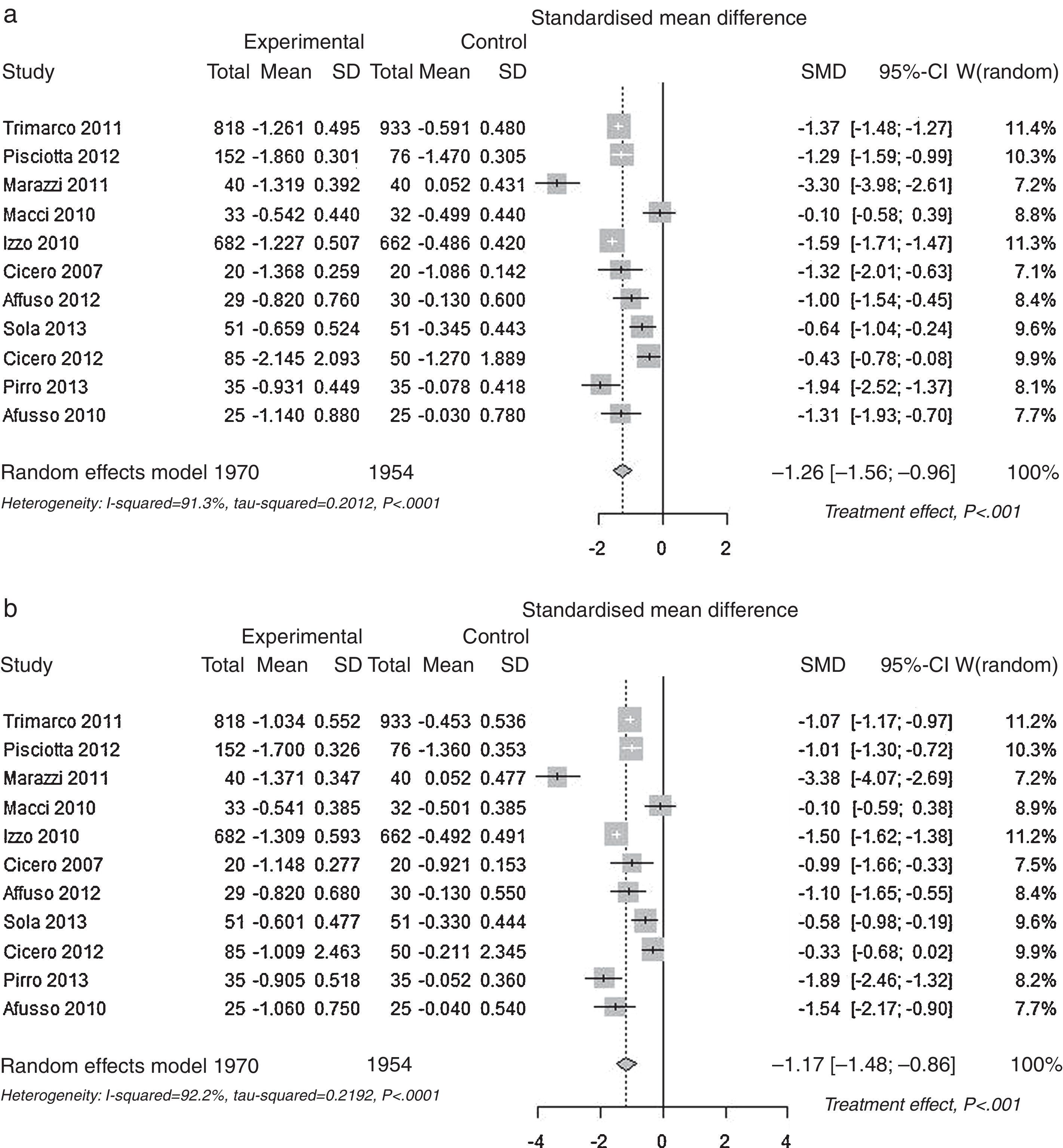
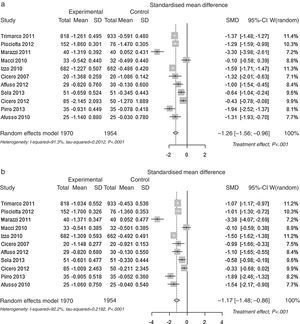
| Standardised Mean Differences (SMD)* | Total Cholesterol p<0.001 | −1.257 | ≫|0.8| | ≫large |
| (0.153) [−1.556 to −0.958] | ||||
| LDL Cholesterol p<0.001 | −1.173 | ≫|0.8| | ≫large | |
| (0.158) [−1.483 to −0.864] | ||||
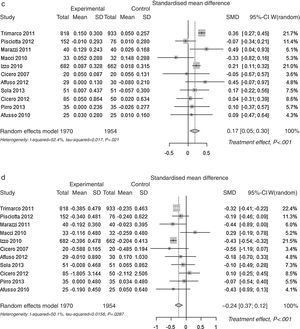
| HDL Cholesterol p<0.001 | 0.174 | <|0.2| | ||
| (0.064) [0.048 to 0.300] | ||||
| Triglycerides p<0.001 | −0.244 | >|0.2| to <|0.5| | ≈small | |
| (0.063) [−0.368 to −0.120] | ||||
| Method | Variable | Mean Differences | Mean baseline# | % Change |
|---|---|---|---|---|
| (SE) [95%CI]$,& | ||||
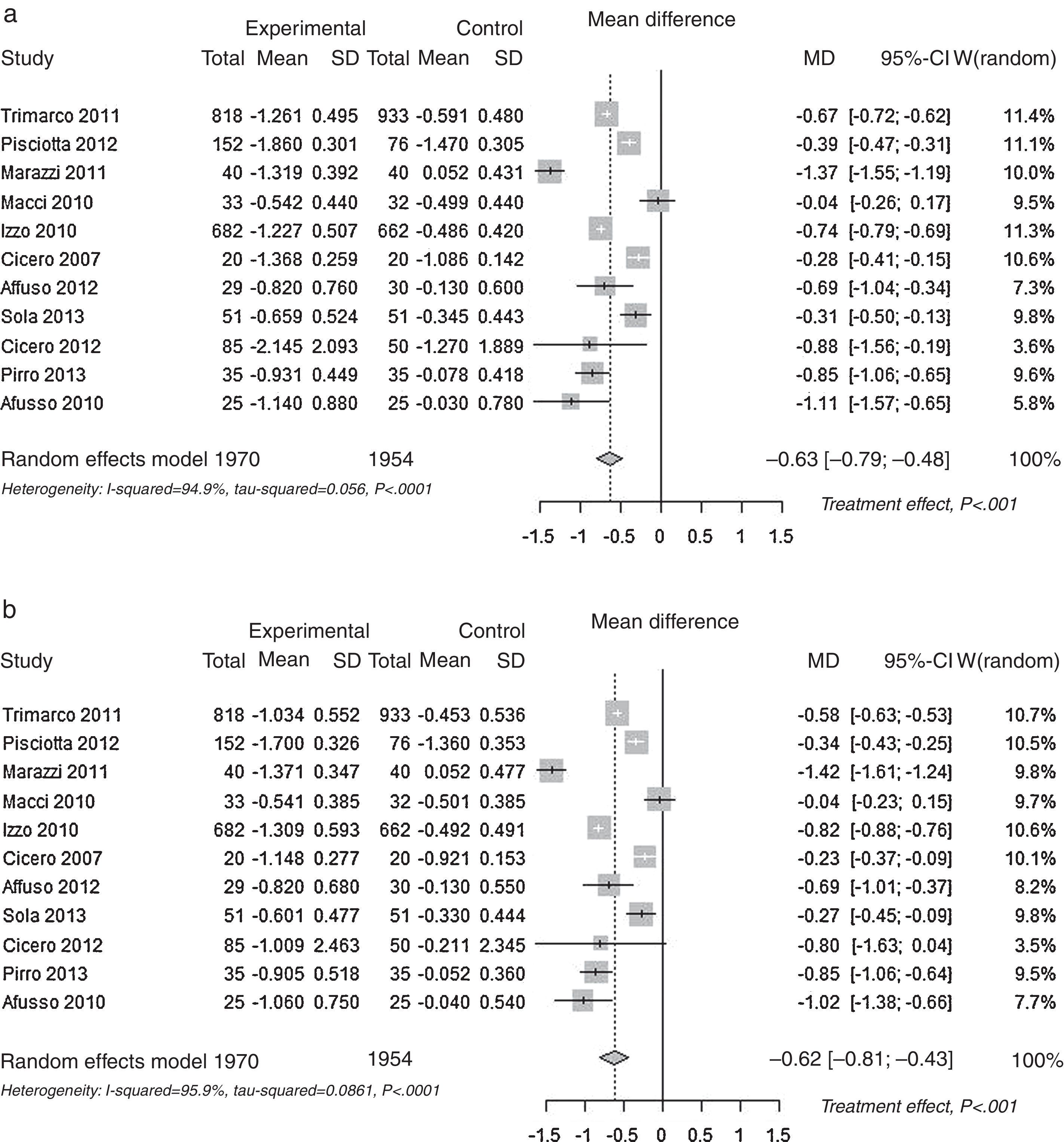
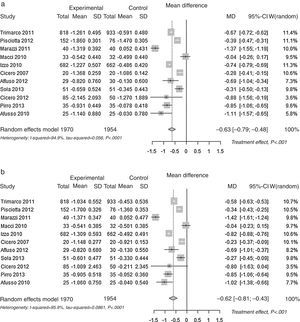
| Mean Differences (MD) (mmol/L) | Total Cholesterol p<0.001 | −0.633 | 6.41 | −9.9% |
| (0.080) [−0.790 to −0.475] | ||||
| LDL Cholesterol p<0.001 | −0.620 | 4.52 | −13.7% | |
| (0.096) [−0.808 to −0.432] | ||||
| HDL Cholesterol p<0.008 | 0.048 | 1.28 | 3.7% | |
| (0.017) [0.013 to 0.082] | ||||
| Triglycerides p<0.001 | −0.142 | 2.01 | −7.0% | |
| (0.021) [−0.184 to −0.100] |
All eligible studies were published between 2007 and 2013 (one submitted in the moment of the meta-analysis, now already published).26 Studies were heterogeneous in many characteristics: (a) sample size (minimum 40, maximum 1751); (b) level of blinding: 3 double-blinded, 3 single-blinded, 1 laboratory blinded and the rest were open or without clear information in this regards; (c) duration (minimum 6 weeks, maximum 1 year); (d) ITT analysis: 5 No, 6 Yes; (e) description of drop-out and adverse events: 2 No, 9 Yes and (f) type of control arm: pure placebo, Diet and lifestyle management, active treatment.
Pooled lipid profile analysisAccording to the predefined method, the unbiased Hedges’ g technique with the random approach, a significant effect was found for all parameters (p<0.01): Total Cholesterol, LDL, HDL and Triglycerides (Table 1). The effect may be considered always as statistically significant, for less than a small magnitude for HDL-Cholesterol, of small magnitude for triglycerides, and of large magnitude for Total Cholesterol and LDL-Cholesterol, according to the Cohen's criterion. As the Hedges’ g approach provides standardised means (means differences divided by its SD), please refer to the difference of means approach for absolute values (mean differences between control and experimental). The Mean Differences approach also lead to significant benefit for all lipid parameters analysed. The relative change was −9.9% for Total Cholesterol, −13.7% for LDL Cholesterol, +3.7% for HDL Cholesterol and −7.0% for Triglycerides. In Fig. 2 the results are shown as random effects pooled analysis of lipid profile variables using Standardised Mean Differences for (a) Total Cholesterol, (b) LDL Cholesterol, (c) HDL Cholesterol and (d) Triglycerides, in Fig. 3 the results are shown as Random effects pooled analysis of lipid profile variables (mmol/L) using Mean Differences for (a) Total Cholesterol, (b) LDL Cholesterol, (c) HDL Cholesterol and (d) Triglycerides.
Increasing evidence supports the antihyperlipidaemic efficacy of some nutraceuticals; so, the use of a combination of nutraceuticals with different but synergic mechanisms of action at lower and safer dosages appears to be an interesting alternative.32 In relation with that some nutraceuticals are prescribed as lipid-lowering substances.
It has been demonstrated that berberine upregulates the LDL-receptor and reduces serum levels of total cholesterol, LDL-cholesterol, and triglycerides.20,24 Furthermore, the efficacy of red yeast rice and policosanoid in reducing cholesterol concentrations were demonstrated in several large prospective clinical trials.20 These effects are related with the synergistic cholesterol lowering effects of policosanol, red yeast extracts and folic acid supported by clinical evidence.33 Since only few natural compounds of vegetal origin are effective in lowering blood triglycerides, could be taken with interest the experimental and clinical studies on berberine that elicits triglyceride-lowering effects, in addition to a significant hypocholesterolemia efficacy.34 In addition to the cholesterol and triglycerides lowering effects, berberine could also exert other relevant activities in cardiovascular prevention. Berberine inhibits platelet aggregation interfering selectively with the collagen-mediated adhesion process35 and could therefore be synergic with the antiplatelet activity shown by policosanol.36 Moreover, despite of its effect on lipid profile, berberine has proved to be effective in improving glycemic control with potential beneficial effect alone or in association with antidiabetic drugs37 and in overweight patients berberine induces improvement in hepatic steatosis index when is administrated as lipid-lowering nutraceuticals in mixed dyslipiemia patients. Once again, in the last paper a clearly effect lowering triglycerides was found.38
The major components of this combination of nutraceuticals are red yeast rice extract, which contains monacolins that compete structurally at HMGCoA reductase levels with HMGCoA, precursor of mevalonate, chemically identical to lovastatin, thus reducing plasma cholesterol; and berberine, a natural extract that reduces circulating LDL-cholesterol levels by increasing LDL receptor on the liver cell surface (up-regulates LDL receptors), and inhibiting triglycerides biosynthesis via the activation of AMP activated proteokinase.39
The results, with the combination of nutraceuticals, in terms of LDL-cholesterol reduction seem to be superior that those observed in trials where different components were used individually: berberine,40 red yeast rice41,42 or policosanol.43,44 This could be explained by a synergistic effect of the components that reduce cholesterol through different mechanisms of action.
The present meta-analysis produced reliable data because of the methodological issues. In fact, PRISMA guidelines have been updated to address several conceptual and practical advances in the science of systematic reviews.13 In our meta-analysis the nutraceutical combination was able to reduce significantly both total cholesterol and LDL-cholesterol. The observed changes in LDL-cholesterol, even if not very large, could be very appropriate in primary prevention subjects and could be useful to monitoring the potential improve of estimated cardiovascular risk by standardised methods (Framingham, SCORE). When combined with policosanol, red yeast extract and berberine increased the antihypercholesterolemic activity and maintained its peculiar triglyceride-lowering and HDL-increasing activity, conferring to the nutraceutical a large spectrum of effects, which could be particularly useful as food supplement for primary prevention of cardiovascular risks in subjects with moderate mixed dyslipidaemias. Furthermore, food supplements, reinforcing the subject's motivation to comply with the lifestyle changes, can lead to postpone the prescription of lipid lowering drugs and the resulting risk of adverse reactions, and even to be used as effective treatment in statin intolerant patients45 and the tolerability has also been confirmed in elderly hypercholesterolemic subjects.24
All lipids parameters showed a benefit for the nutraceutical product compared to control. Using the main approach (Hedges’ method) the effect was of large magnitude particularly for total cholesterol (−9.9%) and LDL-cholesterol (−13.7%) and of less magnitude for HDL-cholesterol (+3.7%) and for triglycerides (−7.0%), and the sensitivity analysis based on mean differences showed similar results. A dietary supplement, Armolipid Plus has shown to be an effective product for the improvement of the lipid profile.
In all of previous trials the tolerability of the product was always very high and the compliance almost always complete. Probably because of the combination of two low-dosed compounds reduce the incidence of the known possible adverse event of the fully dosed single components.46 Furthermore, the insignificant side effects could be considered to justify the choice of this approach, that is also very well accepted by patients as has been recently published in a follow-up of self-paying patients taking nutraceuticals with mild hyperlipidemia.47
Despite this meta-analysis has been designed, conducted and reported in accordance with the scientific recommendations,13 it has an obvious limitation due to the observed between-study heterogeneity. This guidance recommends exploring the source of heterogeneity, however the consensus on how to do it is minimal, and formal recommendations on how to investigate clinical heterogeneity in systematic reviews of controlled trials are required.48 Some authors state that heterogeneity is in general caused by the definitions of the outcome across the individual studies, dosage and schedule of treatments and, inclusion/exclusion criteria for subjects in the individual studies.49 We have studied the sources heterogeneity in our meta-analysis and there was no clear conclusion. Of note, our meta-analysis consists of 11 studies and there was not much room for grouping or excluding studies. In fact, even when considering the evidence of the 3 biggest studies, despite the effect was significant the heterogeneity was still present. Thus, we have to accept that this is a limitation and that heterogeneity is present but without no apparent clinical explanation. However, despite the results should be taken with caution because of the heterogeneity, we consider that the positive conclusions of this meta-analysis may be sufficiently supported by the solid statistical significance, the high precision of the estimates and, the inclusion of all clinical trials in this setting.
As conclusion, the available data supports the rationale for recommending this combination of nutraceuticals with lifestyle changes and a balanced diet as an effective approach to dyslipidemic patients, as has been reported in different papers.20,22,27
FundingThe meta-analysis was funded by Laboratorios Rottapharm, Barcelona, Spain.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe authors declare no conflicts of interest.